Institute of Oceanology, Chinese Academy of Sciences
Article Information
- ZHOU Konglin(周孔霖), SUN Song(孙松)
- Effect of diurnal temperature difference on lipid accumulation and development in Calanus sinicus (Copepoda: Calanoida)
- Chinese Journal of Oceanology and Limnology, 35(4): 745-753
- http://dx.doi.org/10.1007/s00343-017-6039-z
Article History
- Received Feb. 3, 2016
- accepted in principle Mar. 24, 2016
- accepted for publication May. 18, 2016
2 University of Chinese Academy of Sciences, Beijing 100049, China;
3 Laboratory for Marine Ecology and Environmental Science, Qingdao National Laboratory for Marine Science and Technology, Qingdao 266071, China;
4 Jiaozhou Bay Marine Ecosystem Research Station, Institute of Oceanology, Chinese Academy of Sciences, Qingdao 266071, China
As the dominant zooplankton in the coastal waters of East Asia, Calanus sinicus plays a significant role in transferring primary production to higher trophic levels in the pelagic ecosystem. Dominated by C5 copepodites (C5s), C. sinicus migrates into the Yellow Sea Cold Water Mass (YSCWM) in a quiescent state to conserve the population in the Yellow Sea in summer, because the high surface temperature induces high mortality in C. sinicus (Pu et al., 2004; Sun and Zhang, 2005; Zhang et al., 2007; Zhou et al., 2016). The over-summering C5s have a large oil sac, which can provide the energy needed during the entire summer (Sun et al., 2011). Lipids also help to maintain neutral buoyancy at depth, which could reduce energy output (Campbell and Dower, 2003). The lipid reserves are believed to induce dormancy in over-wintering Calanus by accumulating to a high threshold; dormancy is terminated when the lipid reserves have been consumed to low levels (Hirche, 1996; Miller et al., 2000; Irigoien, 2004; Saumweber and Durbin, 2006; Maps et al., 2010; Ji, 2011). Copepods with small oil sacs continue to develop and molt into adults, rather than rest at depth (Johnson et al., 2008). The lipid accumulation and diapause induction might be affected by either various environmental factors (e.g., food, temperature, and predation pressure) or intrinsic processes, but the specific process is unknown (Johnson et al., 2008; Ji, 2011).
Copepods accumulate lipid reserves in oil sacs during the C3–5 copepodite stages (Kattner and Krause, 1987; Lee et al., 2006). Wax ester is the main lipid type stored in the oil sacs of dormant Calanus copepods (Miller et al., 1998; Hagen and Auel, 2001), including C. sinicus (Wang et al., 2014). Additionally, the main fatty alcohols (20:1n–9 and 22:1n–11) in dormant Calanus species' lipid reserves are de novo synthesized from either lipid or non-lipid compounds in food (Kattner and Krause, 1987; Hagen et al., 1993; Hagen and Auel, 2001; Graeve et al., 2005; Falk-Petersen et al., 2009; Bergvik et al., 2012). More lipid is accumulated by copepods when cultured on highconcentration diets (Hakanson, 1984; Escribano and McLaren, 1992; Hygum et al., 2000; Rey-Rassat et al., 2002). In addition to food quantity, food quality can also influence lipid accumulation (Hygum et al., 2000). Pepin (2011) found that C. finmarchicus accumulated more lipids when fed diatoms than those fed mainly on dinoflagellates. However, C. sinicus C5s develop the largest oil sac within the range of the YSCWM in late spring, whereas they store less lipid when inhabiting nearshore areas where Chl a concentrations are much higher (Wang, 2009). Therefore, other factors might affect lipid accumulation in C. sinicus, e.g., temperature.
Diel vertical migration is common in C. sinicus (Huang et al., 1993), which not only allows them to avoid predation (Uye et al., 1990), but also elevates the net energy gain from ingestion (Enright, 1977). In late spring, a thermocline (11–22℃) develops at the subsurface of the Yellow Sea, under which the YSCWM (≤10℃) forms (Hu and Wang, 2004; Wang, 2009). At night, C. sinicus migrates upward to feed in the thermocline, where the Chl a concentration peaks and sea water is warmer (Wei et al., 2013). Copepods then migrate downward into the YSCWM during the day, where the cold water reduces metabolic expenditure and enhances the utilization of ingested food (Enright, 1977). Therefore, C. sinicus could experience a temperature difference (1–12℃) during the diurnal vertical migration. Furthermore, maximum lipid reserves are accumulated in the C5s inhabiting the YSCWM in late spring, whereas C5s outside the YSCWM, where the seawater is vertically mixed, have much smaller oil sacs (Sun et al., 2011). The temperature differences might promote lipid accumulation in C. sinicus to prepare for over-summering.
In this study, C. sinicus C3 copepodites (C3s) were cultured at four constant temperatures (10, 13, 16, and 19℃) and three diurnal temperature ranges (10–13, 10–16, and 10–19℃), to study copepod development and lipid accumulation at different temperatures. The aims of this study were to determine if (1) diurnal temperature differences facilitate lipid accumulation, and (2) temperature differences induce oversummering in C. sinicus.
2 MATERIAL AND METHOD 2.1 Sampling and temperature treatmentsLiving C. sinicus were sampled from Jiaozhou Bay (36°04′N, 120°29′E), China, using a 160-μm mesh zooplankton net (0.32 m mouth diameter) in May 2013. The samples were temporarily kept in a 20-L incubation barrel with ambient seawater (12.2℃) and transferred to a laboratory within 1 h after sampling. Thirty C3–5 copepodites, females and males, respectively, were preserved in 5% formalin seawater solution for morphometrics. Healthy C3s were selected randomly and preserved in 2-L beakers with pre-cooled filtered sea water (FSW, filtered through a 0.45-μm pore size cellulose acetate filter).
As described above, the temperature difference might promote lipid accumulation in C. sinicus in the newly formed YSCWM. Because the optimum temperature for C. sinicus development is < 20℃ (Pu, 2003), the culture temperatures were set at four constant temperatures (10, 13, 16, and 19℃) and three diurnal temperature ranges (10–13, 10–16, and 10–19℃) in this study. In the diurnal temperature range treatments, temperatures were varied in a 12-h low:12-h high-temperature cycle. At the beginning of the experiment, approximately 10 C3s were transferred to a 1-L glass beaker with pre-cooled FSW, with three beakers in each treatment and seven treatments in total. The beakers were placed aPart in seven incubators to maintain the temperatures. The initial temperature setting was 13℃. After acclimating for 1 day, the temperatures were changed by 1℃ per hour until the experimental setting had been achieved. Copepods were fed on a mixed microalgae diet of Skeletonemacostatum, Thalassiosira sp., Prorocentrum micans, and P. minimum at approximately 1 μg C/ mL. The seawater was renewed every other day.
2.2 Phytoplankton culturesMixed microalgae diets provide more nutrition to copepods than mono-algal diets (Irigoien et al., 2000; Camus and Zeng, 2010). The four algae used are common species in the Yellow Sea (Song, 2010; Liu et al., 2011a). The sizes of the microalgae were appropriate for C. sinicus to feed on (Table 1) (Li et al., 2007). The diatoms (S. costatum and Thalassiosira sp.) were cultured in f/2+Si medium in a 12-h light:12-h dark cycle at 18℃ in 2-L glass Erlenmeyer flasks, while the dinoflagellates (P. micans and P. minimum) were incubated in f/2 medium using the same photoperiod. The densities and volume of algae were tested by a Coulter Counter (Multisizer 3) every day. Exponential phase algae were used as food for the copepod cultures, and were added every 2 days. The algae carbon content was estimated on a volume basis (Sun et al., 1999).
Every 3 days, each copepod was observed and photographed under a stereomicroscope (Nikon AZ100, Nikon Corporation, Tokyo, Japan) within 1 min, and then gently placed back into the beakers. The length and width of the prosome (PL and PW) and oil sac (OL and OW) were measured. Oil sac volume (OSV) was calculated by the formula: OSV=0.52×OL×OW2, and prosome volume (PV) by the equation: PV=0.58×PL×PW2 (Svetlichny et al., 2006). OSV was used to represent the degree of lipid reserve (Miller et al., 1998). Because OSV increases with body size (Miller et al., 2000), the OSV/PV ratio was defined as the oil sac proportion (OSV%), to remove the influence of body size and represent the relative degree of lipid reserve (Sun et al., 2011). C. sinicus development stages were recorded. The time that each development stage began was defined as the time when half of the copepods had molted into that stage (Uye, 1988). When the copepods had molted into females, gonad development stages were determined following Niehoff and Runge (2003), and included four different gonad development stages (GS1, GS2, GS3, and GS4). The proportion of GS4 was used as a reproductive index (RI) to describe the level of gonad maturity of the copepods at different temperatures (Wang et al., 2009).
2.4 Statistical analysisAll statistical analysis was performed in SPSS 16.0. The normality and homogeneity of data were tested. One-way analysis of variance (ANOVA) and multiple comparisons (Student-Newman-Keuls: S-N-K) were used to assess differences in prosome lengths and relative lipid reserve levels in each treatment. A significant difference among the various comparisons was accepted when P < 0.05. A Pearson correlation analysis was conducted to determine the relationship between the relative lipid reserve level and development stage.
3 RESULT 3.1 The morphological characteristics of C. sinicus in Jiaozhou BayThe copepods collected in the field differed significantly in OSV% and prosome lengths among the different stages (Fig. 1; ANOVA, F4, 16=80.14, P=0.000). Males stored the most lipid reserves, followed by the C5s (S-N-K, P < 0.05). The OSV% was similar between C4s and females (S-N-K, P=0.523). The C3s had the smallest oil sacs. Most of the females in the field were immature, with a low reproduction index (10%).
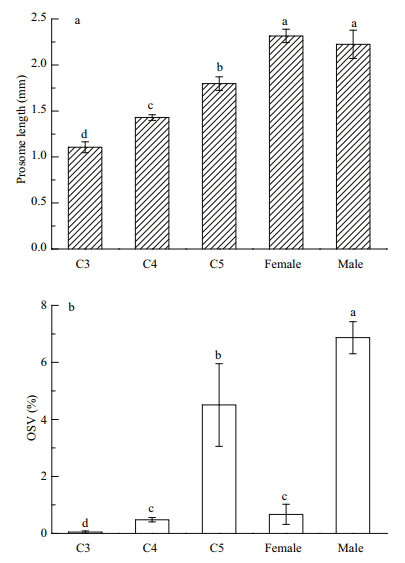
|
| Figure 1 Body size and lipid reserve of Calanus sinicus collected in the field a. prosome lengths (mm); b. oil sac proportions (OSV%). C3: C3 copepodite stage, C4: C4 copepodite stage, C5: C5 copepodite stage. Letters (a, b, c, and d) indicate the significant differences (P < 0.05) among the different development stages. |
Copepodites successfully molted into adults in all of the treatments. The durations of each development stage in all of the treatments are shown in Fig. 2. The duration of copepodite development stages (from C3 to C5) was 15.1, 10.4, 10.5, 7.4, 10.4, 7.3, and 8.0 days at 10, 13, 10–13, 16, 10–16, 19, and 10–19℃, respectively. A low temperature (10℃) extended the development time, while high-temperature treatments (16 and 19℃) accelerated the molting processes (ANOVA, F6, 14= 31.846, P=0.000). The duration of the copepodite stages was similar at 13℃ and 10–13℃ (S-N-K, P=0.987), and at 19℃ and 10–19℃ (S-N-K, P=0.600). However, the copepodite stages were longer at 10–16℃ than at 16℃ (S-N-K, P < 0.05).
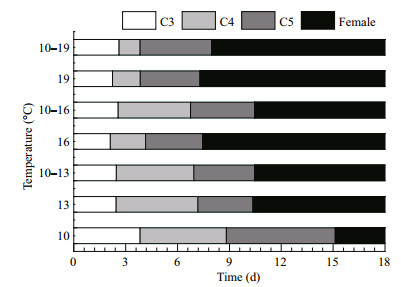
|
| Figure 2 Durations of three copepodite stages and the female stage in Calanus sinicus at seven temperature treatments C3: C3 copepodite stage (white); C4: C4 copepodite stage (light grey); C5: C5 copepodite stage (dark grey); female (black). Treatments: four constant temperatures (10, 13, 16, and 19℃) and three diurnal temperature ranges (10–13, 10–16, and 10–19℃). |
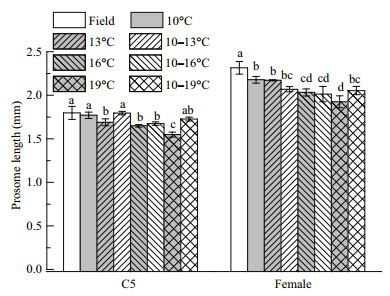
Copepod lipid reserves varied during the experiment (Fig. 3). The OSV% increased and peaked on days 15, 9, 12, 6, 9, 6, and 9 in the 10, 13, 10–13, 16, 10–16, 19, and 10–19℃ treatments, respectively, and decreased thereafter. The copepods were dominated by C5s on these days. The OSV% was positively correlated with the proportion of C5s (Pearson correlation analysis, R=0.499, n=42, P=0.001). When maximum lipid reserves had been accumulated, the OSV% of C5s were lowest at high temperatures (16 and 19℃) (Fig. 4; ANOVA, F7, 16= 5.244, P=0.003). The relevant temperature range treatments (10–16 and 10–19℃) had similar amounts of lipid reserve to the other groups, as well as the C5s in the field (S-N-K, P=0.875). Additionally, the C5 with the biggest oil sac was found in the 10–19℃ treatment (OSV%=16.4%). The temperatures also affected the size of C5 copepodites. C5s in two temperature range treatments (10–13 and 10–19℃) had longer prosome lengths than C5s in the related constant temperature treatments (13 and 19℃), and C5s at lower temperatures had longer prosomes (Fig. 5; ANOVA, F7, 16= 16.148, P=0.000).
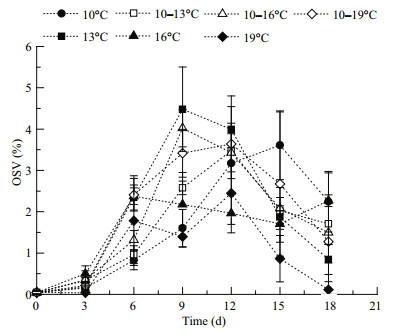
|
| Figure 3 Variations of oil sac proportions (OSV%) in Calanus sinicus cultured in seven temperature treatments Treatments: four constant temperatures (10, 13, 16, and 19℃ (black symbols)) and three diurnal temperature ranges (10–13, 10–16, and 10–19℃ (white symbols)). |
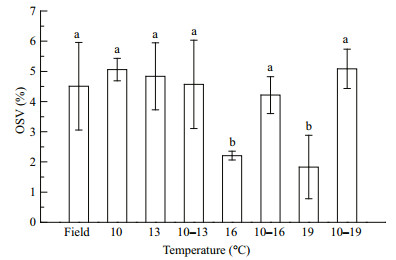
|
| Figure 4 Oil sac proportions (OSV%) of C5s in Calanus sinicus at seven temperature treatments when the OSV% of copepods had reached the maximum Treatments: four constant temperatures (10, 13, 16, and 19℃) and three diurnal temperature ranges (10–13, 10–16, and 10–19℃). Field: copepods collected from Jiaozhou Bay. Letters (a and b) indicate significant differences (P < 0.05) among the different temperature treatments. |
|
| Figure 5 Prosome lengths (mm) of Calanus sinicus C5 copepodites and newly molted females at seven temperature treatments Treatments: four constant temperatures (10, 13, 16, and 19℃ (grey)) and three diurnal temperature ranges (10–13, 10–16, and 10–19℃ (white)). Field: copepods collected from Jiaozhou Bay (white). Letters (a, b, c, and d) indicate significant differences (P < 0.05) among the different temperature treatments for C5s and for females. |
During cultivation, only one copepodite molted into a male (on day 15 in the 10–13℃ temperature range treatment), and had a larger oil sac (OSV%=5.58%) than the females. All of the newly molted females were smaller than the females in the field (Fig. 5), with small oil sacs (OSV%: 1.40%– 3.70%). The female prosomes were longer at lower than at higher temperatures (ANOVA, F7, 16=14.856, P=0.000). Females in the 10–19℃ treatment had longer prosomes than those at 19℃ (S-N-K, P < 0.05), but shorter than those at 10℃.
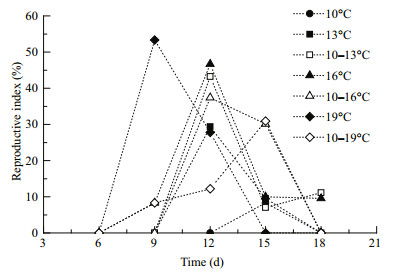
The reproduction indexes increased after the final molting and then decreased in all of the experiment groups (Fig. 6), revealing the gonad development patterns. The indexes peaked on day 15 in the 10 and 10–19℃ treatments, on day 12 in the 13, 10–13, 16, and 10–16℃ treatments, and on day 9 in the 19℃ treatment. However, unfertilized eggs were spawned, leaving immature oocytes in gonads at the end of the experiment.
|
| Figure 6 Variations in the Calanus sinicus reproductive index (%) cultured in seven temperature treatments Treatments: four constant temperatures (10, 13, 16, and 19℃ (black symbols)) and three diurnal temperature ranges (10–13, 10–16, 10– and 19℃ (white symbols)). |
C. sinicus increased lipid reserves during the copepodite stages (C3–5) and peaked in the late C5 stage, which is similar to previous reports for C. finmarchicus (Lee et al., 1972). The C5s cultured in the laboratory had similar sized oil sacs to those in Jiaozhou Bay. Copepods matured within a few days. Thus, a mixed diet with two diatoms and two dinoflagellates met the nutritional needs of C. sinicus.
C. sinicus stored more lipid reserves at low than at high temperatures. Lipid accumulation is more efficient at low temperatures (Chess and Stanford, 1999). The C. sinicus metabolic rate increases as the temperature rises (Zhang et al., 2000). When estimated by body mass and temperature (Ikeda et al., 2001; Li et al., 2004), the daily metabolic loss of C5s at the higher temperature (16 and 19℃) was 2.81–2.97 μg C/(ind.∙d), approximately 1.3–1.4 times that at the low temperature (10℃). Thus, a colder temperature could promote lipid accumulation by reducing metabolic expenditure. Furthermore, C. sinicus development slowed down at lower temperatures, leading more time for copepodites to accumulate lipids in the temperature range treatments.
C. sinicus cultured in two of the temperature range treatments (10–16 and 10–19℃) accumulated 1.9 and 2.1 times the lipid reserves of those cultured at constant temperatures (16 and 19℃) in this study, and might have benefitted from the reduced energy expenditure at 10℃. Additionally, the C. sinicus from the temperature range treatments had a similar oil sac volume to those at 10℃, suggesting that large temperature differences (6–9℃) might promote lipid accumulation in the C5 stage.
Although the concentration of the mixed microalgae diet was over 10 times greater than the Chl a concentration in the Yellow Sea (Wei et al., 2013), the C5s in the laboratory developed much smaller oil sacs than the over-summering C5s (mean OSV%=30%) in late spring (Sun et al., 2011). Given the cold temperature and poor food conditions under the thermocline, C. sinicus could reduce its metabolic loss inside the newly formed YSCWM during the non-feeding hours (Li et al., 2004), and might ingest in the chlorophyll maximum layer at night (Wei et al., 2013). Nocturnal feeding after 12–24 h of starvation would increase the ingestion rate several times in a few hours compared with continuous feeding (McAllister, 1970; Enright, 1977), which, coupled with resting in colder water during the daytime, would maximize the net energetic gain (McLaren, 1963; Enright, 1977). Thus, the conditions in the newly formed YSCWM likely promote lipid accumulation in C. sinicus.
However, the C5s in the laboratory had similar sized oil sacs to the nearshore population in the south Yellow Sea, which have small oil sacs all year round (Sun et al., 2011). The lipid poor C. sinicus molt into adults and spawn rather than quiesce in the YSCWM in summer, even though mortality is high in nearshore waters because of high temperatures (Zhang et al., 2007; Wang et al., 2009). These different life strategies might be attributed to the different degrees of lipid accumulation, which is believed to induce dormancy in Calanus species (Hirche, 1996; Rey-Rassat et al., 2002; Irigoien, 2004; Tarrant et al., 2008). The copepods might be able to either assign the ingested energy to molt and spawn or store enough lipid to induce dormancy (Fiksen, 2000; Johnson et al., 2008; Ji, 2011). Although the diel temperature difference promoted lipid accumulation in this study, the copepods failed to store enough lipid to induce dormancy in the laboratory. No Calanus copepods have been induced into diapause under laboratory conditions to date (Tarrant et al., 2014). Dormancy induction is complex, and is affected by both intrinsic and extrinsic factors. It is likely that the interaction of many environmental factors affects lipid accumulation and fitness maximization, and induces diapause in Calanus copepods (Johnson et al., 2008; Pepin and Head, 2009; Ji, 2011). The physiological processes, such as reduced metabolic rates, suppressed molting, lipid synthesis, and accumulation (Tarrant et al., 2008; Zhou et al., 2016), might be responses to the combined effects of various environmental factors, e.g., temperature and food. However, the interactions between external factors and internal responses are still poorly understood with regard to the induction of dormancy in Calanus copepods. Many factors (e.g., food quality, combined effects of food and temperature) should be considered in future studies to determine the mechanism of dormancy in copepods.
4.2 Lipid consumption and maturityLipid reserves decreased after the copepods had molted into adults, which was consistent with gonad development and maturity. Lipid reserves were used during gonad development; wax esters are transformed into phospholipid to synthesize gonad tissue and support early vitellogenesis in C. finmarchicus after dormancy (Hirche, 1996; Jónasdóttir, 1999; Rey-Rassat et al., 2002; Lee et al., 2006; Kattner et al., 2007; Madsen et al., 2008). The lipid reserves also support final maturity and spawning in C. glacialis and C. hyperboreus in the Arctic Ocean (Hirche and Kattner, 1993; Niehoff et al., 2002; Niehoff and Hirche, 2005; Swalethorp et al., 2011). Additionally, in C. sinicus, final maturity is also supported by an external food supply (Wang et al., 2009). Without mating, unfertilized eggs were spawned by the mature females and subsequently decomposed. Reproduction failed in this study, with highly female-skewed sex ratios. Unlike females, males develop faster and retain more of the lipid reserve (Kattner and Krause, 1987; Sargent and Falk-Petersen, 1988; Miller et al., 2000). Given the degenerated feeding activity, males mainly use the lipid reserve to support active swimming to find females (Miller et al., 2000).
4.3 Skewed sex ratioThe C. sinicus sex ratio was highly skewed toward females, and only one male developed in this study. In C. sinicus, the potential sex ratio of C5s is close to 1:1, and the sex ratio of females:males varies seasonally and geographically (1.6–26:1) in China's coastal waters (Chen, 1964; Pu et al., 2004). Femaleskewed copepod sex ratios are common in both incubation and field studies (Kiørboe, 2006). A biased sex ratio can be attributed to higher mortality in males caused by predation in the field (Hirst et al., 2010). The sexes can also be determined by environmental factors, such as temperature, population density, food quantity, and quality (Kiørboe, 2006; Gusmao and Mckinnon, 2009). Intersexes are common in copepods, including Calanus, which change into females when confronted with poor feeding conditions (Gusmao and Mckinnon, 2009). A high concentration of food might contribute to more males developing in the genus Calanus during incubation (Irigoien et al., 2000). However, most Bestiolina similis cultivated on high-concentration microalgae diets molt into females (Camus and Zeng, 2010), suggesting that the sex ratio is a highly species-specific feature (Camus and Zeng, 2012). Although C. sinicus mainly feeds on microalgae, they have a wider food spectrum, including eggs, organic detritus, and microzooplankton (Zhang et al., 2006; Huo et al., 2008; Liu et al., 2011b). Microzooplankton might provide better nutrition for males (Gusmao and Mckinnon, 2009), which might be the reason why the C. sinicus fed on phytoplankton alone developed into females rather than males in this study.
5 CONCLUSIONThe diurnal temperature differences might promote lipid accumulation during copepodite development in C. sinicus by reducing the energy output at colder temperatures and extending the copepodite duration, which was observed in our laboratory experiments. However, the temperature difference did not induce the C. sinicus to develop a large enough oil sac to begin over-summering in the laboratory. In contrast, the copepods had sufficient ingested energy to molt into adults. The combined effects of many environmental and intrinsic factors and their interactions should be considered in future studies to determine the complex mechanism of Calanus dormancy. Moreover, copepods use their lipid reserves to support gonad development after the final molting. A seriously female-skewed sex ratio occurs in cultivation, which might be attributed to the monotonous diet of microalgae.
6 ACKNOWLEDGEMENTWe thank WANG Shiwei and LIU Mengtan for their guidance with the copepod classification. We thank LUO Xuan for help with microalgae cultivation. We are grateful to TANG Hongjun and FENG Song for assistance during sampling.
| Bergvik M, Leiknes Ø, Altin D, Dahl K R, Olsen Y, 2012. Dynamics of the lipid content and biomass of Calanus finmarchicus (copepodite V) in a Norwegian fjord. Lipids, 47(9): 881–895. Doi: 10.1007/s11745-012-3700-3 |
| Campbell R W, Dower J F, 2003. Role of lipids in the maintenance of neutral buoyancy by zooplankton. Mar. Ecol. Prog. Ser., 263: 93–99. Doi: 10.3354/meps263093 |
| Camus T, Zeng C S, 2010. Roles of microalgae on total egg production over female lifespan and egg incubation time, naupliar and copepodite survival, sex ratio and female life expectancy of the copepod Bestiolina similis. Aquacult. Res., 41(11): 1717–1726. Doi: 10.1111/are.2010.41.issue-11 |
| Camus T, Zeng C S, 2012. Reproductive performance, survival and development of nauplii and copepodites, sex ratio and adult life expectancy of the harpacticoid copepod, Euterpina acutifrons, fed different microalgal diets. Aquacult. Res., 43(8): 1159–1169. Doi: 10.1111/are.2012.43.issue-8 |
| Chen Q C, 1964. A study of the breeding periods, variation in sex ratio and in size of Calanus sinicus Brodsky. Oceanol. Limnol. Sin., 6(3): 272–288. |
| Chess D W, Stanford J A, 1999. Experimental effects of temperature and prey assemblage on growth and lipid accumulation by Mysis relicta loven. Hydrobiologia, 412: 155–164. Doi: 10.1023/A:1003886920400 |
| Enright J T, 1977. Diurnal vertical migration:adaptive significance and timing.Part 1. Selective advantage:a metabolic model. Limnol. Oceanogr., 22(5): 856–872. |
| Escribano R, McLaren I A, 1992. Influence of food and temperature on lengths and weights of marine copepods. J. Exp. Mar. Biol. Ecol., 159(1): 77–88. Doi: 10.1016/0022-0981(92)90259-D |
| Falk-Petersen S, Mayzaud P, Kattner G, Sargent J R, 2009. Lipids and life strategy of Arctic Calanus. Mar. Biol. Res., 5(1): 18–39. Doi: 10.1080/17451000802512267 |
| Fiksen Ø, 2000. The adaptive timing of diapause-a search for evolutionarily robust strategies in Calanus finmarchicus. ICES J. Mar. Sci., 57(6): 1825–1833. Doi: 10.1006/jmsc.2000.0976 |
| Graeve M, Albers C, Kattner G, 2005. Assimilation and biosynthesis of lipids in Arctic Calanus species based on feeding experiments with a 13C labelled diatom. J. Exp. Mar. Biol. Ecol., 317(1): 109–125. Doi: 10.1016/j.jembe.2004.11.016 |
| Gusmao L F M, Mckinnon A D, 2009. Sex ratios, intersexuality and sex change in copepods. J. Plankton Res., 31(9): 1101–1117. Doi: 10.1093/plankt/fbp059 |
| Hagen W, Auel H, 2001. Seasonal adaptations and the role of lipids in oceanic zooplankton. Zoology, 104(3-4): 313–326. Doi: 10.1078/0944-2006-00037 |
| Hagen W, Kattner G, Graeve M, 1993. Calanoides acutus and Calanus propinquus, Antarctic copepods with different lipid storage modes via wax esters or Triacylglycerols. Mar. Ecol. Prog. Ser., 97: 135–142. Doi: 10.3354/meps097135 |
| Hakanson J L, 1984. The long and short term feeding condition in field-caught Calanus pacificus, as determined from the lipid content. Limnol. Oceanogr., 29(4): 794–804. Doi: 10.4319/lo.1984.29.4.0794 |
| Hirche H J, Kattner G, 1993. Egg production and lipid content of Calanus glacialis in spring:indication of a fooddependent and food-independent reproductive mode. Mar. Biol., 117(4): 615–622. Doi: 10.1007/BF00349773 |
| Hirche H J, 1996. Diapause in the marine copepod, Calanus finmarchicus-a review. Ophelia, 44(1-3): 129–143. Doi: 10.1080/00785326.1995.10429843 |
| Hirst A G, Bonnet D, Conway D V P, Kiørboe T, 2010. Does predation controls adult sex ratios and longevities in marine pelagic copepods? Limnol. Oceanogr., 55(5): 2193–2206. |
| Hu D X, Wang Q Y, 2004. Interannual variability of the southern Yellow Sea Cold Water Mass. Chin. J. Oceanol. Limnol., 22(3): 231–236. Doi: 10.1007/BF02842553 |
| Huang C, Uye S, Onbé T, 1993. Ontogenetic diel vertical migration of the planktonic copepod Calanus sinicus in the Inland Sea of Japan. Ⅲ. Early summer and overall seasonal pattern. Mar. Biol., 117(2): 289–299. |
| Huo Y Z, Wang S W, Sun S, Li C L, Liu M T, 2008. Feeding and egg production of the planktonic copepod Calanus sinicus in spring and autumn in the Yellow Sea, China. J.Plankton Res., 30(6): 723–734. Doi: 10.1093/plankt/fbn034 |
| Hygum B H, Rey C, Hansen B W, Tande K, 2000. Importance of food quantity to structural growth rate and neutral lipid reserves accumulated in Calanus finmarchicus. Mar.Biol., 136(6): 1057–1073. Doi: 10.1007/s002270000292 |
| Ikeda T, Kanno Y, Ozaki K, Shinada A, 2001. Metabolic rates of epipelagic marine copepods as a function of body mass and temperature. Mar. Biol., 139(3): 587–596. Doi: 10.1007/s002270100608 |
| Irigoien X, Obermüller B, Head R N, Harris R P, Rey C, Hansen B W, Hygum B H, Heath M R, Durbin E G, 2000. The effect of food on the determination of sex ratio in Calanus spp.:evidence from experimental studies and field data. ICES J. Mar. Sci., 57(6): 1752–1763. |
| Irigoien X, 2004. Some ideas about the role of lipids in the life cycle of Calanus finmarchicus. J. Plankton Res., 26(3): 259–263. Doi: 10.1093/plankt/fbh030 |
| Ji R B, 2011. Calanus finmarchicus diapause initiation:new view from traditional life history-based model. Mar. Ecol. Prog. Ser., 440: 105–114. Doi: 10.3354/meps09342 |
| Johnson C L, Leising A W, Runge J A, Head E J H, Pepin P, Plourde S, Durbin E G, 2008. Characteristics of Calanus finmarchicus dormancy patterns in the Northwest Atlantic. ICES J. Mar. Sci., 65(3): 339–350. Doi: 10.1093/icesjms/fsm171 |
| Jónasdóttir S H, 1999. Lipid content of Calanus finmarchicus during overwintering in the Faroe-Shetland Channel. Fish. Oceanogr., 8(S1): 61–72. |
| Kattner G, Hagen W, Lee R F, Campbell R, Deibel D, FalkPetersen S, Graeve M, Hansen B W, Hirche H J, Jónasdóttir S H, Madsen M L, Mayzaud P, Müller-Navarra D, Nichols P D, Paffenhöfer G A, Pond D, Saito H, Stübing D, Virtue P, 2007. Perspectives on marine zooplankton lipids. Can. J. Fish. Aquat. Sci., 64(11): 1628–1639. Doi: 10.1139/f07-122 |
| Kattner G, Krause M, 1987. Changes in lipids during the development of Calanus finmarchicus s. l. from Copepodid Ⅰ to adult. Mar. Biol., 96(4): 511–518. |
| Kiørboe T, 2006. Sex, sex-ratios, and the dynamics of pelagic copepod populations. Oecologia, 148(1): 40–50. Doi: 10.1007/s00442-005-0346-3 |
| Lee R F, Hagen W, Kattner G, 2006. Lipid storage in marine zooplankton. Mar. Ecol. Prog. Ser., 307: 273–306. Doi: 10.3354/meps307273 |
| Lee R F, Nevenzel J C, Paffenhöfer G A, 1972. The presence of wax esters in marine planktonic copepods. Naturwissenschaften, 59(9): 406–411. Doi: 10.1007/BF00623130 |
| Li C L, Sun S, Wang R, Wang X, 2004. Feeding and respiration rates of a planktonic copepod (Calanus sinicus) oversummering in Yellow Sea Cold Bottom Waters. Mar.Biol., 145(1): 149–157. |
| Li C L, Sun S, Wang R, 2007. An experimental study on grazing selectivity of Calanus sinicus to natural food particles. Oceanol. Limnol. Sin., 38(6): 529–535. |
| Liu M T, Li C L, Sun S, 2011a. Identification of trophic relationships between marine algae and the copepod Calanus sinicus in a fatty acid approach. Acta Ecol. Sin., 31(4): 933–942. |
| Liu M T, Li C L, Sun S, 2011b. Seasonal variation in fatty acid composition of seston and the copepod Calanus sinicus (Brodsky, 1962) in Jiaozhou Bay and its trophic implications. Chin. J. Oceanol. Limnol., 29(6): 1164–1173. Doi: 10.1007/s00343-011-0275-4 |
| Madsen M L, Gaard E, Hansen B W, 2008. Wax-ester mobilization by female Calanus finmarchicus (Gunnerus) during spring ascendance and advection to the Faroe Shelf. ICES J. Mar. Sci., 65(7): 1112–1121. Doi: 10.1093/icesjms/fsn097 |
| Maps F, Plourde S, Zakardjian B, 2010. Control of dormancy by lipid metabolism in Calanus finmarchicus:a population model test. Mar. Ecol. Prog. Ser, 403: 165–180. Doi: 10.3354/meps08525 |
| McAllister C D. 1970. Zooplankton rations, phytoplankton mortality, and the estimation of marine production. In:Steele J H ed. Marine Food Chains. University of California Press, Berkeley. p.419-457. |
| McLaren I A, 1963. Effects of temperature on growth of zooplankton, and the adaptive value of vertical migration. J. Fish. Res. Board Can., 20(3): 685–727. Doi: 10.1139/f63-046 |
| Miller C B, Crain J A, Morgan C A, 2000. Oil storage variability in Calanus finmarchicus. ICES J. Mar. Sci., 57(6): 1786–1799. Doi: 10.1006/jmsc.2000.0975 |
| Miller C B, Morgan C A, Prahl F G, Sparrow M A, 1998. Storage lipids of the copepod Calanus finmarchicus from Georges Bank and the Gulf of Maine. Limnol. Oceanogr., 43(3): 488–497. Doi: 10.4319/lo.1998.43.3.0488 |
| Niehoff B, Hirche H J, 2005. Reproduction of Calanus glacialis in the Lurefjord (western Norway):indication for temperature-induced female dormancy. Mar. Ecol. Prog. Ser., 285: 107–115. Doi: 10.3354/meps285107 |
| Niehoff B, Madsen S, Hansen B, Nielsen T, 2002. Reproductive cycles of three dominant Calanus species in Disko Bay, West Greenland. Mar. Biol., 140(3): 567–576. Doi: 10.1007/s00227-001-0731-3 |
| Niehoff B, Runge J A, 2003. A revised methodology for prediction of egg production of the marine planktonic copepod Calanus finmarchicus from preserved samples. J. Plankton Res., 25(12): 1581–1587. Doi: 10.1093/plankt/fbg104 |
| Pepin P, Head E J H, 2009. Seasonal and depth-dependent variations in the size and lipid contents of stage 5 copepodites of Calanus finmarchicus in the waters of the Newfoundland Shelf and the Labrador Sea. Deep Sea Res., Part Ⅰ, 56(6): 989–1002. Doi: 10.1016/j.dsr.2009.01.005 |
| Pepin P, Parrish C C, Head E J H, 2011. Late autumn condition of Calanus finmarchicus in the northwestern Atlantic:evidence of size-dependent differential feeding. Mar. Ecol. Prog. Ser, 423: 155–166. Doi: 10.3354/meps08952 |
| Pu X M, Sun S, Yang B, Zhang G T, Zhang F, 2004. Life history strategies of Calanus sinicus in the southern Yellow Sea in summer. J. Plankton Res., 26(9): 1059–1068. Doi: 10.1093/plankt/fbh101 |
| Pu X M. 2003. Life strategy of Calanus sinicus in the southern Yellow Sea in summer. University of Chinese Academy of Sciences, Qingdao, China. (in Chinese with English abstract) |
| Rey-Rassat C, Irigoien X, Harris R, Carlotti F, 2002. Energetic cost of gonad development in Calanus finmarchicus and C. helgolandicus. Mar. Ecol. Prog. Ser., 238: 301–306. Doi: 10.3354/meps238301 |
| Sargent J R, Falk-Petersen S. 1988. The lipid biochemistry of calanoid copepods. In:Boxshall G, Schminke H K eds.Biology of Copepods. Netherlands:Springer. p.101-114. |
| Saumweber W J, Durbin E G, 2006. Estimating potential diapause duration in Calanus finmarchicus. Deep Sea Res., Part Ⅱ, 53(23-24): 2597–2617. Doi: 10.1016/j.dsr2.2006.08.003 |
| Song S Q. 2010. Phytoplankton functional groups in the Yellow Sea and the East China Sea. University of Chinese Academy of Sciences, Qingdao, China. (in Chinese with English abstract) |
| Sun J, Liu D Y, Qian S B, 1999. Study on phytoplankton biomass Ⅰ. Phytoplankton measurement biomass from cell volume or plasma volume. Acta Oceanol. Sin., 21(2): 75–85. |
| Sun S, Wang S W, Li C L, 2011. Preliminary study on the oil storage of Calanus sinicus fifth copepodites (C5) in the Yellow Sea. Oceanol. Limnol. Sin., 42(2): 165–169. |
| Sun S, Zhang G, 2005. Over-summering strategy of Calanus sinicus. GLOBEC Int. Newsl., 11: 34. |
| Svetlichny L S, Kideys A E, Hubareva E S, Besiktepe S, Isinibilir M, 2006. Development and lipid storage in Calanus euxinus from the Black and Marmara seas:variabilities due to habitat conditions. J. Mar. Syst., 59(1-2): 52–62. Doi: 10.1016/j.jmarsys.2005.09.003 |
| Swalethorp R, Kjellerup S, Dünweber M, Nielsen T G, Moller E F, Rysgaard S, Hansen B W, 2011. Grazing, egg production, and biochemical evidence of differences in the life strategies of Calanus finmarchicus, C. glacialis and C. hyperboreus in Disko Bay, western Greenland. Mar. Ecol. Prog. Ser., 429: 125–144. |
| Tarrant A M, Baumgartner M F, Hansen B H, Altin D, Nordtug T, Olsen A J, 2014. Transcriptional profiling of reproductive development, lipid storage and molting throughout the last juvenile stage of the marine copepod Calanus finmarchicus. Front. Zool., 11(1): 91–105. Doi: 10.1186/s12983-014-0091-8 |
| Tarrant A M, Baumgartner M F, Verslycke T, Johnson C L, 2008. Differential gene expression in diapausing and active Calanus finmarchicus (Copepoda). Mar. Ecol. Prog. Ser., 355: 193–207. Doi: 10.3354/meps07207 |
| Uye S, Huang C, Onbe T, 1990. Ontogenetic diel vertical migration of the planktonic copepod Calanus Sinicus in the Inland Sea of Japan. Mar. Biol., 104(3): 389–396. Doi: 10.1007/BF01314341 |
| Uye S, 1988. Temperature-dependent development and growth of Calanus sinicus (Copepoda:Calanoida) in the laboratory. Hydrobiologia, 167-168(1): 285–293. Doi: 10.1007/BF00026316 |
| Wang S W, Li C L, Sun S, Ning X R, Zhang W C, 2009. Spring and autumn reproduction of Calanus sinicus in the Yellow Sea. Mar. Ecol. Prog. Ser., 379: 123–133. Doi: 10.3354/meps07902 |
| Wang S W. 2009. Reproduction, population recruitment and life history of Calanus sinicus in the Yellow Sea. University of Chinese Academy of Sciences, Qingdao, China. (in Chinese with English abstract) |
| Wang Y Q, Li C L, Liu M T, Sun X X, 2014. Spatial distribution and lipid related energy-consumption strategies of Calanus sinicus in summer in the southern Yellow Sea and East China Sea. Acta Ecol. Sin., 34(16): 4632–4639. |
| Wei Q S, Fu M Z, Li Y, Wang B D, Yu Z G, 2013. Observation of the seasonal evolution of DO, chlorophyll a maximum phenomena and nutrient accumulating in the southern Huanghai (Yellow) Sea Cold Water Mass area. Acta Oceanol. Sin., 35(4): 142–154. |
| Zhang G T, Li C L, Sun S, Zhang H Y, Sun J, Ning X R, 2006. Feeding habits of Calanus sinicus (Crustacea:Copepoda) during spring and autumn in the Bohai Sea studied with the herbivore index. Sci. Mar., 70(3): 381–388. Doi: 10.3989/scimar.2006.70n3 |
| Zhang G T, Sun S, Yang B, 2007. Summer reproduction of the planktonic copepod Calanus sinicus in the Yellow Sea:influences of high surface temperature and cold bottom water. J. Plankton Res., 29(2): 179–186. Doi: 10.1093/plankt/fbm005 |
| Zhang W C, Wang R, Wang K, 2000. Effect of temperature on metabolic rates of Calanus sinicus. Mar. Sci., 24(2): 42–44. |
| Zhou K L, Sun S, Wang M X, Wang S W, Li C L, 2016. Differences in the physiological processes of Calanus sinicus inside and outside the Yellow Sea Cold Water Mass. J. Plankton. Res., 38(3): 551–563. Doi: 10.1093/plankt/fbw011 |
 2017, Vol. 35
2017, Vol. 35



