Institute of Oceanology, Chinese Academy of Sciences
Article Information
- WU Qingyang(吴清洋), WANG Shuqi(王树启), CHEN Xiaopeng(陈晓鹏), LI Ping(李平)
- Reproductive toxicity assessment of benzo[a]pyrene in the marine polychaete Perinereis nuntia
- Chinese Journal of Oceanology and Limnology, 35(4): 867-873
- http://dx.doi.org/10.1007/s00343-017-6024-6
Article History
- Received Jan. 26, 2016
- accepted in principle Apr. 6, 2016
- accepted for publication May. 11, 2016
2 Administration of Ocean and Fisheries of Shantou, Shantou 515000, China
Polycyclic aromatic hydrocarbons (PAHs) are a considerable concern because they are genotoxic, carcinogenic, and teratogenic to organisms (Kanaly and Harayama, 2000). Benzo[a]pyrene (B[a]P) is a ubiquitous pollutant originating from industrial processes and/or as a result of other human activities (Banni et al., 2009). The marine environment is the ultimate recipient of B[a]P, so concerns about the sublethal biological effects of B[a]P in the marine environment has increased and finding biomarkers to evaluate the potential toxicity of these pollutants has become imperative (Lee et al., 2000). The toxic effects of B[a]P have been explored extensively in a diversity of species, including plankton, crustaceans, fish, turtles, and mollusks (Banni et al., 2010; Mearns et al., 2013; Wen and Pan, 2015).
Polychaetes are the dominant sediment-dwelling fauna in most mud flats, estuaries, and sheltered sandy shores, so they are often exposed to chronic oil pollution or acute spills and accumulate various pollutants through their skin and intestines (Lin and Zheng, 2007). Polychaetes have a wide geographical distribution, short life-cycle, and distinct developmental stages and have been recommended as a potential marine model organism for ecotoxicology (Catalano et al., 2012). Polychaetes have frequently been used to evaluate the impact of anthropogenic disturbances in aquatic systems under field and laboratory conditions. However, most of these studies focused on short and/or long-term bioaccumulation or physiological and biochemical effects of B[a]P on polychaetes (Weston, 1990; Banni et al., 2009), but the reproductive toxicity of B[a]P to benthic invertebrates has not been well studied. Reproductive toxicity will ultimately impact the population of any organism, so it is a hallmark for long-term ecological risk assessment.
The sandworm, Perinereis nuntia, is an economically important polychaete collected by bait diggers for use as fresh fish bait and constitutes an important food source for birds, fish, and other invertebrates (Hardege and Bartels-Hardege, 1995). Furthermore, the reproductive biology of adult P. nuntia has been reported by Du (2004) and Zheng et al. (2010), which facilitate to carry out reproductive toxicity of pollutant to benthic invertebrates. Zheng et al. (2010) has reported that cadmium could inhibit sexual maturation, fertilization, and hatching of P. nuntia and demonstrated that cadmium has strong feminizing effects on polychaetes.
Vitellogenin (VTG) is the major egg-yolk proteinvitellin precursor and provides energy reserves for embryonic development in oviparous organisms. VTGs are synthesized by mature females in response to endogenous estrogens, such as 17β-estradiol (E2), released into the coelomic fluid, and are stored in developing oocytes (Denslow et al., 1999). VTG expression levels have been widely used as biomarker of feminizing effects (Sumpter and Jobling, 1995). The main objectives of this study were to systematically investigate the reproductive toxicity of B[a]P on P. nuntia by evaluating sexual maturation, the sex ratio, number of eggs spawned, and fertilization and hatching rates. In addition, VTG gene mRNA levels in female P. nuntia were analyzed after exposure to B[a]P and possible mechanisms are discussed.
2 MATERIAL AND METHOD 2.1 Animals and B[a]P exposureJuvenile P. nuntia (wet weight: 450±50 mg; standard deviation) were purchased from a bait farm in Fuzhou, China, about 2 months before heteronereis (sexual maturity) and acclimated to clean filtered seawater in pebble-bedded glass tanks (50 cm× 40 cm×40 cm) for 1 week before the experiments. A stock solution of B[a]P (1 μg/μL) was prepared by dissolving B[a]P (Sigma-Aldrich, St. Louis, MO, USA) in dimethyl sulfoxide (DMSO), and 35 or 350 μL of the stock solution was added to 14 L of seawater to prepare sublethal concentrations of 2.5 or 25 μg/L, respectively. A 250-μg/L concentration of B[a]P had long-term lethal effects on P. nuntia (data not shown).
Four groups of P. nuntia were prepared for this study: (1) seawater control; (2) DMSO solvent control, in which a volume of 350 μL DMSO was added to clean seawater; (3) 2.5 μg/LB[a]P exposure group, and (4) 25 μg/L B[a]P exposure group. Three replicates (pebble-bedded glass tanks containing 14 L seawater) were used for each group, and each replicate comprised 50 animals. P. nuntia were cultured under constant laboratory conditions (temperature, 25±1℃; salinity, 30; photoperiod, 12 h light: 12 h dark; dissolved oxygen ≥6 mg/L) and fed 2 g (dry weight) fish powder once per day. The experiment ran 60 days, and half of the aquarium water was changed with newly prepared artificial seawater every morning.
2.2 Analysis of reproductive effects 2.2.1 Sexual maturation and the sex ratioThe ratio of sexually mature worms and the ratio of identified sexes (female to male) were calculated for each treatment during the 60-day exposure. Morphological changes (body length and color) appear when P. nuntia attains heteronereis. The dorsal color of a mature female is pink, whereas that of the male is dark green (Du, 2004).
2.2.2 Number of eggs spawned and fertilization and hatching ratesSix pairs of sexually mature worms were selected randomly from each group on day 60 and placed in a 2-L glass beaker with 1 L of their treatment seawater. The male of the pair began to swim around the female and ejected brume-like sperm, whereas the female spawned green eggs from the front of her body. The worms continued to release sperm or eggs until they died and fell to the bottom (about 3 h). Fertilization was usually completed in 15–30 min. We removed the dead worms from the beaker 4 h later and mixed the eggs and sperm slowly. Then, 1.0 mL of solution was sampled randomly to calculate the number of fertilized and unfertilized eggs by microscopy (Carl Zeiss AG, Oberkochen, Germany). Fertilized eggs are covered with a colloid membrane, which develops into the embryo (Zheng et al., 2010). Fertilization rate was defined as the ratio of fertilized eggs to total egg number.
Two hundred zygotes (fertilized eggs) from each group were selected and assigned to another beaker with the same treatment to determine the effect of B[a]P on hatching. Zygotes generally hatch within 65 h after fertilization (Du, 2004). In this study, hatching rate in each beaker was calculated on day 4 (96 h) after fertilization as the number of larvae per 200 zygotes. This calculation was repeated six times under the same laboratory experimental conditions described above.
2.3 Relative quantitative real-time reversetranscription polymerase chain reaction (RTPCR) assay for VTGSix sexually mature female P. nuntia from each treatment group were selected randomly and ground individually into powder in liquid nitrogen with a ceramic mortar. Total RNA of each worm was isolated following the RNAiso Reagent protocol. cDNA was synthesized with the RevertAidTM First Strand cDNA Synthesis Kit (#K1622; ThermoFisher, Rockford, IL, USA). The relative quantitative real-time RT-PCR assay was performed using a thermal cycler (GeneAmp PCR System 2720; ABI, Foster City, CA, USA). The primers (5′-CGTCACCAAAGTCCGAAAC-3′ and 5′-TCCATCAAAGGAAGCGAGA-3′) for the VTG cDNA sequence and the internal control 18s rRNA (5′-CCCGTCTCGTTGGTGACTCTGG-3′ and 5′-TGCTGCCTTCCTTGGATGTGGT-3′) (NCBI No. EU340097.1) were designed. PCR was performed in a 20 μL volume containing 10 μL Fast SYBR Green Master Mix (Applied Biosystems, Foster City, CA, USA), 2 μL cDNA, 2 μL of each forward and reverse primers (2 μmol/L each), 0.4 μL ROX and 3.6 μL RNase-free water. The PCR program included one cycle of 95℃ for 30 s, 40 cycles of 95℃ for 5 s, and 60℃ for 10 s. Data from the quantitative real-time RTPCR analysis were determined by the formula of 2-ΔΔCt, in which mean ΔΔCt=[(exposed worm VTG-exposed worm 18S RNA)-(seawater control worm VTG-seawater control worm 18s RNA)].
2.4 Statistical analysisMean number of eggs, fertilization and hatching rates, and VTG mRNA expression levels with homogenous variances were compared between treatments using one-way analysis of variance (ANOVA) followed by Tukey's multiple comparisons test. The non-parametric Kruskal-Wallis test followed by Dunn's multiple comparisons test were used to compare data with heterogeneous variances (Zar, 1999). All statistical computations were performed using SPSS software (SPSS Inc., Chicago, IL, USA). A P-value < 0.05 was considered significant.
3 RESULTB[a]P concentrations in the fish powder fed to the sandworms and in the seawater were below the detection limits of 0.01 mg/(g dw) and 1.0 ng/L of the gas chromatography/mass spectroscopy system. Cumulative survival rates in the groups of seawater control, solvent control, 2.5 and 25 μg/L B[a]Pexposed groups were 93%±1.2%, 91%±3.1%, 87%±3.1% and 86%±2.0%, respectively, by the end of the experiment.
3.1 Sexual maturation and sex ratiosThe sexual maturation rates of the two control groups (seawater and DMSO) and the two B[a]P exposed groups (2.5 and 25 μg/L) were 67%±5.2%, 65%±6.1%, 70%±6.2%, and 40%±3.2%, respectively during the 60-day exposure (Fig. 1a), and the sex ratios (female: male) in these respective groups were 1.6:1, 1.6:1, 2.3:1, and 2.5:1 (Fig. 1b). The sexual maturation rate in the 25 μg/L group was significantly lower than that in the other three groups (P < 0.05). No differences in sex ratios (female: male) were observed between the solvent and seawater controls, but the sex ratio increased significantly after exposing P. nuntia to B[a]P. The high B[a]P concentration (25 μg/L) caused a great change in the sex ratio compared with that of the low concentration (2.5 μg/L) (P < 0.05).
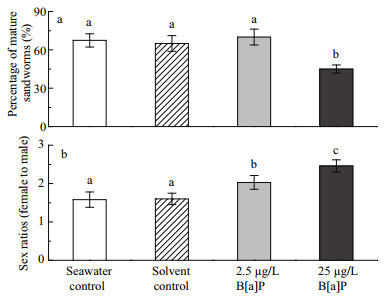
|
| Figure 1 Chronic effect of benzo[a]pyrene (B[a]P) on sexual maturation (a) and the sex ratio (female: male) (b) in the sandworm Perinereis nuntia after a 60-day exposure, compared with that in the two controls Data are mean±standard deviation (n=3). Different letters at the top of each bar indicate significant differences between treatments (P < 0.05, one-way ANOVA followed by Tukey test). |
No differences in the number of eggs spawned by female worms were detected between the two B[a]Pexposed groups or the two control groups (P > 0.05) following after the 60-days treatment (Fig. 2). Oneway ANOVA showed that the fertilization rates among the two control groups and 2.5 μg/L B[a]Pexposed group were not different (P > 0.05). However, the fertilization rate in the 25 μg/L B[a]P-exposed group was significantly lower than that in the other three groups (P < 0.05) (Fig. 3).
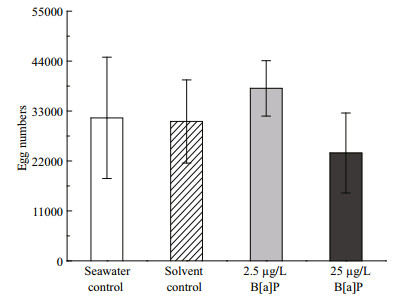
|
| Figure 2 Chronic effect of benzo[a]pyrene (B[a]P) on the number of eggs spawned by female Perinereis nuntia after a 60-day exposure, compared with that in the two controls Data are mean±standard deviation (n=6). |
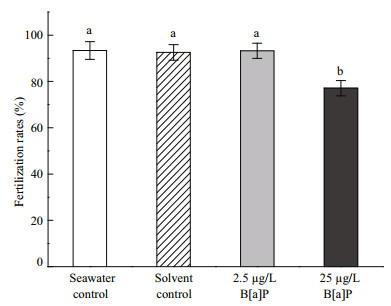
|
| Figure 3 Fertilization rates of Perinereis nuntia eggs in the two benzo[a]pyrene (B[a]P)-exposed groups (2.5 and 25 μg/L) and the seawater and solvent controls Data are mean±standard deviation (n=6), Different letters at the top of each bar indicate significant differences between treatments (P < 0.05, one-way ANOVA followed by Tukey test). |
The changes in hatching rates are shown in Fig. 4. Hatching rates decreased significantly after exposure to 2.5 and 25 μg/L B[a]P. However, no difference was found between the high (25 μg/L) and low concentration (2.5 μg/L) groups.

|
| Figure 4 Perinereis nuntia hatching rates in the two benzo[a] pyrene (B[a]P)-exposed groups (2.5 and 25 μg/L) compared with the seawater and solvent controls Data are mean±standard deviation (n=6). Different letters at the top of each bar indicate significant differences between treatments (P < 0.05, one-way ANOVA followed by Tukey test). |
VTG mRNA levels in female P. nuntia changed significantly after exposure to B[a]P at the end of experiment (Fig. 5). No difference in VTG mRNA expression level was observed between the two control groups (P > 0.05). VTG mRNA expression levels in female P. nuntia exposed to 2.5 and 25 μg/L B[a]P increased significantly by 3.7±0.4-and 7.1±1.1-fold, respectively, compared with that in the DMSO solvent control group (P < 0.05).
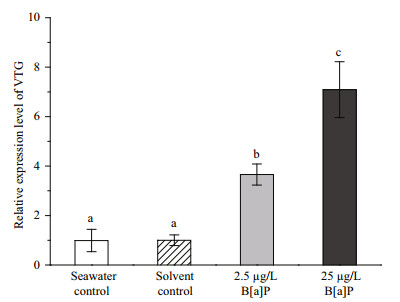
|
| Figure 5 Relative vitellogenin (VTG) gene mRNA expression levels in female Perinereis nuntia from the two benzo[a]pyrene (B[a]P)-exposed groups (2.5 and 25 μg/L) compared with those in the seawater and solvent controls using quantitative real-time reversetranscription polymerase chain reaction and the 2-ΔΔCt method (n=6) Data are mean±standard deviation. Different letters at the top of each bar indicate significant differences between treatments (P < 0.05, one-way ANOVA followed by Tukey test). |
In our study, the sexual maturation rate of P. nuntia was inhibited significant following the 60-day B[a]P exposure to 25 μg/L B[a]P. A delay in sexual maturation is commonly observed in response to oxidative stresses, such as lipid peroxidation, as well as neuromodulated energy stores, steroidal androgens and estrogens upon exposure to endocrine disruptors (Wang and Croll, 2004; Oakes et al., 2005; Sternberg et al., 2008). Polychaetes possess cytochrome P450s that biotransform PAHs just like fishes (McElroy et al., 2000; Rust et al., 2004), which will result in the production of reactive oxygen species (Livingstone, 1994). Malondialdehyde increases significantly when the other polychaete, Hediste diversicolor, is exposed to B[a]P (Bouraoui et al., 2009). Moreover, Tian et al. (2013) reported that a 10-day B[a]P exposure significantly impaired ovarian function and delayed sexual development in mature female Chlamys farreri. We found a clear feminizing effect of B[a]P, as the sex ratios (female: male) were 1.6:1, 1.6:1, 2.3:1, and 2.5:1 in the seawater, DMSO, and 2.5 and 25 μg/L B[a]P groups, respectively. Many endocrine disruptors exert estrogenic activity, and feminization has been reported in all classes of vertebrates including fish (Munkittrick et al., 1991), reptiles (Guilette and Crain, 1996), and birds (Fry and Toone, 1981). However, this is the first demonstration of a feminizing effect in a polychaetes. A wide range of estrogens and estrogen mimics stimulate increases in plasma VTG levels in male fish (Rose et al., 2002; Van den Belt et al., 2003), and consequently increased proportions of intersex fish have been reported, which could affect sexual differentiation (Jobling et al., 1998; Gibson et al., 2005). In addition, spawning by male bivalves exposed to PAHs is delayed more than that of females, as PAHs interact with steroid synthesis and prevent conversion of testosterone to estradiol (E2) (Denslow et al., 1999). In our study, VTG mRNA expression levels increased significantly after the 60-day exposure of P. nuntia to both B[a]P concentrations, suggesting that changes in estrogen also play a key role determining the sex ratio.
Feminization is a very serious problem in polychaetes. The population dynamics in many freespawning marine invertebrates is thought to be sperm limited (Yund, 2000), and a proportion of oocytes may remain unfertilized in the wild (Bode and Marshall, 2007). In this study, no difference in the number of eggs spawned per female was found between the four groups, so increasing the number of females may further affect the fertilization rates. As shown in Fig. 3, the fertilization rates of 25μg/L B[a]Pexpose group was significantly inhibited (P < 0.05), but not that in the 2.5 μg/L B[a]P-expose group, so further studies are needed to clarify this effect. Furthermore, the impaired sperm quality may be another factor decreasing fertilization success. PAHs, such as B[a]P, have been reported to preferably distribute in the gonad owing to high lipid content, where they cause deleterious effects, including reduced sperm counts, increased number of abnormal cells, and chromosomal abnormalities in humans (Lopes et al., 1998), fish (Monteiro et al., 2000) and some marine invertebrates (Jeong and Cho, 2005). It is now clear that PAHs can affect ATP content and depress the sperm motility (Rurangwa et al., 2002). This impairment in sperm quality and decreased fertilization rates suggests that fertilized egg quality may also be lower. Hatching rates in the B[a]P (2.5 and 25 μg/L) treatment groups were inhibited significantly (P < 0.05) in this study, suggesting that early developmental stages of P. nuntia are sensitive to B[a]P.
VTG mRNA expression levels after the 60-day B[a]P exposure were also assessed in females in our study. The results indicate that VTG mRNA expression levels in female P. nuntia exposed to 2.5 and 25 μg/L of B[a]P increased significantly by 3.7-and 7.1-fold, respectively, compared with those in both controls (P < 0.05). As mentioned above, VTGs are the major precursor to vitellins (egg-yolk proteins), and are good biomarkers of exposure to estrogenic compounds (Jobling et al., 1998; Denslow et al., 1999). VTGs are detectable in vertebrates, as well as aquatic invertebrates, such as mollusks, crustaceans, aquatic insects, and polychaetes, and they are induced following exposure to xenoestrogens by stimulating the E2 receptor (Matozzo et al., 2008; Keay and Thornton, 2009). Interestingly, eleocytes from immature polychaetes, such as N. virens, do not respond to oestradiol-17β signaling by secreting VTG, possibly due to absence of the estrogen receptor (García-Alonso et al., 2006).
5 CONCLUSIONThis is the first study to demonstrate the PAH B[a]P adversely affected reproductive performance in the sandworm P. nuntia, including changes in sexual maturation, the sex ratio, and fertilization and hatching rates after a 60-day exposure. VTG mRNA expression levels in female worms increased significantly in response to 2.5 and 25 μg/L B[a]P exposure. PAH pollution is a very serious problem in coastal waters around highly urbanized and industrialized areas of China. The highest PAH concentrations in the coastal sediment samples in China are 5.8–11.0 μg/g sediment (Zheng et al., 2004), so more studies are necessary to detect the potential effects of endocrine disrupting chemicals and their mechanisms of action to properly assess the cause and effect and clearly address their ecological relevance.
| Banni M, Bouraoui Z, Clerandeau C, Narbonne J F, Boussetta H, 2009. Mixture toxicity assessment of cadmium and benzo[a]pyrene in the sea worm Hediste diversicolor. Chemosphere, 77(7): 902–906. Doi: 10.1016/j.chemosphere.2009.08.041 |
| Banni M, Negri A, Dagnino A, Jebali J, Ameur S, Boussetta H, 2010. Acute effects of benzo[a]pyrene on digestive gland enzymatic biomarkers and DNA damage on mussel Mytilus galloprovincialis. Ecotoxicology and Environmental Safety, 73(5): 842–848. Doi: 10.1016/j.ecoenv.2009.12.032 |
| Bode M, Marshall D J, 2007. The quick and the dead? Sperm competition and sexual conflict in sea. Evolution, 61(11): 2 693–2 700. Doi: 10.1111/evo.2007.61.issue-11 |
| Bouraoui Z, Banni M, Ghedira J, Clerandeau C, Narbonne J F, Boussetta H, 2009. Evaluation of enzymatic biomarkers and lipoperoxidation level in Hediste diversicolor exposed to copper and benzo[a]pyrene. Ecotoxicology and Environmental Safety, 72(7): 1 893–1 898. Doi: 10.1016/j.ecoenv.2009.05.011 |
| Catalano B, Moltedo G, Martuccio G, Gastaldi L, VirnoLamberti C, Lauria A, Ausili A, 2012. Can Hediste diversicolor (Nereidae, Polychaete) be considered a good candidate in evaluating PAH contamination? A multimarker approach. Chemosphere, 86(9): 875–882. Doi: 10.1016/j.chemosphere.2011.10.040 |
| Denslow N D, Chow M C, Kroll K J, Green L, 1999. Vitellogenin as a biomarker of exposure for estrogen or estrogen mimics. Ecotoxicology, 8(5): 385–398. Doi: 10.1023/A:1008986522208 |
| Du R B. 2004. The study on ecological condition and reproductive biology of adult Perinereis nuntia Savigny under artificial culture condition. Ocean University of China, Qingdao, China, p. 18-38. (in Chinese) |
| Fry D M, Toone C K, 1981. DDT-induced feminization of gull embryos. Science, 231(4510): 922–924. |
| García-Alonso J, Hoeger U, Rebscher N, 2006. Regulation of vitellogenesis in Nereis virens (Annelida:Polychaeta):Effect of estradiol-17β on eleocytes. Comparative Biochemistry and Physiology Part A:Molecular & Integrative Physiology, 143(1): 55–61. |
| Gibson R, Smith M D, Spary C J, Tyler C R, Hill E M, 2005. Mixtures of estrogenic contaminants in bile of fish exposed to wastewater treatment works effluents. Environmental Science & Technology, 39(8): 2 461–2 471. |
| Guilette L J, Crain D A, 1996. Endocrine-disrupting contaminants and reproductive abnormalities in reptiles. Communications Toxicology, 5(4): 381–399. |
| Hardege J D, Bartels-Hardege H D, 1995. Spawning behaviour and development of Perinereis nuntia var brevicirrus(Annelida:Polychaeta). Invertebrate Biology, 114(1): 39–45. Doi: 10.2307/3226951 |
| Jeong W G, Cho S M, 2005. The effects of polycyclic aromatic hydrocarbon exposure on the fertilization and larval development of the Pacific oyster, Crassostrea gigas. Journal of Shellfish Research, 24(1): 209–213. Doi: 10.2983/0730-8000(2005)24[209:TEOPAH]2.0.CO;2 |
| Jobling S, Nolan M, Tyler C R, Brighty G, Sumpter J P, 1998. Widespread sexual disruption in wild fish. Environmental Science & Technology, 32(17): 2 498–2 506. |
| Kanaly R A, Harayama S, 2000. Biodegradation of highmolecular-weight polycyclic aromatic hydrocarbons by bacteria. Journal of Bacteriology, 182(8): 2 059–2 067. Doi: 10.1128/JB.182.8.2059-2067.2000 |
| Keay J, Thornton J W, 2009. Hormone-activated estrogen receptors in annelid invertebrates:implications for evolution and endocrine disruption. Endocrinology, 150(4): 1 731–1 738. Doi: 10.1210/en.2008-1338 |
| Lee B G, Griscom S B, Lee J S, Choi H J, Koh C H, Luoma S N, Fisher N S, 2000. Influences of dietary uptake and reactive sulfides on metal bioavailability from aquatic sediments. Science, 287(5451): 282–284. Doi: 10.1126/science.287.5451.282 |
| Lin J H, Zheng F W, 2007. Preliminary study on ecology of benthic polychaeta in Quanzhou Bay and its adjacent waters. Journal of Oceanography Taiwan Strait, 26(2): 281–288. |
| Livingstone D R, 1994. Recent developments in marine invertebrate organic xenobiotic metabolism. Toxicology and Ecotoxicology News, 1(3): 88–94. |
| Lopes S, Jurisicova A, Sun J G, Casper R F, 1998. Reactive oxygen species:potential cause for DNA fragmentation in human spermatozoa. Human Reproduction, 13(4): 896–900. Doi: 10.1093/humrep/13.4.896 |
| Matozzo V, Gagné F, Marin M G, Ricciardi F, Blaise C, 2008. Vitellogenin as a biomarker of exposure to estrogenic compounds in aquatic invertebrates:a review. Environment International, 34(4): 531–545. Doi: 10.1016/j.envint.2007.09.008 |
| McElroy A, Leitch K, Fay A, 2000. A survey of in vivo benzo[α]pyrene metabolism in small benthic marine invertebrates. Marine Environmental Research, 50(1-5): 33–38. Doi: 10.1016/S0141-1136(00)00054-4 |
| Mearns A J, Reish D J, Oshida P S, Ginn T, Rempel-Hester M A, Arthur C, Rutherford N, 2013. Effects of pollution on marine organisms. Water Environment Research, 85(10): 1 828–1 933. Doi: 10.2175/106143013X13698672322949 |
| Monteiro P R R, Reis-Henriques M A, Coimbra J, 2000. Plasma steroid levels in female flounder (Platichthys flesus) after chronic dietary exposure to single polycyclic aromatic hydrocarbons. Marine Environmental Research, 49(5): 453–467. Doi: 10.1016/S0141-1136(99)00085-9 |
| Munkittrick K R, Portt C B, Van Der Kraak G J, Smith I R, Rokosh D A, 1991. Impact of bleached kraft mill effluent on population characteristics, liver MFO activity, and serum steroid levels of a Lake Superior white sucker(Catostomus commersoni) Population. Canadian Journal of Fisheries and Aquatic Sciences, 48(8): 1 371–1 380. Doi: 10.1139/f91-164 |
| Oakes K D, Hewitt L M, McMaster M E, Wood C, Munkittrick K R, Van Der Kraak G J, 2005. Oxidative stress and sex steroid levels in fish following short-term exposure to pulp-mill effluents. Journal of Toxicology and Environmental Health, Part A, 68(4): 267–286. Doi: 10.1080/15287390590895621 |
| Rose J, Holbech H, Lindholst C, Nørum U, Povlsen A, Korsgaard B, Bjerregaard P, 2002. Vitellogenin induction by 17β-estradiol and 17α-ethinylestradiol in male zebrafish (Danio rerio). Comparative Biochemistry and Physiology Part C:Toxicology & Pharmacology, 131(4): 531–539. |
| Rurangwa E, Biegniewska A, Slominska E, Skorkowski E F, Ollevier F, 2002. Effect of tributyltin on adenylate content and enzyme activities of teleost sperm:a biochemical approach to study the mechanisms of toxicant reduced spermatozoa motility. Comparative Biochemistry and Physiology Part C:Toxicology & Pharmacology, 131(3): 335–344. |
| Rust A J, Burgess R M, Brownawell B J, McElroy A E, 2004. Relationship between metabolism and bioaccumulation of benzo[α]pyrene in benthic invertebrates. Environmental Toxicology and Chemistry, 23(11): 2 587–2 593. Doi: 10.1897/03-354 |
| Sternberg R M, Hotchkiss A K, LeBlanc G A, 2008. The contribution of steroidal androgens and estrogens to reproductive maturation of the eastern mud snail Ilyanassa obsoleta. General and Comparative Endocrinology, 156(1): 15–26. Doi: 10.1016/j.ygcen.2007.12.002 |
| Sumpter J P, Jobling S, 1995. Vitellogenesis as a biomarker for estrogenic contamination of the aquatic environment. Environmental Health Perspectives, 103(Supplement 7): 173–178. |
| Tian S M, Pan L Q, Sun X H, 2013. An investigation of endocrine disrupting effects and toxic mechanisms modulated by benzo[a]pyrene in female scallop Chlamys farreri. Aquatic Toxicology, 144-145: 162–171. Doi: 10.1016/j.aquatox.2013.09.031 |
| Van den Belt K, Verheyen R, Witters H, 2003. Comparison of vitellogenin responses in zebrafish and rainbow trout following exposure to environmental estrogens. Ecotoxicology and Environmental Safety, 56(2): 271–281. Doi: 10.1016/S0147-6513(03)00004-6 |
| Wang C D, Croll R P, 2004. Effects of sex steroids on gonadal development and gender determination in the sea scallop, Placopecten magellanicus. Aquaculture, 238(1-4): 483–498. Doi: 10.1016/j.aquaculture.2004.05.024 |
| Wen J M, Pan L Q, 2015. Short-term exposure to benzo[a] pyrene disrupts reproductive endocrine status in the swimming crab Portunus trituberculatus. Comparative Biochemistry and Physiology Part C:Toxicology & Pharmacology, 174-175: 13–20. |
| Weston D P, 1990. Hydrocarbon bioaccumulation from contaminated sediment by the deposit-feeding polychaete Abarenicola pacifica. Marine Biology, 107(1): 159–169. Doi: 10.1007/BF01313253 |
| Yund P O, 2000. How severe is sperm limitation in natural populations of marine free-spawners?. Trends in Ecology & Evolution, 15(1): 10–13. |
| Zar J H. 1999. Biostatistical Analysis. 5th edn. Prentice Hall Inc. , New Jersey, p. 189-248. |
| Zheng J S, Richardson B J, Shouming O, Zheng J H, 2004. Distribution and sources of polycyclic aromatic hydrocarbon (PAH) in marine environment of China. Chinese Journal of Oceanology and Limnology, 22(2): 136–145. Doi: 10.1007/BF02842584 |
| Zheng S L, Chen B, Wang Z, Qiu X Y, Yu X, Freestone D, Liu Z H, Huang H, Yu W W, Xu X Z, 2010. Reproductive toxic effects of sublethal cadmium on the marine polychaete Perinereis nuntia. Ecotoxicology and Environmental Safety, 73(6): 1 196–1 201. Doi: 10.1016/j.ecoenv.2010.05.022 |
 2017, Vol. 35
2017, Vol. 35


