Institute of Oceanology, Chinese Academy of Sciences
Article Information
- WANG Baoqiang(王宝强), WANG Hongzhu(王洪铸), CUI Yongde(崔永德)
- Pectinatella magnifica (
Leidy, 1851 ) (Bryozoa, Phylactolaemata), a biofouling bryozoan recently introduced to China - Chinese Journal of Oceanology and Limnology, 35(4): 815-820
- http://dx.doi.org/10.1007/s00343-017-6052-2
Article History
- Received Feb. 22, 2016
- accepted in principle Mar. 30, 2016
- accepted for publication Apr. 26, 2016
2 University of Chinese Academy of Sciences, Beijing 100049, China
Biofouling animals accumulate on underwater substrates and adversely affect human infrastructure and activities. Freshwater biofoulers are much less well-known than their marine counterparts, but can have an important economic impact (Callow, 1993). Nakano and Strayer (2014) estimate the potential global cost of freshwater biofouling to be ~277US$ million per year. As one of the most important groups of freshwater biofoulers, bryozoans have the ability to accumulate in large masses of living and non-living materials on submerged surfaces, which can seriously damage artificial equipment and disrupt human activity (Wood, 2010).
Bryozoans are tiny coelomate animals. They can form coherent colonies which often grow to a surprisingly large size. Most freshwater species belong to the class Phylactolaemata, and we here report an exotic bryozoan, Pectinatella magnifica, which has caused serious biofouling problems in aquaculture systems in several freshwater lakes and ponds in China. Due to its large size (sometimes exceeding 0.5 m in diameter), P. magnifica also has a history of clogging water intake structures in North America (Geiser, 1937). Although seldom acknowledged publically, it is capable of causing serious damage to irrigation and other water supply systems.
Our primary intent in this paper was to document P. magnifica occurrence in China, giving the first full description of the species. We also summarized the fouling problems and discussed the dispersal mode of P. magnifica. Although we discuss several possible methods for suppressing bryozoan populations, thorough studies on the effectiveness of such measures are still under way.
2 MATERIAL AND METHOD 2.1 Collection and preservationWe used a sharp knife to remove colonies, along with the underlying substratum to keep them intact. Living colonies were put in a standard 1-L wide-mouth polyethylene bottle and taken to the laboratory for further observation and final identification. Specimens were narcotized with menthol, then fixed and preserved in 75% ethanol.
2.2 Treatment of statoblastStatoblasts were measured with a compound microscope fitted with an ocular micrometer. For scanning electron microscopy, we removed the closefitting membranous envelope of the statoblasts and dried them in a household freezer, sputtered them in gold, and examined and photographed them through a Hitachi S-4800 microscope (Wood, 2005).
2.3 Data sources and analysisData on the distribution and biofouling of P. magnifica in China were collected from author's field investigations, literature (Wang et al., 2009) and news report (http://news.163.com/05/0929/21/1URLF3BU0001122C.html).
3 RESULT 3.1 General description and identification of Pectinatella magnificaColonies are globular and gelatinous, ranging from a small flat sheet to a large mass the size of a rugby ball (sometimes exceeding 0.5 m in diameter). They can be free floating or attached to submerged solid materials in freshwater rivers and lakes (Fig. 1). They consist of a single layer of zooids attached to a thick substratum of firm, transparent, colorless jelly. Zooids are arranged in clusters, or "rosettes", with many slender, radiating lobes, each bordered by a double row of actively-contracting polypides (Fig. 1). The mouth area of each zooid is red, and each tip of a lophophore arm bears a single white glandular organ.

|
| Figure 1 Pectinatella magnifica found in Poyang Lake in June 2012 a. habitat; b. colonies; c. statoblast. |
The free statoblast of P. magnifica is discoidal, laterally asymmetrical and about 1 mm in diameter (Fig. 1c). Hooked spines radiate from the annulus periphery, each spine composed of fused extensions of the dorsal periblast (Fig. 2a, b, c, and d). There is a single large tubercle in the center of the ventral fenestra (Fig. 2b). The surface of dorsal and ventral periblasts are both reticulated with a raised net-like pattern (Fig. 2a, b, c, and d), but ventral valve's reticulation becomes weak in the center of the fenestra (Fig. 2b). No sessile statoblasts are produced.

|
| Figure 2 Scanning electron Microscopy of Pectinatella magnifica a. statoblast, dorsal views (left), ventral views (right); b. higher magnification enlargement of ventral statoblast; c. hooked spines; d. higher magnification enlargement of spine. |
A pair of conspicuous white spots on the lophophore is diagnostic for this species. Red pigmentation around the mouth is much more pronounced and consistent than in any other phylactolaemate species. The statoblast bears a superficial resemblance to that of Cristatella mucedo, but the spines of P. magnifica originate at the periphery of the annulus, not at the periphery of the fenestra.
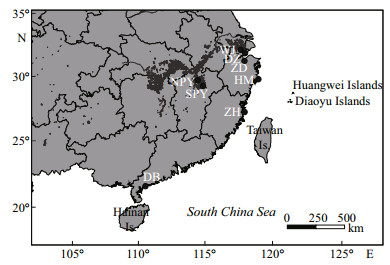
3.2 Distribution of Pectinatella magnifica in ChinaIn September 2005, P. magnifica was recorded on leaves and stems of aquatic plants in Human Reservoir (28°21′38″N, 121°24′56″E) on the eastern coast of Zhejiang Province. This is the first record of P. magnifica from China. Within a short period of time, the presence of this species was also confirmed in Poyang Lake, Zuohai Lake and the Wujiang River. In addition, P. magnifica was also found in Dianbai Pond, where colonies grow to a giant size of up to 2 m in diameter. Until now, P. magnifica had been found in 8 localities, including 5 different provinces in China (Table 1, Fig. 3).
|
| Figure 3 Localities (black dots) of Pectinatella magnifica in China from 2005 to 2012 HM: Human Reservoir; SPY: Southeast of Poyang Lake; ZH: Zuohai Lake; WJ: Wujiang River; ZD: Zhudong Lake; DB: Dianbai Pond; NPY: Northeast of Poyang Lake; DZ: Dingzha Pond. |
The known distribution so far suggests the species prefers a sluggish lentic habitat and flourished from the early summer to the late autumn. The colony substrate included long-submerged wood, bamboo, aquaculture cage, dangling ropes and dock pilings.
3.3 The fouling problems of Pectinatella magnificaAlthough a single bryozoan species fouled all eight sites, the problems they caused were somewhat different at each site (Table 1). In the Human Reservoir and Wujiang River, the colonies were a nuisance and a worrisome presence, but they did not noticeably impair any human activity. In Poyang Lake (2012), Zuohai Lake, Zhudong Lake, Dianbai Pond and Dingzha Pond the fouling problems were more serious. Thousands of colonies were attached to fish cages, which can reduce interstitial water flow, with consequent effects on water oxygen and chemistry causing adverse impacts on aquaculture and ecosystems. In a mussel farm of Poyang Lake (2007), the fouling problem was the most serious case occurring in China. Vigorous colonies of P. magnifica on mussel cages had lethal and sublethal effects on Cristaria plicata, because of the strong competition for space. Moreover, they belong to the same functional feeding group, and thus probably compete for food as well as space. Large accumulations of P. magnifica attached on mussel cage can generate a hypoxic environment, which would lead to a fall in production and even widespread death of C. plicata for lacking of food and oxygen.
Due to its large size (sometimes exceeding 0.5 m in diameter), P. magnifica can block water intake structures. Although not reported in China, P. magnifica is capable of causing serious damage to irrigation and other water supply systems.
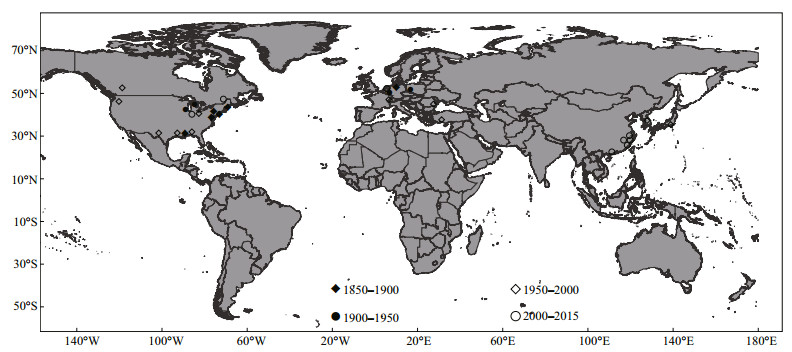
4 DISCUSSIONLeidy found and described Pectinatella magnifica in 1851 near Philadelphia. He placed it in the genus Cristatella and called this new species Cristatella magnifica (Leidy, 1851a). However, he soon discovered that this new species differs from others in the genus Cristatella and established a new genus for this species: Pectinatella (Leidy, 1851b). Early records of P. magnifica mainly focused on the east of the Mississippi River, and the northeastern coast of the Atlantic Ocean (Leidy, 1851a; Kellicott, 1882; Jullien, 1885; Kraepelin, 1887; Davenport, 1898). Subsequently it was found in a relatively wide area from the Great Lakes on the border with Canada to Florida, and from the Mississippi to the Atlantic Ocean (Davenport, 1904; Dendy, 1963; Bushnell, 1965; Everitt, 1975; Wood, 1989; Joo et al., 1992). It now appears to be well established across North America, except in some cold areas (Wood, 2001; Rodriguez and Vergon, 2002; Barnes and Lauer, 2003). In addition to its occurrence in the United States and Canada, P. magnifica had been recorded in scattered sites in Europe, including Germany, Poland, Luxemburg, Turkey, Romania, France, the Czech Republic and the Netherlands (Kraepelin, 1887; Lacourt, 1968; Bernauer and Jansen, 2006; Balounová et al., 2013), Japan (Mawatari, 1973; Oda, 1974) and Korea (Seo, 1998; Jo et al., 2014) (Fig. 4). Since 2005, this species had been found in several areas of China, including Jiangxi, Jiangsu, Zhejiang, Fujian and Guangdong Provinces.
|
| Figure 4 Historical global records of Pectinatella magnifica |
According to many authors, P. magnifica originated from the east of North America. Its occurrence outside this area is very likely only a result of being introduced (Bushnell, 1965; Everitt, 1975; Wood, 1989; Barnes and Lauer, 2003). The origin of P. magnifica in China remains unclear, but historical records of this species indicate that anthropochory is the most probable and coherent reason. Some researchers had reported statoblasts of P. magnifica in the stomach contents of fish, which were largely unharmed by the digestive system. This provides the most likely possibility for the introduction of P. magnifica; its association with imported commercial fish, clams, and aquatic plants (Osburn, 1921; Seo, 1998). P. magnifica was first introduced in Zhejiang Province and then spread to nearby provinces over the next few years. The principal pathway for dispersal of this invasive species is likely to be anthropochory. The big anchors on the statoblasts enable them to attach to different objects in the water, such as ships and fishing equipment (Lacourt, 1968, Seo, 1998). Zoochory is considered to be another important pathway for its dispersal (Oda, 1974; Wood, 2001). Waterfowling carries viable bryozoan statoblasts on their feathers, feet and even in their digestive tracts, and this may be especially important vectors of dispersal (Brown, 1933; Wood et al., 2006). Apart from anthropochory and zoochory, water flow may be another important agent in the dispersal of P. magnifica (Rodriguez and Vergon, 2002).
Freshwater biofouling threatens a number of human activities, from the supply of water and energy to recreation. Several species of freshwater bryozoans are notorious for clogging pipes and filters and there is no effective method for prevention (Wood, 2010). Spraying hot water and thorough drying of fouled surfaces are suitable methods for temporarily reducing the living biomass of bryozoans. However, these physical methods require high labor and time costs. Various chemicals, including chlorine and BOD, can prevent the accumulation of bryozoans by means of their toxic effects (Wood and Marsh, 1999). However, this is not a long-term solution, since dormant statoblasts remain and can instigate a new generation. Canning et al. (1997) used microsporidium or myxosporidium parasites to disable bryozoans, which suggests biological control could be a future possibility, but further research is needed. Although we suggest several possible methods for suppressing bryozoan populations, detailed studies on the effectiveness of measures are still under way.
5 ACKNOWLEDGEMENTWe acknowledge the efforts of reviewers for their helpful comments on this manuscript. The authors are much indebted to Prof. Timothy S. Wood (Bryo Technologies LLC, USA), whose comments greatly improved the manuscript.
| Balounová Z, Pechoušková E, Rajchard J, Joza V, Šinko J, 2013. World-wide distribution of the Bryozoan Pectinatella magnifica (Leidy 1851). European Journal of Environmental Sciences, 3(2): 96–100. |
| Barnes D K, Lauer T E, 2003. Distribution of freshwater sponges and bryozoans in northwest Indiana. Proceedings of the Indiana Academy of Science, 112(1): 29–35. |
| Bernauer D, Jansen W, 2006. Recent invasions of alien macroinvertebrates and loss of native species in the upper Rhine River, Germany. Aquatic Invasions, 1(2): 55–71. Doi: 10.3391/ai |
| Brown C J D, 1933. A limnological study of certain freshwater Polyzoa with special reference to their statoblasts. Transactions of the American Microscopical Society, 52(4): 271–316. Doi: 10.2307/3222415 |
| Bushnell Jr J H, 1965. On the taxonomy and distribution of freshwater Ectoprocta in Michigan. Part Ⅰ. Transactions of the American Microscopical Society, 84(2): 231–244. Doi: 10.2307/3224290 |
| Callow M E, 1993. A review of fouling in freshwaters. Biofouling:The Journal of Bioadhesion and Biofilm Research, 7(4): 313–327. Doi: 10.1080/08927019309386262 |
| Canning E U, Okamura B, Curry A, 1997. A new Microsporidium, Nosema cristatellae n. sp. in the Bryozoan Cristatella mucedo (Bryozoa, Phylactolaemata). Journal of Invertebrate Pathology, 70(3): 177–183. Doi: 10.1006/jipa.1997.4687 |
| Davenport C B, 1898. The fauna and flora about Coldspring Harbor, L. I. Science, 8(203): 685–689. Doi: 10.1126/science.8.203.685 |
| Davenport C B, 1904. Report on the fresh-water bryozoa of the United States. Proceedings of the United States National Museum, 27(1355): 211–221. Doi: 10.5479/si.00963801.27-1355.211 |
| Dendy J S, 1963. Observations on bryozoan ecology in farm ponds. Limnology and Oceanography, 8(4): 478–482. Doi: 10.4319/lo.1963.8.4.0478 |
| Everitt B, 1975. Fresh-water ectoprocta:distribution and ecology of five species in southeastern Louisiana. Transactions of the American Microscopical Society, 94(1): 130–134. Doi: 10.2307/3225540 |
| Geiser S W, 1937. Pectinatella magnifica Leidy, an occasional river-pest in Lowa. Field and Laboratory, 5(2): 65–76. |
| Jo H, Joo G J, Byeon M, Hong D G, Gim J S, Kim J Y, Choi J Y, 2014. Distribution pattern of Pectinatella magnifica(Leidy, 1851), an invasive species, in the Geum River and the Nakdong River, South Korea. Journal of Ecology and Environment, 37(4): 217–223. Doi: 10.5141/ecoenv.2014.026 |
| Joo G J, Ward A K, Ward G M, 1992. Ecology of Pectinatella magnifica (Bryozoa) in an Alabama oxbow lake:colony growth and association with algae. Journal of the North American Benthological Society, 11(3): 324–333. Doi: 10.2307/1467652 |
| Jullien J, 1885. Monographie des Bryozoaires d'eau douce. Bulletin de la Société Zoologique de France, 10: 91–207. |
| Kellicott D S, 1882. Polyzoa:observations on species detected near Buffalo, N. Y. Proceedings of the American Society of Microscopists, 4: 217–229. Doi: 10.2307/3220534 |
| Kraepelin K, 1887. Die deutschen Süsswasser-Bryozoen eine Monographie. L. Friederichsen & Co., Hamburg, 10: 1–168. |
| Lacourt A W, 1968. A monograph of the freshwater Bryozoa-Phylactolaemata. Zoologische Verhandelingen, 93(1): 1–155. |
| Leidy J, 1851a. Cristatella magnifica, n. s. Proceedings of the Academy of Natural Sciences of Philadelphia, 5: 265–266. |
| Leidy J, 1851b. On some American fresh-water Polyzoa. Proceedings of the Academy of Natural Sciences of Philadelphia, 5: 320–322. |
| Mawatari S, 1973. New occurrence of Pectinatella magnifica(Leidy) in a Japanese lake. Proceedings of the Japanese Society of Systematic Zoology, 9: 41–44. |
| Nakano D, Strayer D L, 2014. Biofouling animals in fresh water:biology, impacts, and ecosystem engineering. Frontiers in Ecology and the Environment, 12(3): 167–175. Doi: 10.1890/130071 |
| Oda S, 1974. Pectinatella magnifica occurring in Lake Shoji, Japan. Proceedings of the Japanese Society for Systematic Zoology, 10: 31–39. |
| Osburn R C, 1921. Bryozoa as food for other animals. Science, 53(1376): 451–453. Doi: 10.1126/science.53.1376.451 |
| Rodriguez S, Vergon J P, 2002. Pectinatella magnifica Leidy 1851 (Phylactolaemates), a species of Bryozoa introduced in the north of Franche-Comté. Bulletin Français de la pêChe et de la Pisciculture, 365-366: 281–296. |
| Seo J E, 1998. Taxonomy of the freshwater bryozoans from Korea. Korean Journal of Systematic Zoology, 14(4): 371–381. |
| Wang F, Gao Y S, Liu H J, Li R, Tu Q G, 2009. The preliminary report of unknown gathered water living organisms discovered for the first time in Jiangxi Province. Acta Agriculturae Universitatis Jiangxiensis, 31(1): 124–131. |
| Wood T S, Anurakpongsatorn P, Mahujchariyawong J, 2006. Freshwater bryozoans of Thailand (Ectoprocta and Entoprocta). The Natural History Journal of Chulalongkorn University, 6(2): 83–119. |
| Wood T S, Marsh T G, 1999. Biofouling of wastewater treatment plants by the freshwater bryozoan, Plumatella Vaihiriae (Hastings, 1929). Water Research, 33(3): 609–614. Doi: 10.1016/S0043-1354(98)00274-7 |
| Wood T S, 1989. Ectoproct bryozoans of Ohio. Bulletin of the Ohio Academy of Science, 8: 1–66. |
| Wood T S. 2001. Freshwater bryozoans: a zoogeographical reassessment. In: Bryozoan Studies 2001-Proceedings of the 12th International Bryozoology Association Conference, Dublin, Ireland. p. 339-345. |
| Wood T S, 2005. Study methods for freshwater bryozoans. Denisia, 16: 103–110. |
| Wood T S. 2010. Bryozoans. In: Thorp J H, Covich A P eds. Ecology and Classification of North American Freshwater Invertebrates. 3rd edn. Academic Press, San Diego, CA. p. 437-454. |
 2017, Vol. 35
2017, Vol. 35



