Institute of Oceanology, Chinese Academy of Sciences
Article Information
- JIANG Haifeng(姜海峰), CHENG Xiaofei(程小飞), GENG Longwu(耿龙武), TANG Shizhan(汤施展), TONG Guangxiang(佟广香), XU Wei(徐伟)
- Comparative study of the nutritional composition and toxic elements of farmed and wild Chanodichthys mongolicus
- Chinese Journal of Oceanology and Limnology, 35(4): 737-744
- http://dx.doi.org/10.1007/s00343-017-6119-0
Article History
- Received Apr. 18, 2016
- accepted in principle Jun. 13, 2016
- accepted for publication Jun. 16, 2016
2 Fisheries Institute Science of Hunan Province, Changsha 410153, China
Fish represent an excellent protein source in healthy human diet are owing to the richness in highquality proteins, n-3 polyunsaturated fatty acids (PUFA), especially eicosapentaenoic acid (EPA: C20:5n3) and docosahexaenoic acid (DHA: C22:6n3), as well as other micronutrients such as vitamins, minerals and trace elements that play critical roles in human nutrition, disease prevention and health promotion (Nakamura et al., 2007). In recent decades, aquaculture has contributed most in the increase of world fish production as a result of the growing demand for fish products combined with the serious depletion in wild resources. China ranks the first in world aquaculture, where the aquaculture production occupied up to 60% of the total at the same time accounted for more than 75% of the national fish production in 2013 (CMAFB, 2014; FAO, 2014). However, aquaculture industry faces an important challenge in providing consumers a final product that resembles the wild equivalent, ideally, a product with superior quality (González et al., 2006).
Quality of fish involves several aspects such as sensory, nutritional composition and edible safety. Indeed, numerous studies were undertaken to compare the quality of farmed fish species to their wild counterpart (Alasalvar et al., 2002; González et al., 2006; Fuentes et al., 2010; Rodríguez-Barreto et al., 2012; Wang et al., 2014). However, results can hardly reach a consensus since the quality traits can be strongly affected by various factors including species, life history, environment salinity and temperature, food sources, rearing systems, feeding regimes etc (Calabretti et al., 2003; Xiccato et al., 2004; Fuentes et al., 2010; Nurnadia et al., 2013). Therefore, scientifically examine and consider these factors and variation is required when culturing, fishing and consuming fish and fish products. Furthermore, despite the recognized benefits in human nourishment, fish can also accumulate many notorious contaminations that will counteract the beneficial effects from fish consumption. The teratogenic and carcinogenic effects of toxic elements like arsenic, cadmium, mercury and lead (As, Cd, Hg and Pb), and persistent organic pollutants (POPs) have been extensively documented (Battershill, 1994; Weyandt et al., 2008; Ginsberg and Toal, 2009). Given the growing concerns regarding the nutrition and safety of farmed and wild fish, it is of great importance to study both of the nutrient and contaminant contents in fish. On the other hand, the essential amino acid and fatty acid profiles in body composition have been regarded as good indicators of amino acids and fatty acid requirements in each fish species (Saavedra et al., 2006; Rodríguez-Barreto et al., 2012), therefore this information is not only useful for formulating a more suitable diet, but also for better understanding the nutritional influence in all life stages of fish.
Chanodichthys mongolicus, commonly known as red tail, is a cyprinid fish widely distributed in Amur, Huanghe (Yellow), Huaihe, Changjiang (Yangtze), Qiantang, Zhujiang (Pearl) Rivers and other freshwater bodies in China (Lin et al., 2012). This species has high price and market potential because their delicious taste. However, the wild population has undergo severe decline in recent decades due to overfishing, habitat degradation and water pollution. Aquaculture of C. mongolicus has been initiated in several regions in China to meet the need of stock enhancement and market demand (Lin et al., 2012; Geng et al., 2017). The differences between farmed and wild fish with respect to their quality traits is an important issue to all the actors of the sector and for consumer. Therefore, the objective of this study was to determine the variability in nutritional composition (proximate, fatty acid and amino acid) and toxic elements (As, Cd, Pb and Hg) of C. mongolicus from farmed and wild origin.
2 MATERIAL AND METHOD 2.1 SamplingIn July 2015, twenty wild C. mongolicus were caught from the Jingpo Lake (Heilongjiang Province, China) by local fishermen using fish net. Another twenty farmed C. mongolicus were obtained from the Hulan Experimental Station, Heilongjiang River Fisheries Research Institute. Farmed fish were fed a commercial feed for crucian carp (crude protein 30%, crude fat 5%). All the fish specimens were at age 3 and of similar sizes: the average weight and length (mean±SD) were 134.9±25.3 g and 26.42±1.82 cm for wild fish and 113.6±16.6 g, 24.96±1.20 cm for farmed fish. After measurement of biometric data, the axial muscle without skin were immediately dissected and pooled as three samples. Samples were packed in polyethylene pouches on ice then kept in a deep freezer at-20℃ until analysis.
2.2 Chemical analysisThe nutrient components analysis including proximate composition, amino acids and fatty acids and the toxic elements analysis were As, Cd, Pb and Hg. All analysis were performed in triplicate with the procedure applied as follows:
The proximate composition was determined following the standard methods of AOAC (2002). Moisture content was estimated by oven-drying at 105℃ to reach a constant weight; Crude protein (N96.25) was determined by the Kjeldahl method using an Auto Kjeldahl System (K9860; HANON, DE, USA); crude lipid was extracted by the etherextraction method using an Auto Soxtec System (E-816; BUCHI, Switzerland), while ash contents was carried out by ignition the samples in a muffle furnace at 600℃ for 24 h. The amino acid contents were determined using an automatic amino acid analyzer (L-8900; Auto analyzer, HITACHI, Japan) according to the Chinese national standard (Ministry of Health of the People's Republic of China and Standardization Administration of the People's Republic of China, 2004). Because of the great loss and break down in acid hydrolysis, tryptophan and cysteine were respectively prepared by means of formic acid oxidation hydrolysis method and alkaline hydrolysis method (Valverde et al., 2013). Analysis of fatty acids was conducted by gas chromatography-mass spectrometer (GC-MS) (7820A; Agilent, CA, USA), according to the Chinese national standard (General Administration of Quality Supervision, Inspection and Quarantine of the People's Republic of China and Standardization Administration of the People's Republic of China, 2008), and area normalization method was used to make quantitative analysis of their compositions. All analyses described above were calculated and presented as percentages (%).
For the determination of Hg, homogenized fish samples were directly subjected to an Automatic Hg Analyser (Leeman labs, NH, USA). Prior the determination of As, Cd and Pb, a microware digestion process was conducted, then performed using an inductively-coupled plasma mass spectrometry (ICPMS) (7500 cx; Agilent, CA, USA), according to the method described by Jiang et al. (2015).
2.3 Evaluation of nutritional valuesThe amino acid scores (AAS), chemical score (CS) and essential amino acid index (EAAI) were used to represent the nutritional values of the fish (FAO/WHO, 1973). A high score means high quality in protein amino acid components. The formulas for calculation are as follows:
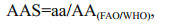 (1)
(1)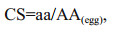 (2)
(2)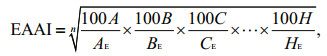 (3)
(3)where aa, AA(FAO/WHO) and AA(egg) are the amount of amino acid in the test protein, FAO/WHO reference pattern and whole egg protein reference pattern (%), respectively; n is the number of amino acid for comparison; A, B, C, …, H are the contents of essential amino acids (EAA) (%, dry weight) in the test sample; AE, BE, CE, …, HE are the EAA contents (%, dry weight) in egg reference pattern.
2.4 Statistical analysisIn order to compare the differences between wild and farmed C. mongolicus, simple t-tests were performed using SPSS for windows (Version 19.0). Data are expressed as means±standard deviation. A P-value of less than 0.05 was considered as significance level in this study.
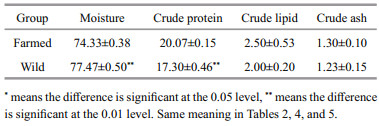
3 RESULT AND DISCUSSION 3.1 Proximate compositionsComparison of proximate compositions between the farmed and wild C. mongolicus is presented in Table 1. Farmed C. mongolicus showed comparable results in lipid and ash levels with wild C. mongolicus (P>0.05), whereas possessed a significant lower moisture content and higher protein content than their wild counterpart (P < 0.05). Actually, some farmed fish species were reported have similar proximate composition with wild individuals of the same species like Sepia pharaonis (Wen et al., 2014), Lota lota (Huang et al., 2015) and Seriola lalandi (O'Neill et al., 2015), whereas more studies observed significant different results. For example, numerous studies observed a trend that farmed fish tend to contain lower moisture content and higher fat content compared to the same species captured in natural environment (González et al., 2006; Fuentes et al., 2010; Zhao et al., 2010; Rodríguez-Barreto et al., 2012; Wang et al., 2014). Generally, the differences obtained by different studies were highly species-specific and largely dependent on the environmental factors and rearing techniques of the farm (Cardinal et al., 2011; Nurnadia et al., 2013). As one of the life basic materials, protein is an important indicator of evaluating food nutrition. Therefore, the significant higher protein levels in farmed fish found in this study indicated that the nutrient value did not decrease under culture environment.
|
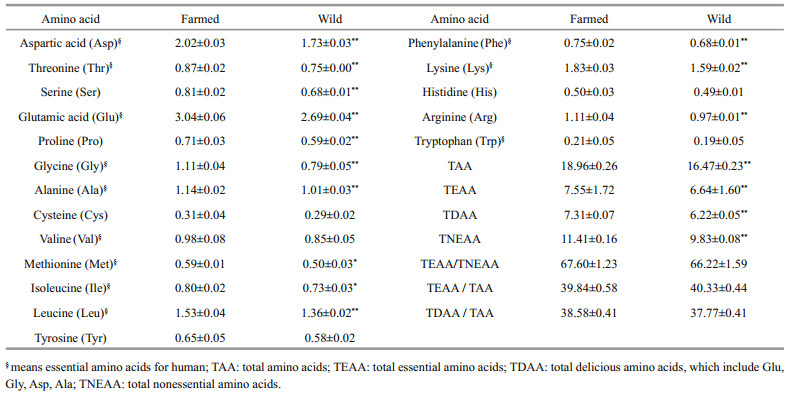
The comparison in amino acid profiles of farmed and wild C. mongolicus is depicted in Table 2. A total of 18 amino acids were detected in the muscles of farmed and wild C. mongolicus, including eight essential amino acids (EAA: Lys, Trp, Phe, Met, Thr, Ile, Leu, Val), ten non-essential amino acids (NEAA: Gly, Ala, Ser, Asp, Glu, Pro, Arg, His, Tyr, Cys). With the exception of Cys, Val, Tyr, His and Trp, which did not vary significantly (P>0.05), all other amino acids constituents showed significant higher concentrations (P < 0.05) in farmed fish in comparison with the wild equivalent (P < 0.05). The amino acid concentrations in farmed and wild C. mongolicus shared the same trend: Glu was the most abundant (2.69% in wild fish, 3.04% in farmed fish) followed by Asp, Lys, Leu and Ala, with Trp being the lowest (0.19% in wild fish, 0.21% in farmed fish).
|
The nutritional quality of a protein source is governed by its amino acid composition and bioavailability. In the present study, the levels of total amino acid (TAA), total essential amino acids (TEAA), total non-essential amino acid (TNEAA) and total delicious amino acid (TDAA) in farmed C. mongolicus were all significantly higher (P < 0.05) than those in wild fish. The TEAA/TAA values were 39.84% in farmed fish and 40.33% in wild fish, and the TEAA /TNEAA values were 67.60% and 66.22% respectively, which were comparable to the reference values of 40% and above the reference values of 60% recommended by FAO/WHO, indicating that this species can become a food protein source of high quality. Moreover, it has been suggested that the TDAA components including Glu, Asp, Ala and Gly are related to the characteristic taste and flavor of fish, of which different contents may cause variations in fish flavor (Ruiz-Capillas and Moral, 2004). The TDAA/TAA values in farmed and wild C. mongolicus were 38.58% and 37.77% (P>0.05) respectively, indicating that the flavor of the farmed fish was no inferior to wild fish. Overall, our results showed superior quality in farmed C. mongolicus when compared to the wild equivalent in respect to protein quality.
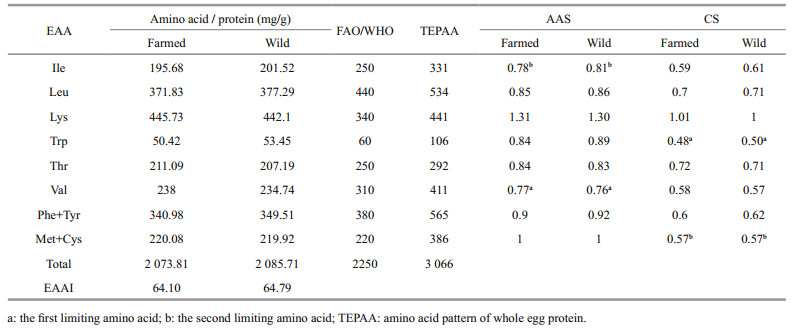
With regard to the evaluation of protein quality, amino acid scores (AAS) are worldwide used method (Iqbal et al., 2006). Prior to compare with the FAO/ WHO reference pattern (AA(FAO/WHO)) and whole egg protein reference pattern (AA(egg)), the data in Table 2 were converted to milligrams of amino acids in per gram protein by multiplying 0.625 (Wang et al., 2014). As Table 3 shows, the TEAA contents of C. mongolicus were below the FAO/WHO reference pattern (2 250 mg/g N), and the TEAA contents of the farmed fish (2 073.81 mg/g N) was a slightly lower than wild fish (2 085.71 mg/g N). The first limiting amino acid and second limiting amino acid for C. mongolicus were Val and Ile according to the AAS, while they were Trp and Met+Cys based on the CS. Moreover, Lys displayed the highest score in both farmed and wild C. mongolicus, which means C. mongolicus are rich in Lys. Lysine is the first limiting amino acid in cereal-based diets population, and therefore the Lys in C. mongolicus can be a good supplement to the corresponding deficiency. The EAAI in farmed and wild C. mongolicus were 64.10 and 64.79, indicating that the protein in farmed and wild C. mongolicus muscle is well-balanced in EAA composition and is of high quality.
|
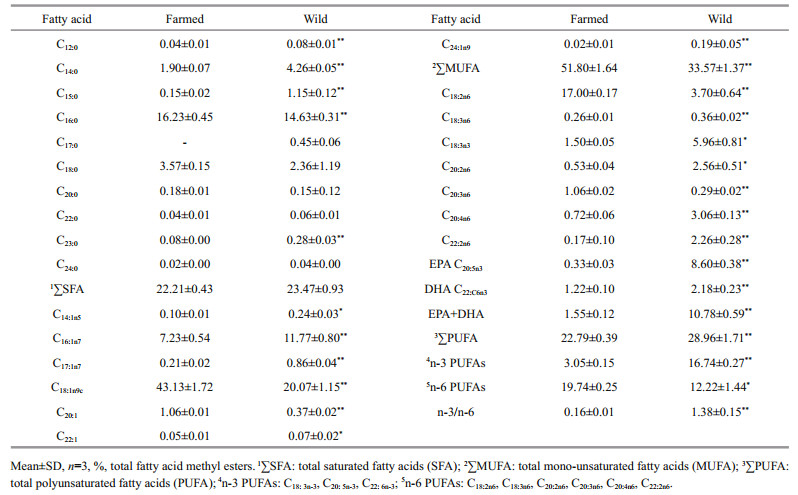
The total saturated fatty acids (SFAs) content were comparable between the two fish groups (22.21% for farmed fish; 23.47% for wild fish), with palmitic acid (C16:0) the most dominant (Table 4). Half of the saturated fatty acids (SFAs) measured (n=5) were significantly different (C12:0, C14:0, C15:0, C16:0 & C23:0) (P < 0.05) and methylheptadecanoate (C17:0) was only detected in wild fish. The fatty acid profiles in farmed and wild C. mongolicus were dominated by monounsaturated fatty acid (MUFA) with farmed fish contained much higher MUFA content than wild fish (P < 0.05). This result is in good agreement with the general trend observed in numerous studies, where the muscle of farmed fish contains higher total MUFA compared to wild fish of the same species (Fuentes et al., 2010; Norambuena et al., 2012; Wen et al., 2014; O'Neill et al., 2015). All components within the MUFA were significantly different in concentrations (P < 0.05). Oleic acid (C18:1n9c) was the highest amount in both fish groups, and about 2.14 times higher in farmed C. mongolicus. Generally, the high C18:1 level found in this study is a typical character of fish muscle composition, and a high C18:1 content mayreduce the risk of cardiovascular diseases (CVD) and numerous inflammatory disorders (O'Neill et al., 2015).
In contrast, total polyunsaturated fatty acid (PUFA) including eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) were all significantly higher in wild fish (28.96%) than those in farmed fish (22.79%). EPA and linoleic acid (C18:2n6) were the most abundant in wild and farmed C. mongolicus respectively. All PUFAs displayed significantly higher concentration in wild fish except for the linoleic acid (C18:2n6), which was significant higher in farmed fish (P < 0.05). Generally, fish recognized as healthy food for people are largely owing to the richness in omega-3 PUFAs that benefit lowering the risk of CVD and enhancing brain development (Ginsberg and Toal, 2009; Hossain, 2011). However, in this study, the most renowned omega-3 PUFAs i.e. EPA and DHA, presented about 26.1 and 1.79 times higher concentration in wild fish compared to the farmed equivalent, and the EPA+DHA was up to 10.78% in wild fish whereas accounted for 1.55% in farmed fish. This result is consistent with the observations of numerous studies that wild fish commonly contain higher levels of EPA and DHA compared to their farmed counterpart (Zhao et al., 2010; Hossain, 2011). Accordingly, our result suggests that regular intake of wild C. mongolicus can exert more nutritional benefits than farmed C. mongolicus. Moreover, the EPA+DHA value in wild C. mongolicus was much higher than those reported in other extensively consumed freshwater fish species in China such as Cyprinus carpio (0.3%), Ctenopharyngodon idellus (0.2%), Carassius auratus (3.3%), Hypophthalmichthys molitrix (5.8%) (Du et al., 2012). In fact, it is well established that fatty acid profile in fish flesh reflects the dietary fatty acid composition (Torstensen et al., 2000; Cejas et al., 2003; Cardinal et al., 2011). Hence, the differences could be explained by the fatty acid composition of the diet of both farmed and wild fishes. Typically, wild C. mongolicus is carnivores and eat small fish which are rich in n-3 PFUAs with low proportion of n-6 PUFAs, whereas the farmed C. mongolicus lives on commercial feed which usually use cereal and vegetable oils containing more of the n-6 PUFAs and less n-3 PUFAs. In view of that amino acid profile can be customized by adjusting dietary intakes, increasing the n-3 and n-6 PUFAs proportion in the diet or selecting appropriate fatty acid sources which can facilitate biosynthesis of n-3 and n-6 PUFAs should be emphasized in order to improve the nutritional composition of farmed C. mongolicus in the future.
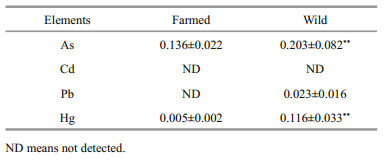
3.4 Toxic elements in fishConcentrations of toxic elements As, Cd, Pb and Hg in farmed and wild C. mongolicus are shown in Table 5. Wild fish had significantly higher levels of As and Hg than farmed fish; Pb was only detected in wild fish and Cd was undetected in both fish groups (Table 5). The overall lower toxic metals levels in farmed fish may be attributed to the strict supervision of feed quality and the relatively controlled conditions compared to wild fish. Based on the established limits of edible parts of fish in China (General Administration of Quality Supervision, Inspection and Quarantine of the People's Republic of China, 2013), the maximum limits (MLs) for inorganic As, Pb, Cd and methyl Hg (MeHg) were 0.1 mg/kg, 0.5 mg/kg, 0.5 mg/kg (1.0 mg/kg for carnivorous fishes), respectively. Assuming that inorganic As accounted for 10% of total As and methyl Hg accounted for 75% of total Hg in fish (Qin et al., 2015), all the toxic elements detected in both fish groups were far below the MLs.
The present study has provided novel nutritional and safety information on farmed and wild C. mongolicus, which can inform all actors in the aquaculture sectors and consumers. Significant variations in proximate, amino acid and fatty acid composition, and toxic elements contents in farmed and wild fish were observed. Compared to wild fish, farmed fish had significantly higher crude protein content while lower moisture content. Both groups (farmed and wild C. mongolicus) contained high quality protein with a well-balanced composition of EAA, and farmed fish had an overall higher amino acid contents, whereas displayed considerable lower PUFA content than their wild counterpart, particularly for the EPA and DHA. Moreover, although wild fish presented higher concentrations of toxic elements, both groups are of high edible safety. Therefore, regular consumption of wild C. mongolicus can have superior health benefits than farmed fish to some extent. In view of that there has no specific feed for C. mongolicus, further studies should pay close attention to adjust the feed formulation to improve the proportion of n-3 and n-6 PUFAs in farmed fish.
5 ACKNOWLEDGMENTThe authors sincerely thank Dr. DU Xue, Mr. SONG Dan and Ms. BAI Shuyan at Heilongjiang River Fisheries Research Institute, Chinese Academy of Fishery Sciences for help in sample collection.
| Alasalvar C, Taylor K D A, Zubcov E, Shahidi F, Alexis M, 2002. Differentiation of cultured and wild sea bass (Dicentrarchus labrax):total lipid content, fatty acid and trace mineral composition. Food Chemistry, 79(2): 145–150. Doi: 10.1016/S0308-8146(02)00122-X |
| AOAC. 2002. Official Methods of Analysis of AOAC International. 17th ed. AOAC, Arlington, Virginia. |
| Battershill J M, 1994. Review of the safety assessment of polychlorinated biphenyls (PCBs) with particular reference to reproductive toxicity. Human & Experimental Toxicology, 13(9): 581–597. |
| Calabretti A, Cateni F, Procida G, Favretto L G, 2003. Influence of environmental temperature on composition of lipids in edible flesh of rainbow trout (Oncorhynchus mykiss). Journal of the Science of Food and Agriculture, 83(14): 1493–1498. Doi: 10.1002/(ISSN)1097-0010 |
| Cardinal M, Cornet J, Donnay-Moreno C, Gouygou J P, Bergé J P, Rocha E, Soares S, Escórcio C, Borges P, Valente L M P, 2011. Seasonal variation of physical, chemical and sensory characteristics of sea bream (Sparus aurata) reared under intensive conditions in Southern Europe. Food Control, 22(3-4): 574–585. Doi: 10.1016/j.foodcont.2010.10.007 |
| Cejas J R, Almansa E, Villamandos J E, Badía P, Bolaños A, Lorenzo A, 2003. Lipid and fatty acid composition of ovaries from wild fish and ovaries and eggs from captive fish of white sea bream (Diplodus sargus). Aquaculture, 216(1-4): 299–313. Doi: 10.1016/S0044-8486(02)00525-2 |
| CMAFB. 2014. Chinese Ministry of Agriculture Fisheries Bureau. China Fishery Statistical Yearbook 2014. China Agriculture Press, Peking. (in Chinese) |
| Du Z Y, Zhang J, Wang C R, Li L X, Man Q Q, Lundebye A K, Frøyland L, 2012. Risk-benefit evaluation of fish from Chinese markets:nutrients and contaminants in 24 fish species from five big cities and related assessment for human health. Science of the Total Environment, 416: 187–199. Doi: 10.1016/j.scitotenv.2011.12.020 |
| FAO. 2014. Food and Agriculture Organization of the United Nations. The State of World Fisheries and Aquaculture 2014. Food and Agriculture Organization of the United Nations, Rome. http://www.fao.org/3/a-i3720e.pdf. Accessed on 2014-11. p. 3-92. |
| FAO/WHO. 1973. Energy and Protein Requirements. Technical Report Series No. 522. WHO, Geneva, Switzerland. |
| Fuentes A, Fernández-Segovia I, Serra J A, Barat J M, 2010. Comparison of wild and cultured sea bass (Dicentrarchus labrax) quality. Food Chemistry, 119(4): 1514–1518. Doi: 10.1016/j.foodchem.2009.09.036 |
| General Administration of Quality Supervision, Inspection and Quarantine of the People's Republic of China. GB 2762-2012. 2013. Maximum levels of contaminants in foods. China Standard Press, Beijing. (in Chinese) |
| General Administration of Quality Supervision, Inspection and Quarantine of the People's Republic of China and Standardization Administration of the People's Republic of China. GB/T 9695. 2-2008. 2008. Meat and meat products-Determination of fatty acids. China Standard Press, Beijing. (in Chinese) |
| Geng L W, Jiang H F, Tong G X, Xu W. 2017. Determining oxygen consumption rate and asphyxiation point in Chanodichthys mongolicus using an improved respirometer chamber. Chinese Journal of Oceanology and Limnology, 35(2): 294-302, http://dx.doi.org/10.1007/s00343-016-5293-9. |
| Ginsberg G L, Toal B F, 2009. Quantitative approach for incorporating methylmercury risks and omega-3 fatty acid benefits in developing species-specific fish consumption advice. Environmental Health Perspectives, 117(2): 267–275. Doi: 10.1289/ehp.11368 |
| González S, Flick G J, O'keefe S F, Duncan S E, McLean E, Craig S R, 2006. Composition of farmed and wild yellow perch (Perca flavescens). Journal of Food Composition and Analysis, 19(6-7): 720–726. Doi: 10.1016/j.jfca.2006.01.007 |
| Hossain M A, 2011. Fish as source of n-3 polyunsaturated fatty acids (PUFAs), which one is better-farmed or wild?. Advance Journal of Food Science & Technology, 3(6): 455–466. |
| Huang W, Sheng Z M, Yu S B, Ma L M, Sun Y, 2015. Comparative analysis of farmed and wild burbot muscle nutrients. Journal of Zhejiang Ocean University (Natural Science), 34(1): 36–39. |
| Iqbal A, Khalil I A, Ateeq N, Khan M S, 2006. Nutritional quality of important food legumes. Food Chemistry, 97(2): 331–335. Doi: 10.1016/j.foodchem.2005.05.011 |
| Jiang H F, Tang S Z, Qin D L, Chen Z X, Wang J L, Bai S Y, Mou Z B, 2015. Heavy metals in sea cucumber juveniles from coastal areas of Bohai and Yellow Seas, North China. Bulletin of Environmental Contamination and Toxicology, 94(5): 577–582. Doi: 10.1007/s00128-014-1432-1 |
| Lin M L, Wang Q D, Xia Y G, Murphy B R, Li Z J, Liu J S, Zhang T L, Ye S W, 2012. Effects of two anesthetics on survival of juvenile Culter mongolicus during a simulated transport experiment. North American Journal of Aquaculture, 74(4): 541–546. Doi: 10.1080/15222055.2012.700905 |
| Ministry of Health of the People's Republic of China and Standardization Administration of the People's Republic of China. GB/T 5009. 124-2003. 2004. Determination of amino acids in foods. China Standard Press, Beijing. (in Chinese) |
| Nakamura Y N, Ando M, Seoka M, Kawasaki K, Tsukamasa Y, 2007. Changes of proximate and fatty acid compositions of the dorsal and ventral ordinary muscles of the fullcycle cultured Pacific bluefin tuna Thunnus orientalis with the growth. Food Chemistry, 103(1): 234–241. Doi: 10.1016/j.foodchem.2006.07.064 |
| Norambuena F, Estevez A, Bell G, Carazo I, Duncan N, 2012. Proximate and fatty acid compositions in muscle, liver and gonads of wild versus cultured broodstock of Senegalese sole (Solea senegalensis). Aquaculture, 356-357: 176–185. Doi: 10.1016/j.aquaculture.2012.05.018 |
| Nurnadia A A, Azrina A, Amin I, Mohd Y A S, Mohd I E H, 2013. Mineral contents of selected marine fish and shellfish from the west coast of Peninsular Malaysia. Food Research International, 20(1): 431–437. |
| O'Neill B, Le Roux A, Hoffman L C, 2015. Comparative study of the nutritional composition of wild versus farmed yellowtail (Seriola lalandi). Aquaculture, 448: 169–175. Doi: 10.1016/j.aquaculture.2015.05.034 |
| Qin D L, Jiang H F, Bai S Y, Tang S Z, Mou Z B, 2015. Determination of 28 trace elements in three farmed cyprinid fish species from Northeast China. Food Control, 50: 1–8. Doi: 10.1016/j.foodcont.2014.08.016 |
| Rodríguez-Barreto D, Jerez S, Cejas J R, Martin M V, Acosta N G, Bolaños A, Lorenzo A, 2012. Comparative study of lipid and fatty acid composition in different tissues of wild and cultured female broodstock of greater amberjack (Seriola dumerili). Aquaculture, 360-361: 1–9. Doi: 10.1016/j.aquaculture.2012.07.013 |
| Ruiz-Capillas C, Moral A, 2004. Free amino acids in muscle of Norway lobster (Nephrops novergicus (L.)) in controlled and modified atmospheres during chilled storage. Food Chemistry, 86(1): 85–91. |
| Saavedra M, Conceição L E C, Pousão-Ferreira P, Dinis M T, 2006. Amino acid profiles of Diplodus sargus (L., 1758) larvae:implications for feed formulation. Aquaculture, 261(2): 587–593. Doi: 10.1016/j.aquaculture.2006.08.016 |
| Torstensen B E, Lie Ø, Frøyland L, 2000. Lipid metabolism and tissue composition in Atlantic salmon (Salmo salar L.)-effects of capelin oil, palm oil, and oleic acid-enriched sunflower oil as dietary lipid sources. Lipids, 35(6): 653–664. |
| Valverde J C, Martínez-Llorens S, Vidal A T, Jover M, Rodríguez C, Estefanell J, Gairín J I, Domingues P M, Rodríguez C J, García B G, 2013. Amino acids composition and protein quality evaluation of marine species and meals for feed formulations in cephalopods. Aquaculture International, 21(2): 413–433. Doi: 10.1007/s10499-012-9569-6 |
| Wang Y Y, Yu S L, Ma G J, Chen S B, Shi Y, Yang Y H, 2014. Comparative study of proximate composition and amino acid in farmed and wild Pseudobagrus ussuriensis muscles. International Journal of Food Science & Technology, 49(4): 983–989. |
| Wen J, Chen D H, Zeng L, 2014. Comparison in nutritional quality between wild and cultured cuttlefish Sepia pharaonis. Chinese Journal of Oceanology and Limnology, 32(1): 58–64. Doi: 10.1007/s00343-014-3083-9 |
| Weyandt J, Ellsworth R E, Hooke J A, Shriver C D, Ellsworth D L, 2008. Environmental chemicals and breast cancer risk-a structural chemistry perspective. Current Medicinal Chemistry, 15(26): 2680–2701. Doi: 10.2174/092986708786242930 |
| Xiccato G, Trocino A, Tulli F, Tibaldi E, 2004. Prediction of chemical composition and origin identification of European sea bass (Dicentrarchus labrax L.) by near infrared reflectance spectroscopy (NIRS). Food Chemistry, 86(2): 275–281. |
| Zhao F, Zhuang P, Zhang L Z, Shi Z H, 2010. Biochemical composition of juvenile cultured vs. wild silver pomfret, Pampus argenteus:determining the diet for cultured fish. Fish Physiology and Biochemistry, 36(4): 1105–1111. |
 2017, Vol. 35
2017, Vol. 35