Institute of Oceanology, Chinese Academy of Sciences
Article Information
- YANG Yang(杨洋), SUN Xiaoxia(孙晓霞), ZHAO Yongfang(赵永芳)
- Effects of Lugol's iodine solution and formalin on cell volume of three bloom-forming dinoflagellates
- Chinese Journal of Oceanology and Limnology, 35(4): 858-866
- http://dx.doi.org/10.1007/s00343-017-5378-0
Article History
- Received Dec. 30, 2015
- accepted in principle Mar. 3, 2016
- accepted for publication Jun. 12, 2016
2 University of Chinese Academy of Sciences, Beijing 100049, China;
3 Laboratory for Marine Ecology and Environmental Science, Qingdao National Laboratory for Marine Science and Technology, Qingdao 266071, China
Phytoplankton blooms may cause mortality across several trophic levels (Stephen and Hockey, 2007), leading to changes in the pelagic food web. Prorocentrum micans, Scrippsiella trochoidea and Noctiluca scintillans are bloom-forming dinoflagellates found in coastal aquatic ecosystems of the world. Their blooms have been widely observed, e.g. in the coastal area of Korea (Lee and Lim, 2006), the coast of western South Africa (Stephen and Hockey, 2007), the coast of India (Naik et al., 2011), and in Greek coastal waters (Ignatiades and Gotsis-Skretas, 2010). P. micans and S. trochoidea blooms have an effect on the level of chlorophyll a and dissolved oxygen (Pybus, 1990; Hallegraeff, 1992). N. scintillans was reported to induce predation pressure on phytoplankton, copepods, crustacean larvae, and fish eggs and larvae (Huang and Qi, 1997). Therefore, accurate biomass estimates of these bloom-forming dinoflagellate species are required to evaluate their quantitative roles in microbial food webs and help to assess the state of marine ecosystems
Phytoplankton biomass is traditionally estimated based on empirical relationships between carbon biomass and the cell volume of planktonic organisms (Mullin et al., 1966; Strathmann, 1967; Eppley et al., 1970; Montagnes et al., 1994; Menden-Deuer and Lessard, 2000). Besides the bias introduced from sampling procedure (e.g. where the sample was taken), counting and measuring procedure (e.g. variations in the estimates by different analysts) (Majaneva et al., 2009; Jakobsen et al., 2015), and uncertainly in the empirical conversion from biovolume to carbon (Jakobsen et al., 2015), preservation may also bring potential errors in the biomass estimate. Swelling of phytoplankton cells in fixatives will lead to overestimate of the carbon biomass based on the carbon to volume relationship and vice versa. In large-scale marine ecology surveys, plankton samples are commonly preserved using fixatives for subsequent species identification and cell size measurement. Inaccurate estimate of cell volume for each species would be one important component of the measurement uncertainty in carbon biomass. Thus, the accurate estimate of cell volume from preserved samples is essential.
Lugol's iodine (hereafter referred to as Lugol's), formalin, and glutaraldehyde are traditional fixatives widely used in phytoplankton preservation. Lugol's and glutaraldehyde have been reported to induce changes in the biovolume of some species of dinoflagellates and diatoms (Verity et al., 1992; Montagnes et al., 1994; Menden-Deuer et al., 2001; Zarauz and Irigoien, 2008; Mukherjee et al., 2014), and formalin has been reported to induce changes in planktonic protozoa (Choi and Stoecker, 1989; Zinabu and Bott, 2000; Chaput and Carrias, 2002; Karayanni et al., 2004). However, few studies have estimated the long-term and short-term effect of Lugol's or formalin on P. micans, S. trochoidea and especially N. scintillans.
The objective of this study was to determine the degree of change in the biovolume of these three bloom-forming dinoflagellates brought about by traditional fixatives during short-and long-term fixation. We also examined the effects of fixative concentration on N. scintillans.
2 MATERIAL AND METHODP. micans is tear drop-or heart-shaped cell of medium-size (42–57 μm long and 23–33 μm wide); S. trochoidea is pear-shaped in relatively small size (16– 36 μm long and 20–23 μm wide) (Dodge, 1975; Horner, 2002; Long et al., 2013). Both are thecate dinoflagellates with cellulosic thecal plates. P. micans and S. trochoidea were isolated from the coastal water of the East China Sea. To simulate normal natural conditions, Whatman GF/F-filtered seawater was collected where the P. micans and S. trochoidea were sampled. Cultures were kept in a 14 h:10 h light/dark cycle at 18℃. Samples were obtained from cultures in the late logarithmic growth phase. N. scintillans is an athecate, non-photosynthetic dinoflagellate, roughly spherical with diameter larger than 200 μm (Tada et al., 2000; Horner, 2002). N. scintillans was sampled using a phytoplankton net (mesh size, 76 μm) in the coastal water of the East China Sea.
Lugol's and formalin were freshly prepared in accord with China's Standard Method, the Specifications for Oceanographic Surveys, Marine Biological Survey (GB/T 12763.6-2007), which was also in accord with the method of Throndsen (1978). P. micans and S. trochoidea samples were divided into two sub-samples and fixed immediately after the initial measurements. Applying the standard methods, one sub-sample was preserved with Lugol's at a final concentration of 0.6%–0.8% and the other was preserved with formalin at a final concentration of 5%, respectively. The samples of N. scintillans were divided into four sub-samples after measurement, two of which were preserved with the standard methods as mentioned above. To exclude the potential impact of microbial intervention, the other two N. scintillans samples were preserved with higher concentrations for comparing the effects induced by fixative concentration, i.e. a final concentration of 2% for Lugol's and 10% for formalin, respectively. Both of these two concentrations were used in previous plankton preservation experiments (Graham and Sprules, 1992; Montagnes et al., 1994; Menden-Deuer et al., 2001; Coyle and Pinchuk, 2002; Palardy et al., 2006). All the samples were preserved in dark at room temperature after adding the fixatives. The dimensions of the cells in each sub-sample were measured again after 2 h, 12 h, 24 h, 72 h, 168 h, 720 h, and 2 160 h, respectively.
A VS-IV FlowCAM (Fluid Imaging Inc., Edgecomb, ME, USA) under the control of VisualSpreadsheetTM software version 2.4.8 was used to perform the analysis. Samples were analyzed in autoimage mode (Sieracki et al., 1998) following standard procedure. A ×40 magnification with a ×4 objective was used. A digital camera, which has a resolution of 1 024×768 pixels, photographs the cells. A calibration factor is used in the software to determine the pixel to micron conversion into μm units. The default calibration factor for a ×4 objective is 1.362 2, i.e. each 1.362 2 pixels are used to size 1 μm in the output of the cell size. Several studies have applied FlowCAM in the size analysis of phytoplankton (Zarauz and Irigoien, 2008; Álvarez et al., 2011; Jakobsen and Carstensen, 2011). The FlowCAM in this study was calibrated using beads of known size. To further verify the measurement accuracy of the FlowCAM, we have also compared the measurement results with those of a BX-51 microscope (Olympus, Melville, NY, USA). Results showed that no significant differences existed between these two methods (Yang et al., 2016). The images were checked manually to eliminate invalid images such as bubbles, detritus, and repeated images. Damaged cells of preserved N. scintillans were excluded. The biovolume of each cell was calculated from the equivalent sphere diameter (ESD), which was the mean feret distance based on 36 sample measurements by VisualSpreadsheetTM software. The analysis of each live sample was conducted three times. Means and standard deviations were calculated. Each treatment sample of P. micans and S. trochoidea was measured three times and at least 300 cells in total were measured. Each treatment sample of N. scintillans was measured twice and at least 60 cells in total were measured.
Differences in cell size induced by fixation time were tested by one-way ANOVA. Kruskal-Wallis was used when the data failed to meet the assumption of normality or equal variance. Student's t-test and Wilcoxon rank sum test were used to compare the differences in cell size between the two fixation treatments. P < 0.05 was considered statistically significant. All P-values reported are two-tailed. Statistical analyses were carried out using SAS software (SAS Institute Inc., Cary, NC, USA).
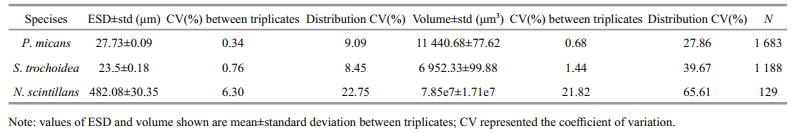
3 RESULT 3.1 Error of the measurementThe size information of the live cells are listed in Table 1. The coefficient of variation (CV) between triplicates reflected the measurement precision of the FlowCAM and the variation in cell size. The CVs for P. micans and S. trochoidea were rather small, indicating a small method variation between triplicates. The CVs for N. scintillans were relatively larger. The cell volume of N. scintillans had a CV of 21.82%. It was mainly induced by the variability in the natural sample of N. scintillans population. The distribution CV of N. scintillans was 65.61%. Besides, the number of observations were relatively larger for P. micans and S. trochoidea. We analyzed the shortterm and long-term effects of Lugol's and formalin on P. micans, S. trochoidea and N. scintillans by comparing the ESD, which was equivalent to using a cube-root transformation of volume.
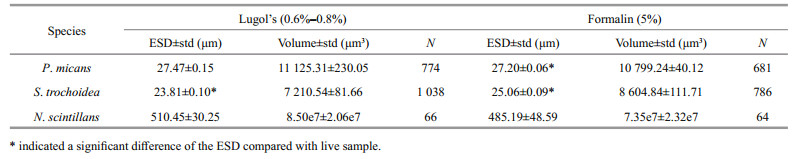
There was significant difference among fixation time for each tested species (P < 0.000 1 for each treatment). We first analyzed the short-term effects of Lugol's and formalin on P. micans, S. trochoidea and N. scintillans by comparing the ESD of live cells and cells preserved for 2 h. The results showed that P. micans shrank slightly in Lugol's, but the difference was not significant (Table 2). However, P. micans preserved with formalin showed statistically significant shrinkage of 1.91% in ESD within 2 h compared with live cells. S. trochoidea was significantly swollen within 2 h with an increase of 1.32% in ESD when fixed with Lugol's and 6.64% when fixed with formalin, respectively. No statistically significant changes were found in N. scintillans with short-term preservation.
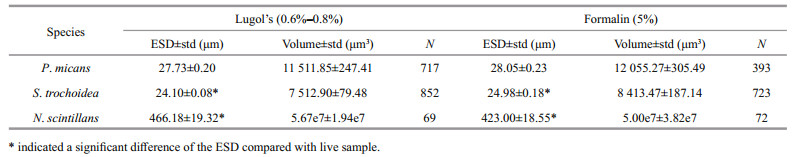
The ESDs of P. micans, S. trochoidea, and N. scintillans cells preserved for 720 h were not significantly different from those preserved for 2 160 h (P > 0.05 for P. micans, S. trochoidea, and N. scintillans, respectively), which indicated that after fixation for one month, the cell volume tended to be stable. Thus, we analyzed the long-term effects of Lugol's and formalin by comparing the ESDs of live cells and cells in the presence of fixatives for 2 160 h (Table 3). These results showed that long-term preservation made no significant change on the ESD of P. micans. However, S. trochoidea cells were significantly swollen in both fixatives and the swelling percentages were 2.55% with Lugol's and 6.30% with formalin, respectively. In contrast, N. scintillans shrank significantly in both fixatives, i.e. 3.30% with Lugol's and 12.26% with formalin. According to these results, the long-term effects of these fixatives were species-specific.
Higher concentration of fixatives was applied for long-term preservation to avoid microbial intervention. For N. scintillans, the ESD of cells preserved for 720 h was not significantly different to that of cells preserved for 2 160 h (P > 0.05) in all the treatments. The differences between different concentrations were assessed following fixation for 2 160 h. The results showed that N. scintillans preserved with formalin at a concentration of 10% was not significantly different to that with the traditionally used concentration of 5% (P > 0.05). However, N. scintillans preserved with 2% Lugol's shrank by 28.27%, which was significantly different to that with the traditionally used concentration of 0.6%–0.8% with a shrinkage of 3.30% (P < 0.000 1).
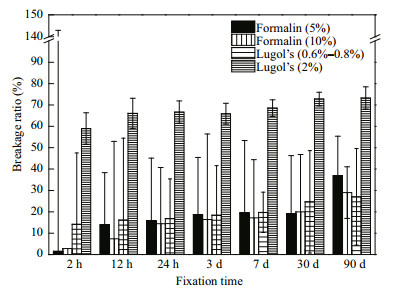
3.4 The breakage ratio of N. scintillans in fixativesApart from size change, broken N. scintillans was also found during the fixation experiments. Damage induced by Lugol's was obvious during short-term fixation (Fig. 1). The concentration of fixative had a greater effect on N. scintillans cell breakage ratio than fixation time. Lugol's at a concentration of 2% caused the greatest damage to N. scintillans. The breakage ratio caused by Lugol's at a concentration of 2% was 58.95% when preserved for 2 h and 73.29% when preserved for 2 160 h, almost three-fold that at a concentration of 0.6%–0.8%, which caused a 14.16% breakage when preserved for 2 h and a 27.05% breakage when preserved for 2 160 h. These results indicated that Lugol's (2%) was not suitable for the preservation of N. scintillans. The breakage ratio when preserved for 2 h were 1.52% and 2.78% for formalin at a concentration of 5% and 10%, respectively. Cell damage in the first 2 hours' preservation caused by formalin was less obvious than that caused by Lugol's. The breakage ratio increased with the fixation time, and small differences were found in different concentrations of formalin. The final breakage ratio was nearly 30% for N. scintillans preserved with formalin.
|
| Figure 1 The breakage percentage of N. scintillans in different fixatives |
Fixation time is an important factor that impacts the volume changes of preserved cells. In our study, P. micans preserved with Lugol's showed no obvious changes in the first 2 h, but then showed shrinkage (Fig. 2). The maximum percentage decrease in P. micans volume was 6.16% after preservation for 72 h. After that, the cells regained their volume and no differences were found after 720 h when the equilibrium was reached, compared with live cells. When P. micans was preserved in formalin, the cells initially shrank and then regained their original volume. Long-term fixation resulted in no obvious size changes in P. micans.
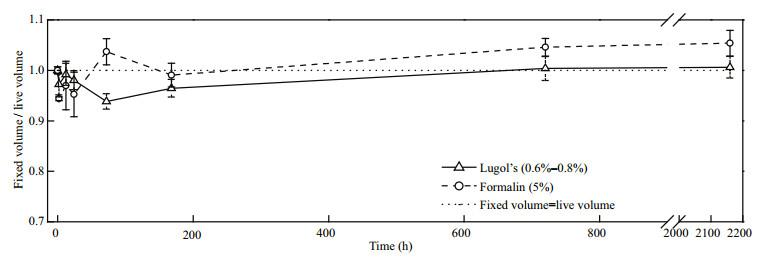
|
| Figure 2 Effects of the fixatives on P. micans over time |
Preserved with Lugol's, S. trochoidea swelled immediately and the maximum percentage increase in cell volume was 6.84% after preservation for 12 h (Fig. 3). The final increase in percentage was 8.06% after preservation for 2 160 h. When preserved with formalin, S. trochoidea cells swelled within the first 2 h with a maximum percentage increase of 23.77%, and was 20.97% after reaching a steady state. When preserved with the fixatives, S. trochoidea cells quickly swelled initially and then swelled at a much lower rate. Thus, we recommend rectifying the volume of a single S. trochoidea cell during long-term preservation according to the following formula:
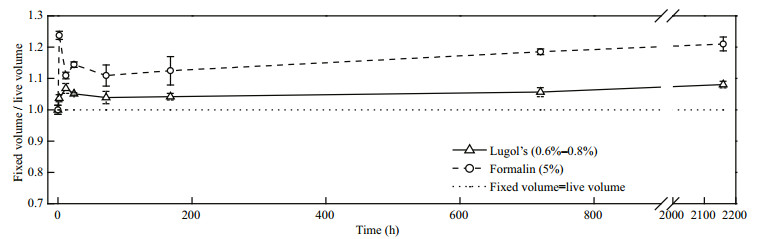
|
| Figure 3 Effects of the fixatives on S. trochoidea over time |
For Lugol's at a concentration of 0.6%–0.8%,
volume of live cell=volume of fixed cell/1.08.
For formalin at a concentration of 5%,
volume of live cell=volume of fixed cell/1.21.
4.1.2 For N. scintillansN. scintillans shrank following preservation with the fixatives (Fig. 4). Shrinkage began to stabilize after 168 h, longer than the previous report by Børsheim and Bratbak (1987) who found on average an 85% change in the volume of preserved samples during the first 2 h of storage. The marine ciliate, Strombidium sp., were also reported to shrink immediately and then relatively small changes occurred after 24 h in 2% Lugol's (Ohman and Snyder, 1991). During long-term preservation, N. scintillans cell volume showed a decrease of approximately 39.36% in formalin at a concentration of 5% and a decrease of 27.59% in Lugol's at a concentration of 0.6%–0.8%, respectively. Thus, we recommend rectifying the volume of a single N. scintillans cell according to the following formula:
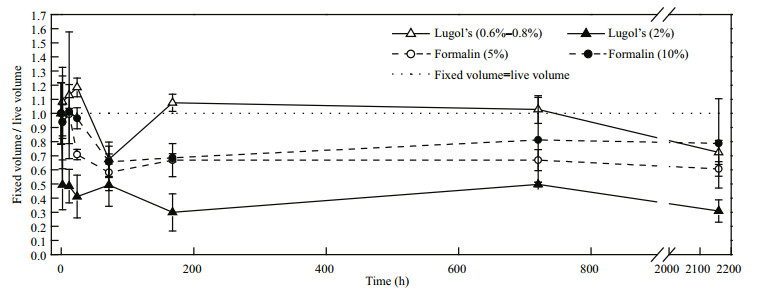
|
| Figure 4 Effects of the fixatives on N. scintillans over time |
volume of live cell=volume of intact fixed cell/0.72 for Lugol's,
volume of live cell=volume of intact fixed cell/0.61 for formalin, respectively.
The high rate of cell breakage should be considered. Because the accurate volume of ruptured cell could not be available, we recommend using the average volume of intact N. scintillans cells combined with all the cell counts, including the ruptured ones, to estimate the total biomass of N. scintillans.
Thus, we summarized the uncertainty associated with the biovolume estimates of the three dinoflagellates in the present study: a) uncertainty arising from the number of cells measured. More cells measured would decrease this error. In the present study, the counting error of N. scintillans was potentially large since only 120 cells were analyzed, while for P. micans and S. trochoidea the data sets were relatively larger; b) the variation of FlowCAM. The FlowCAM was of high measurement precision since the CVs between triplicates were rather small (Table 1); c) the natural variation of a population. The magnitude of population variation was 27.86% for P. micans and 39.67% for S. trochoidea, respectively. The magnitude of N. scintillans population variation, 65.61%, was relatively larger (Table 1); d) fixation effects on the volume change. The magnitude for cell swell of fixed S. trochoidea ranged from 3.71% to 23.77% (Fig. 3). The magnitude of cell shrinkage of N. scintillans due to long-term fixation ranged from 21.24% to 69.11% (Fig. 4); e) breakage of fixed N. scintillans. Except for the shortterm fixation effects of formalin, the breakage of N. scintillans in fixatives was rather obvious. The magnitude of errors induced by Lugol's with a concentration of 0.6%–0.8% ranged from 14.16% to 27.05% and those induced by formalin with a concentration of 5% ranged from 1.52% to 36.83% (Fig. 1); f) error from cell shapes. Dinoflagellate species are of irregular shapes, thus variations existed between the real cell volume and the volume calculated from the commonly used ESD which based on two-dimensional images.
4.2 The effects of different fixativesIn this study, the effects of different fixatives were species-specific. When preserved with Lugol's and formalin, respectively, P. micans showed no significant change, S. trochoidea swelled significantly, and N. scintillans significantly shrank (Table 3). Species showed the same responses to different fixatives. However, extent of the changes was different. For S. trochoidea, the swelling induced by formalin was statistically different from that induced by Lugol's (t=5.45, P < 0.01), and the effect on the former was greater (Fig. 3). N. scintillans is relatively larger in size and has a large vesicle. For N. scintillans, after preserved for 72 h, the shrinkage induced by 0.6%–0.8% Lugol's was weaker than that induced by formalin. Lugol's at a concentration of 2% induced the greatest shrinkage. Despite this, N. scintillans was prone to damage due to Lugol's as mentioned above.
4.3 The effect of fixative concentrationCell shrinkage varied with the concentration of fixative (Verity et al., 1992). In this study, the concentration of formalin used for fixation had little effect on N. scintillans cell shrinkage while N. scintillans cells preserved using a higher concentration of Lugol's were significantly smaller than those preserved using a lower concentration.
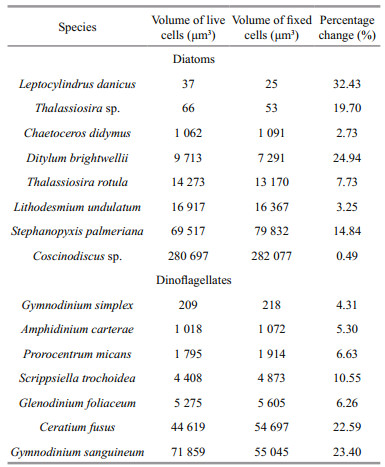
Different effects on cell volume induced by fixative concentration were reported to be less important (Montagnes et al., 1994; Mukherjee et al., 2014), i.e. compared with the volume differences induced by fixative concentrations, the differences between fixed cell volume and live cell volume were far greater. In addition, a specific fixative is not suitable for all plankton species. We reviewed previous work that carried out on the concentration effect of Lugol's on plankton (Table 4). It revealed that most significant effect of Lugol's concentration was on dinoflagellates and ciliate and least on chlorophytes. In the this study, Lugol's at a concentration of 0.6%–0.8% reduced N. scintillans volume by 8.34%, while Lugol's at a concentration of 2% shrank N. scintillans by 50.60% after preservation for 2 h (almost six-fold greater). Preserved for 2 160 h, shrinkage was 27.59% and 69.11% for Lugol's 0.6%–0.8% and 2%, respectively. Therefore, difference induced by fixative concentration cannot be ignored when considering dinoflagellates, especially N. scintillans. The effect of Lugol's concentration was species-specific as Thalassiosira sp. shrank more in 0.5% Lugol's than in 2% Lugol's (Table 3), while Thalassiosira weissflogii shrank a little more in 2% Lugol's than in 0.5% Lugol's. Apart from its effect on biovolume, Lugol's with a concentration of 3.5% or higher has been reported to cause frustule rupture or cellular disintegration in diatoms and blue-green algal cells over a period of 7 to 14 days (Mukherjee et al., 2014). The former has siliceous frustules that may limit the volume change if frustules were not ruptured. We also found broken N. scintillans during fixation. A high concentration of Lugol's induced obvious shrinkage of N. scintillans, which indicated that the fixative had a significant effect on N. scintillans volume and the measurement of N. scintillans volume should be carried out during early sampling. The results showed that Lugol's was not suitable for the preservation of N. scintillans. With fixation longer than 2 h, the breakage caused by formalin could not be ignored either, suggesting that live N. scintillans cells should be measured to avoid errors from breakage and shrinkage.
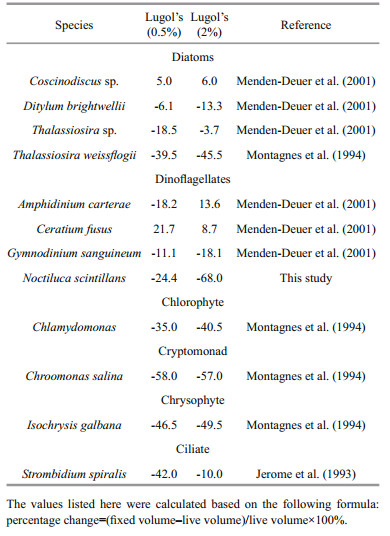
|
N. scintillans differs from the other two species in several aspects. N. scintillans is relatively larger in size, approximately 200–750 μm in diameter in this study. To verify whether the species size was the major cause of cell shrinkage, we extracted data from the study by Menden-Deuer et al. (2001) and calculated the change ratio of each species under the same treatment (Table 5). The results indicated that the change ratio did not regularly change with the increase in cell volume. Thus, volume is likely not the main cause leading to different levels of shrinkage. N. scintillans lacks armor plates, thus is more fragile during preservation. The nutritional state and the size of ingested prey were reported to be the factors that impacted cell shrinkage in a study of fixed protozoa (Choi and Stoecker, 1989). N. scintillans is heterotrophic and has a phagotrophic food vacuole that often contains prey organisms, such as diatoms and ciliates (Horner, 2002), so the size of a live N. scintillans cell (and the degree of shrinkage) may also be affected by what the cell has previously ingested. Thus, the reaction of cells to a fixative may be influenced by a combination of factors and N. scintillans should be discriminated from the other dinoflagellates.
|
The effect of fixatives on dinoflagellates is speciesspecific. S. trochoidea tended to swell when preserved, whereas P. micans showed no significant change. N. scintillans significantly shrank in both Lugol's and formalin. Different fixatives had different levels of effect on cell volume. The traditional used concentration of Lugol's (0.6%–0.8%) resulted in a relatively small volume change. However, N. scintillans was more prone to damage due to fixation by Lugol's or formalin, which may introduce notable measurement errors. In order to accurately estimate the volume of fixed samples, we recommend the application of conversion relationships during long-term preservation of S. trochoidea according to the following formula:
for Lugol's at a concentration of 0.6%–0.8%,
volume of live cell=volume of fixed cell/1.08; and for formalin at a concentration of 5%,
volume of live cell=volume of fixed cell/1.21.
Apart from size change, damage induced by fixatives on N. scintillans is obvious. Therefore, the measurement of N. scintillans is recommend to conduct on live cells during early sampling. As the effects of fixatives on plankton are complex and species-specific, new approaches such as FlowCAM, CytoSence and Phyto-PAM that permit rapid analysis of live specimens would provide more reliable and accurate estimate of biovolume.
6 ACKNOWLEDGEMENTWe thank Q. C. ZHANG and X. LUO for help with P. micans and S. trochoidea culture. We also thank the anonymous reviewers for their valuable comments.
| Álvarez E, López-Urrutia Á, Nogueira E, Fraga S, 2011. How to effectively sample the plankton size spectrum? A case study using FlowCAM. Journal of Plankton Research, 33(7): 1 119–1 133. Doi: 10.1093/plankt/fbr012 |
| Børsheim K Y, Bratbak G, 1987. Cell volume to cell carbon conversion factors for a bacterivorous Monas sp. enriched from seawater. Marine Ecology Progress Series, 36(2): 171–175. |
| Chaput O, Carrias J F, 2002. Effects of commonly used fixatives on size parameters of freshwater planktonic protists. Archiv für Hydrobiologie, 155(3): 517–526. Doi: 10.1127/archiv-hydrobiol/155/2002/517 |
| Choi J W, Stoecker D K, 1989. Effects of fixation on cell volume of marine planktonic protozoa. Applied and Environmental Microbiology, 55(7): 1 761–1 765. |
| Coyle K O, Pinchuk A I, 2002. Climate-related differences in zooplankton density and growth on the inner shelf of the southeastern Bering Sea. Progress in Oceanography, 55(1-2): 177–194. Doi: 10.1016/S0079-6611(02)00077-0 |
| Dodge J D, 1975. The Prorocentrales (Dinophyceae).Ⅱ. Revision of the taxonomy within the genus Prorocentrum. Botanical Journal of the Linnean Society, 71(2): 103–125. Doi: 10.1111/boj.1975.71.issue-2 |
| Eppley R W, Reid F M H, Strickland J D H. 1970. Estimates of phytoplankton crop size, growth rate and primary production. In: Strickland J D H ed. The Ecology of the Plankton off La Jolla, California, in the Period April through September, 1967. University of California Press, Berkeley, USA. p. 33-42. |
| Graham D M, Sprules W G, 1992. Size and species selection of zooplankton by larval and juvenile walleye (Stizostedion vitreum vitreum) in Oneida Lake, New York. Canadian Journal of Zoology, 70(10): 2 059–2 067. Doi: 10.1139/z92-277 |
| Hallegraeff G M, 1992. Harmful algal blooms in the Australian region. Marine Pollution Bulletin, 25(5-8): 186–190. Doi: 10.1016/0025-326X(92)90223-S |
| Horner R A. 2002. A Taxonomic Guide To Some Common Marine Phytoplankton. Biopress Limited, Dorset Press, Dorchester, UK. 200p. |
| Huang C, Qi Y, 1997. The abundance cycle and influence factors on red tide phenomena of Noctiluca scintillans(Dinophyceae) in Dapeng Bay, the South China Sea. Journal of Plankton Research, 19(3): 303–318. Doi: 10.1093/plankt/19.3.303 |
| Ignatiades L, Gotsis-Skretas O, 2010. A review on toxic and harmful algae in Greek coastal waters (E.Mediterranean Sea).. Toxins, 2(5): 1 019–1 037. Doi: 10.3390/toxins2051019 |
| Jakobsen H H, Carstensen J, Harrison P J, Zingone A, 2015. Estimating time series phytoplankton carbon biomass:inter-lab comparison of species identification and comparison of volume-to-carbon scaling ratios. Estuarine, Coastal and Shelf Science, 162: 143–150. Doi: 10.1016/j.ecss.2015.05.006 |
| Jakobsen H H, Carstensen J, 2011. FlowCAM:sizing cells and understanding the impact of size distributions on biovolume of planktonic community structure. Aquatic Microbial Ecology, 65(1): 75–87. Doi: 10.3354/ame01539 |
| Jerome C A, Montagnes D J S, Taylor F J R, 1993. The effect of the quantitative protargol stain and Lugol's and bouin's fixatives on cell size:a more accurate estimate of ciliate species biomass. The Journal of Eukaryotic Microbiology, 40(3): 254–259. Doi: 10.1111/jeu.1993.40.issue-3 |
| Karayanni H, Christaki U, Van Wambeke F, Dalby A P, 2004. Evaluation of double formalin-Lugol's fixation in assessing number and biomass of ciliates:an example of estimations at mesoscale in NE Atlantic. Journal of Microbiological Methods, 56(3): 349–358. Doi: 10.1016/j.mimet.2003.11.002 |
| Lee C, Lim W, 2006. Variation of harmful algal blooms in Masan-Chinhae Bay. Science Asia, 32(S1): 51–56. |
| Long C, Chen B, He B J, Gao C H, 2013. Morphological and phylogenetic analysis of Prorocentrum micans isolated from the Beibu Gulf. Journal of Tropical and Subtropical Botany, 21(4): 332–338. |
| Majaneva M, Autio R, Huttunen M, Kuosa H, Kuparinen J, 2009. Phytoplankton monitoring:the effect of sampling methods used during different stratification and bloom conditions in the Baltic Sea. Boreal Environment Research, 14: 313–322. |
| Menden-Deuer S, Lessard E J, Satterberg J, 2001. Effect of preservation on dinoflagellate and diatom cell volume and consequences for carbon biomass predictions. Marine Ecology Progress Series, 222: 41–50. Doi: 10.3354/meps222041 |
| Menden-Deuer S, Lessard E J, 2000. Carbon to volume relationships for dinoflagellates, diatoms, and other protist plankton. Limnology and Oceanography, 45(3): 569–579. Doi: 10.4319/lo.2000.45.3.0569 |
| Montagnes D J S, Berges J A, Harrison P J, Taylor F J R, 1994. Estimating carbon, nitrogen, protein, and chlorophyll a from volume in marine phytoplankton. Limnology and Oceanography, 39(5): 1 044–1 060. Doi: 10.4319/lo.1994.39.5.1044 |
| Mukherjee A, Das S, Bhattacharya T, De M, Maiti T, Kumar De T, 2014. Optimization of phytoplankton preservative concentrations to reduce damage during long-term storage. Biopreservation and Biobanking, 12(2): 139–147. Doi: 10.1089/bio.2013.0074 |
| Mullin M M, Sloan P R, Eppley R W, 1966. Relationship between carbon content, cell volume, and area in phytoplankton. Limnology and Oceanography, 11(2): 307–311. Doi: 10.4319/lo.1966.11.2.0307 |
| Naik R K, Hegde S, Anil A C, 2011. Dinoflagellate community structure from the stratified environment of the Bay of Bengal, with special emphasis on harmful algal bloom species. Environmental Monitoring and Assessment, 182(1-4): 15–30. Doi: 10.1007/s10661-010-1855-z |
| Ohman M D, Snyder R A, 1991. Growth kinetics of the omnivorous oligotrich ciliate Strombidium sp. Limnology and Oceanography, 36(5): 922–935. Doi: 10.4319/lo.1991.36.5.0922 |
| Palardy J E, Grottoli A G, Matthews K A, 2006. Effect of naturally changing zooplankton concentrations on feeding rates of two coral species in the Eastern Pacific. Journal of Experimental Marine Biology and Ecology, 331(1): 99–107. Doi: 10.1016/j.jembe.2005.10.001 |
| Pybus C, 1990. Blooms of Prorocentrum micans (Dinophyta) in the Galway Bay area. Journal of the Marine Biological Association of the United Kingdom, 70(4): 697–705. Doi: 10.1017/S0025315400058987 |
| Sieracki C K, Sieracki M E, Yentsch C S, 1998. An imagingin-flow system for automated analysis of marine microplankton. Marine Ecology Progress Series, 168(1): 285–296. |
| Stephen V C, Hockey P A R, 2007. Evidence for an increasing incidence and severity of Harmful Algal Blooms in the southern Benguela region. South African Journal of Science, 103(5-6): 223–231. |
| Strathmann R R, 1967. Estimating the organic carbon content of phytoplankton from cell volume or plasma volume. Limnology and Oceanography, 12(3): 411–418. Doi: 10.4319/lo.1967.12.3.0411 |
| Tada K, Pithakpol S, Yano R, Montani S, 2000. Carbon and nitrogen content of Noctiluca scintillans in the Seto Inland Sea, Japan. Journal of Plankton Research, 22(6): 1 203–1 211. Doi: 10.1093/plankt/22.6.1203 |
| Throndsen J. 1978. Preservation and storage. In: Sournia A ed. Phytoplankton Manual. UNESCO, Paris, France. p. 69-74. |
| Verity P G, Robertson C Y, Tronzo C R, Andrews M G, Nelson J R, Sieracki M E, 1992. Relationships between cell volume and the carbon and nitrogen content of marine photosynthetic nanoplankton. Limnology and Oceanography, 37(7): 1 434–1 446. Doi: 10.4319/lo.1992.37.7.1434 |
| Yang Y, Sun X X, Zhu M L, Luo X, Zheng S. 2016. Estimating the carbon biomass of marine net phytoplankton from abundance based on samples from China seas. Marine and Freshwater Research, http://dx.doi.org/10.1071/MF15298. |
| Zarauz L, Irigoien X, 2008. Effects of Lugol's fixation on the size structure of natural nano-microplankton samples, analyzed by means of an automatic counting method. Journal of Plankton Research, 30(11): 1 297–1 303. Doi: 10.1093/plankt/fbn084 |
| Zinabu G M, Bott T L, 2000. The effects of formalin and Lugol's iodine solution on protozoal cell volume. Limnologica-Ecology and Management of Inland Waters, 30(1): 59–63. Doi: 10.1016/S0075-9511(00)80044-4 |
 2017, Vol. 35
2017, Vol. 35