Institute of Oceanology, Chinese Academy of Sciences
Article Information
- MA Lin(马林), LI Xinzheng(李新正)
- Benthic harpacticoid copepods of Jiaozhou Bay, Qingdao
- Chinese Journal of Oceanology and Limnology, 35(5): 1127-1133
- http://dx.doi.org/10.1007/s00343-017-6031-7
Article History
- Received Jan. 28, 2016
- accepted in principle Mar. 21, 2016
- accepted for publication Jun. 27, 2016
2 Laboratory for Marine Biology and Biotechnology, Qingdao 266237, China
Harpacticoid copepods are a highly diverse group, comprising over 4 300 described species (Wells, 2007). They are primarily marine, are particularly abundant in soft sediments and on macroalgae (Huys et al., 1996), and are known from muds to sands from the intertidal zone to the deepest oceanic ooze (Huys and Boxshall, 1991). Despite their small size, harpacticoids play important roles in marine ecosystems (Willen, 2000; Mu et al., 2001). They are highly nutritious and provide food for many fish, shrimp and benthic bivalves (Huys and Boxshall, 1991; Lee et al., 2012). Harpacticoids are also good indicators of the marine sedimentary environment, which have a potential value using as a monitor of environmental quality (Ahnert and Schriever, 2001; Araújo-Castro et al., 2009).
Jiaozhou Bay is a semi-enclosed bay located on the southern coast of Shandong Peninsula, near Qingdao City, in the western part of the Yellow Sea. Expansion in mariculture and industry, has affected marine ecosystems in this region. Global warming, sea level rising, melting of ice sheets, and extreme weather events are important aspects of climate change (Scavia et al., 2002; Zeppilli et al., 2015), which have significant impacts on marine ecosystem and can affect biodiversity and community structure of marine organisms (Harley et al., 2006).
With the exception of the Bohai Gulf, the harpacticoid fauna of the Chinese marine environment is less well known than its freshwater fauna, as few investigations have been undertaken (Gee and Mu, 2000; Mu and Gee, 2000; Mu et al., 2001; Mu and Huys, 2002, 2004; Huys and Mu, 2008). Though meiofaunal ecology and the free-living benthic harpacticoid fauna of Jiaozhou Bay have been studied (Zhang et al., 2001; Yang et al., 2009; Zhang, 2009), the marine harpacticoid fauna of this region remains poorly known (Ma and Li, 2011). In this paper we present additional data on the marine benthic harpacticoid fauna of Jiaozhou Bay, an inventory of taxa, a key to assist in species identification, and a brief synopsis of taxon distributions.
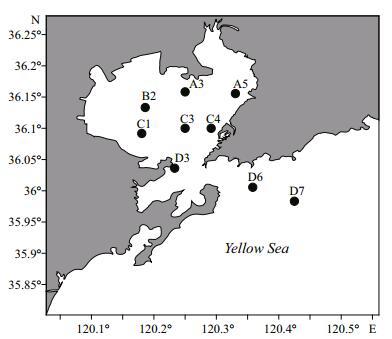
2 MATERIAL AND METHOD 2.1 Harpacticoid sampling and identificationSediment box-core (0.05 m2) samples were taken at nine stations in Jiaozhou Bay from 2011 to 2012. Stations A3, A5, B2, C1, C2, C3 and C4 were located inside the bay; station D3 was located in the mouth of the bay; stations D6 and D7 were located outside the bay (Fig. 1; Table 1). Subsamples were collected by acrylic corers (surface area: 14 cm2) for quantitative analysis of harpacticoid abundance, and surface sediments were collected for sediment grain-size analysis. Samples were fixed in buffered formalin solution, washed through 45 μm-mesh sieves, then centrifuged with colloidal silica (Ludox TM-50) as the flotation mesium. The proportion between sediment and flotation mesium is 1:3. Specimens were preserved in 75% ethanol. Harpacticoids were dissected in lactic acid, mounted on slides using lactophenol as a mounting medium for further examination. The slides were sealed with clear nail varnish. Observations were done using an Olympus BH-2 phase contrast microscope and a Zeiss Axioskop equipped with phase contrast microscope. Vouchers of species are deposited in the Marine Biological Museum of Chinese Academy of Sciences (MBMCAS), in the Institute of Oceanology, Chinese Academy of Sciences, Qingdao, China (IOCAS).
|
| Figure 1 Jiaozhou Bay sampling stations |

|
Abbreviations used in the text are: exp-1(-2-3), the first (second, third) segment of the exopod; enp-1(-2-3), the first (second, third) segment of the endopod; benp, baseoendopod of P5; P1–P6, swimming legs 1–6.
2.2 Sediment characteristicsGrain-size analysis was determined by laser particle-size analysis, with sediments were divided into four fractions: medium sand (500–250 μm), fine sand (250–63 μm), fine silt (63–4 μm), and clay (4–1 μm) (Zhang et al., 2013).
2.3 Data analysisRedundancy analysis (RDA) was used to correlate environmental variables and species distribution, the maximum gradient length of axes in detrended correspondence analysis (DCA) was 2.467 SD < 3 SD. In addition, the species/genera ratio was also used to analyze local faunistic complexes. The relationship between this index and species richness has been previously described for the harpacticoid fauna of the seas of Russia (Chertoprud and Garlitska, 2007). The fauna similarity was evaluated using Hacker-Dice index (H) for the qualitative data, which was used by Chertoprud et al. (2009).

where a is the number of shared characteristics for the subjects x and y; and b, c are the number of unique characteristics.
3 RESULT 3.1 Harpacticoid abundance and species richnessHarpacticoid abundance (mean 5.31 ind./cm2) was greatest at station C4 (8.35 ind./cm2) and least at station D7 (1.54 ind./cm2) (Table 1). Within-bay mean abundance (stations A3, A5, B2, C1, C3, C4, D3) was 6.13 ind./cm2, slightly higher than outside the bay (stations D6, D7), 2.45 ind./cm2.
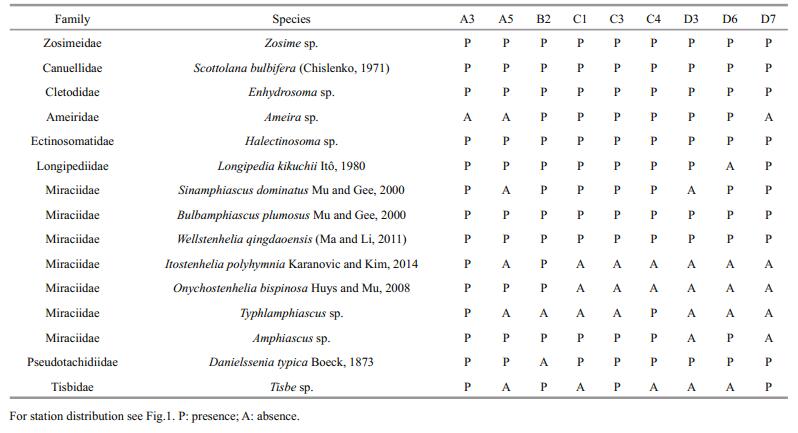
Fifteen species in 15 genera and nine families were found (Table 2). The most speciose family, the Miraciidae (seven species), was also the most frequently encountered, with six species occurring at all stations (Table 2). Neither Typhlamphiascus nor Amphiascus and none of Zosimeidae, Cletodidae, Ameiridae, Ectinosomatidae nor Tisbidae could be identified to species level given both sexes were not represented in samples. Each of the Canuellidae, Longipediidae, and Pseudotachidiidae were represented by single species. The species/genus ratio was low (1.0). The highest number of species was found at station A3 (14), close to Hongdao (inner bay), and the least (9) was found at station D3.
We first report Itostenhelia polyhymnia, a species formerly known from muds in Gwangyang Bay, South Korea (Karanovic and Kim, 2014), from Chinese waters. All of Scottolana bulbifera, Longipedia kikuchii, Sinamphiascus dominatus, Bulbamphiascus plumosus, Onychostenhelia bispinosa, Danielssenia typica had been previously reported from Bohai Gulf (Mu, 2000).
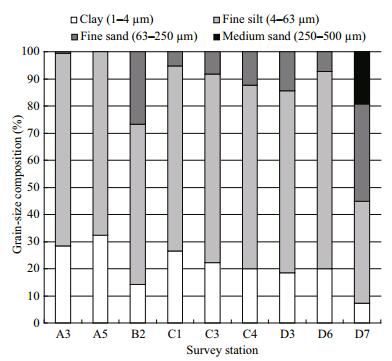
3.2 Sediment characteristicsThe sediment was sort into medium sand (500– 250 μm), fine sand (250–63 μm), fine silt (63–4 μm), and clay (4–1 μm). Textural types were observed in Jiaozhou Bay with four textural grades of medium sand, fine sand, fine silt and clay mixture (Fig. 2, Table 1). Medium sand occurred only at station D7, where it comprised 19.3% of the total sediment. The highest proportion of fine sand was also found at station D7 (35.93%), whereas the lowest was found at A5 (0.07%). The highest proportion of fine silt was found at station D6 (72.8%), the lowest at D7 (37.42%). The highest proportion of clay occurred at station D6 (32.34%), while the lowest occurred at station D7 (7.35%).
|
| Figure 2 Grain-size composition at survey stations in Jiaozhou Bay |
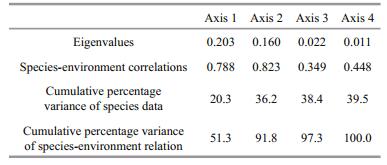
Redundancy analysis (RDA) was used to determine the extent to which depth and sediment characteristics affected the harpacticoid distribution (Table 3). All canonical axes explained approximately 39.5% variance of species data, and 100% variance of species-environment relation, among which the cumulative explanation of the first two axes reached 36.2% of species data and 91.8% of speciesenvironment relation. The eigenvalues of the first two canonical axes were higher than the other axes, indicating these two axes to the most explanatory. For species-environment relation, the first and second axes explained approximately 51.3% and 40.5% of variation, respectively. On the whole, the first two canonical axes explained the relationship between species and the environmental variables well. The biplot of the overall species distribution and environmental variables (Fig. 3) reveals the proportions of fine silt and clay have positive correlation with the distributions of most species, especially the species Zosime sp. Depth and the proportion of medium sand was negatively correlated with species distribution, except the species Enhydrosoma sp. and Amphiascus sp.
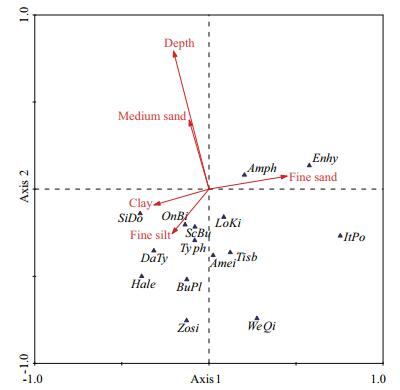
|
| Figure 3 RDA ordination biplot of species distribution and environmental variables in Jiao zhou Bay Species codes: Zosi: Zosime sp.; ScBu: Scottolana bulbifera; Enhy: Enhydrosoma sp.; Amei: Ameira sp.; Hale: Halectinosoma sp.; LoKi: Longipedia kikuchii; SiDo: Sinamphiascus dominatus; BuPl: Bulbamphiascus plumosus; WeQi: Wellstenhelia qingdaoensis; ItPo: Itostenhelia polyhymnia; OnBi: Onychostenhelia bispinosa; Typh: Typhlamphiascus sp.; Amph: Amphiascus sp.; DaTy: Danielssenia typica; Tisb: Tisbe sp. |
RDA reveals harpacticoid distribution throughout Jiaozhou Bay to be primarily affected by certain sedimentary characteristics and depth, with the proportion of fine silt and clay in sediment being critical variables. The proportions of fine silt and clay were marginally greater inside the bay than outside it, and the proportions of fine and medium sands were marginally lower within the bay. The water was a little deeper inside the bay than outside it. Harpacticoid species richness within the bay was marginally greater than that outside of it.
4.2 Comparative analysis with other coastal regionMean harpacticoid abundance (5.31 ind./cm2) was slightly higher than reported from this region (3.54 ind./cm2) by Zhang et al. (2001), but considerably lower than Bohai Gulf (6.63 ind./cm2) (Mu et al., 2001). More is known of the benthic harpacticoid fauna of Bohai Gulf than elsewhere in Chinese seas. The number of harpacticoid species from Jiaozhou Bay is comparatively lower than Bohai Gulf, though this could be an artefact of the limited sampling effort. Of the 15 genera in Jiaozhou Bay, 12 are known also from the Bohai Gulf. Though the genera Wellstenhelia and Itostenhelia were recently separated from Delavalia by Karanovic and Kim (2014), and species were divided between the three, all Delavalia taxa from Bohai Gulf were identified as Delavalia spp. As we have been unable to examine that material earlier reported from Bohai Gulf, we cannot determine whether the genera Wellstenhelia and Itostenhelia occurred there. Six species (40% of species found in Jiaozhou Bay) were also recorded from Bohai Gulf (Bulbamphiascus plumosus, Danielssenia typica, Longipedia kikuchii, Onychostenhelia bispinosa, Sinamphiascus dominatus and Scottolana bulbifera). The fauna similarity of Jiaozhou Bay and Bohai Gulf is 0.4 (the Hacker-Dice index). Four species (26.7% of species found in Jiaozhou Bay) were also recorded from Gwangyang Bay, South Korea (Itostenhelia polyhymnia, Onychostenhelia bispinosa, Scottolana bulbifera, Wellstenhelia qingdaoensis) (Lee et al., 2012; Karanovic and Kim, 2014). The fauna similarity of Jiaozhou Bay and Gwangyang Bay is also 0.4 (the Hacker-Dice index). The species/genus ratio in Jiaozhou Bay (1.0) was lower than Bohai Gulf (1.925, 77 species, 40 genera) and Gwangyang Bay (1.429, 10 species, 7 genera). This ratio is affected both by the biodiversity and the level of species knowledge for each region (Chertoprud and Garlitska, 2007).
4.3 Species distributions and sediment preferencesAll Jiaozhou Bay species have Indo-West Pacific distributions (Lang, 1948; Wells, 1980; Huys and Gee, 1993; Mu et al., 2001; Dvoretsky and Dvoretsky, 2010; Lee et al., 2012; Karanovic and Kim, 2014), as follows:
Scottolana bulbifera. China: Bohai Gulf, Jiaozhou Bay, South China Sea; Russia: Possjet Bay in the East Sea; South Korea: Kwangyang Bay; mud and sandy mud sediments.
Longipedia kikuchii. China: Bohai Gulf, Jiaozhou Bay; Japan: Amakusa; Singapore; India: Andaman and Nicobar Islands, Bay of Bengal, Porto Novo, Tamil Nadu; mud and sandy mud sediments.
Sinamphiascus dominatus. China: Bohai Gulf, Jiaozhou Bay; South Korea; subtidal muddy sediment. Bulbamphiascus plumosus. China: Bohai Gulf, Jiaozhou Bay; subtidal muddy sediment.
Wellstenhelia qingdaoensis. China: Jiaozhou Bay; South Korea: Gwangyang Bay; mud and sandy mud sediments.
Itostenhelia polyhymnia. China: Jiaozhou Bay; South Korea: Gwangyang Bay; mud and sandy mud sediments.
Onychostenhelia bispinosa. China: Bohai Gulf, Jiaozhou Bay; South Korea: Gwangyang Bay; subtidal muddy sediment.
Danielssenia typica. Europe; Canada; China: South China Sea, Yellow Sea (Jiaozhou Bay, Yantai), Bohai Gulf; mud and sandy mud sediments.
4.4 Key to harpacticoid species in Jiaozhou Bay (amended from Huys et al., 1996; Boxshall and Halsey, 2004; Wells, 2007):1. P1 coxa with inner spine or seta; antennary exopod at least 6-segmented................................................. 2
P1 coxa without inner spine or seta; antennary exopod at most 4-segmented.................................... 3
2. P2 enp-3 extremely elongated, longer than the total length of P2 enp-1 and enp-2; P5 well developed.......................................................... Longipedia kikuchii
P2 enp-3 not elongated; P5 vestigial and incorporated into somite............. Scottolana bulbifera
3. P5 exopod fused with baseoendopod......................................................................................... Zosime sp.
P5 exopod not fused with baseoendopod............. 4
4. Body typically fusiform; maxilla with elongate basis and armed with geniculate setae distally on endopod; maxilliped stenopodial...................................................................................... Halectinosoma sp.
Body variable, not fusiform; maxilla not of this form; maxilliped prehensile or subchelate............... 5
5. P1 endopod 2-segmented..................................... 6
P1 endopod 3-segmented................................... 10
6. P2–P4 endopod 2-segmented............................... 7
P2–P4 endopod 3-segmented............................... 8
7. P3 and P4 enp-1 without inner setae................................................................................ Enhydrosoma sp.
P3 and P4 enp-1 with one inner setae............................................................... Onychostenhelia bispinosa
8. Mandibular endopod elongated, twisted over exopod and with long setae.......................................9
Mandibular endopod not elongated............................................................................. Danielssenia typica
9. P3 and P4 exopods all with 7 setae and spines..................................................... Itostenhelia polyhymnia
P3 and P4 exopods all with 8 setae and spines................................................ Wellstenhelia qingdaoensis
10. Antennary exopod with 3 segments at most......11
Antennary exopod with 4 segments....... Tisbe sp.
11. Rostrum large, defined at base, reaching to at least the distal margin of the first antennulary segment; P2 endopod male 2-segmented; two egg-sacs............. 12
Rostrum small; P2 endopod male 3-segmented; one egg-sac............................................... Ameira sp.
12. P3 enp-3 with 5 setae........................................ 13
P3 enp-3 with 6 setae................... Amphiascus sp.
13. Caudal rami longer than broad................................................................................ Typhlamphiascus sp.
Caudal rami not longer than broad................... 14
14. P1 endopod non-prehensile, segments almost equal in length................... Sinamphiascus dominatus
P1 endopod prehensile, enp-1 longer than enp-2 and enp-3 combined.......... Bulbamphiascus plumosus
5 CONCLUSIONOurs is the first report of marine benthic harpacticoid species richness of Jiaozhou Bay, relating their distributions to sedimentary variables. We report 15 species from the region, and recognize species richness to be marginally higher within the bay than outside it. We also determine the proportions of fine silt and clay to positively affect the species richness and distributions of most harpacticoid species, demonstrate the proportion of medium sand and depth to negatively correlate with harpacticoid species richness and distribution. Finally, we provide a brief discussion on the geographic distribution of species, their known sedimentary preference, and a key to taxa of the region.
6 ACKNOWLEDGEMENTWe would like to thank our colleagues (DONG Dong, GAN Zhibin, KOU Qi, PENG Songyao, SUI Jixing, WANG Jinbao, XU Peng) for collecting sediment samples and providing us with depth and sediment information; and very appreciated to XU Yong for RDA analysis.
| Ahnert A, Schriever G, 2001. Response of abyssal Copepoda Harpacticoida (Crustacea) and other meiobenthos to an artificial disturbance and its bearing on future mining for polymetallic nodules. Deep-Sea Research Part Ⅱ:Topical Studies in Oceanography, 48(17-18): 3779–3794. Doi: 10.1016/S0967-0645(01)00067-4 |
| Araújo-Castro C M V, Souza-Santos L P, Torreiro A G, Garcia K S, 2009. Sensitivity of the marine benthic copepod Tisbe biminiensis (Copepoda, Harpacticoida) to potassium dichromate and sediment particle size. Brazilian Journal of Oceanography, 57(1): 33–41. Doi: 10.1590/S1679-87592009000100004 |
| Boxshall G A, Halsey S H. 2004. An Introduction to Copepod Diversity. The Ray Society, London. 966p. |
| Chertoprud E S, Garlitska L A, 2007. A comparative analysis of the Harpacticoida (Copepoda) faunas from the northern and southern seas of Russia. Oceanology, 47(6): 814–823. Doi: 10.1134/S0001437007060069 |
| Chertoprud E S, Gómez S, Gheerardyn H, 2009. Harpacticoida(Copepoda) fauna and the taxocene diversity of the South China Sea. Oceanology, 49(4): 488–498. Doi: 10.1134/S0001437009040079 |
| Chislenko L L, 1971. New common forms of harpacticids(Copepoda, Harpacticoida) from Possjet Bay of the Sea of Japan. Issledovaniya Fauny Morei, 8(14): 151–181. |
| Dvoretsky V G, Dvoretsky A G, 2010. Checklist of fauna found in zooplankton samples from the Barents Sea. Polar Biology, 33(7): 991–1005. Doi: 10.1007/s00300-010-0773-4 |
| Gee J M, Mu F H, 2000. A new genus of Cletodidae (Copepoda; Harpacticoida) from the Bohai Sea, China. Journal of Natural History, 34(6): 809–822. Doi: 10.1080/002229300299273 |
| Harley C D G, Hughes A R, Hultgren K M, Miner B G, Sorte C J B, Thornber C S, Rodriguez L F, Tomanek L, Williams S L, 2006. The impacts of climate change in coastal marine systems. Ecology Letters, 9(2): 228–241. Doi: 10.1111/j.1461-0248.2005.00871.x |
| Huys R, Boxshall G A. 1991. Copepod Evolution. 1st edn. The Ray Society, London. 468p. |
| Huys R, Gee J M, Moore C G, Hamond R. 1996. Synopses of the British Fauna (New Series) No. 51. Marine and Brackish Water Harpacticoids, Part 1. Field Studies Council, Shrewsbury. 352p. |
| Huys R, Gee J M, 1993. A revision of Danielssenia Boeck and Psammis Sars with the establishment of two new genera Archisenia and Bathypsammis (Harpacticoida:Paranannopidae). Bulletin of the Natural History Museum Zoology, 59(1): 45–81. |
| Huys R, Mu F H, 2008. Description of a new species of Onychostenhelia Itô (Copepoda, Harpacticoida, Miraciidae) from the Bohai Sea, China. Zootaxa, 1706: 51–68. |
| Karanovic T, Kim K, 2014. New insights into polyphyly of the harpacticoid genus Delavalia (Crustacea, Copepoda) through morphological and molecular study of an unprecedented diversity of sympatric species in a small South Korean bay. Zootaxa, 3783(1): 1–96. Doi: 10.11646/zootaxa.3783.1 |
| Lang K. 1948. Monographie der Harpacticiden. Hakan Ohlsson, Lund. 1683p. |
| Lee W, Park E, Song S J. 2012. Invertebrate Fauna of Korea, Marine Harpacticoida. National Institute of Biological Resources, Ministry of Environment, South Korea. 276p. |
| Ma L, Li X Z, 2011. Delavalia qingdaoensis sp.nov.(Harpacticoida, Miraciidae), a new copepod species from Jiaozhou Bay, Yellow Sea. Crustaceana, 84(9): 1085–1097. Doi: 10.1163/001121611X584334 |
| Mu F H, Gee J M, 2000. Two new species of Bulbamphiascus(Copepoda:Harpacticoida:Diosaccidae) and a related new genus, from the Bohai Sea, China. Cahiers de Biologie Marine, 41(2): 103–135. |
| Mu F H, Huys R, 2002. New species of Stenhelia (Copepoda, Harpacticoida, Diosaccidae) from the Bohai Sea (China) with notes on subgeneric division and phylogenetic relationships. Cahiers de Biologie Marine, 43(2): 179–206. |
| Mu F H, Huys R, 2004. Canuellidae (Copepoda, Harpacticoida) from the Bohai Sea, China. Journal of Natural History, 38(1): 1–36. Doi: 10.1080/00222930210138935 |
| Mu F H, Zhang Z N, Guo Y Q, 2001. The study on the community structure of benthic copepods in the Bohai Sea. Acta Oceanologica Sinica, 23(6): 120–127. |
| Mu F H. 2000. Studies on Taxonomy and Ecology of Benthic Copepods in the Bohai Sea, China. Ocean University of China, Qingdao, China. 220p. (in Chinese with English abstract) |
| Scavia D, Field J C, Boesch D F, Buddemeier R W, Burkett V, Cayan D R, Fogarty M, Harwell M A, Howarth R W, Mason C, Reed D J, Royer T C, Sallenger A H, Titus J G, 2002. Climate change impacts on U.S. coastal and marine ecosystems. Estuaries, 25(2): 149–164. Doi: 10.1007/BF02691304 |
| Wells J B J, 1980. A revision of the genus Longipedia Claus(Crustacea:Copepoda:Harpacticoida). Zoological Journal of the Linnean Society, 70(2): 103–189. Doi: 10.1111/zoj.1980.70.issue-2 |
| Wells J B J, 2007. An annotated checklist and keys to the species of Copepoda Harpacticoida (Crustacea). Zootaxa, 1568: 1–872. |
| Willen E. 2000. Phylogeny of the Thalestridimorpha Lang, 1944 (Crustacea, Copepoda). Cuvillier Verlag, Göttingen. 246p. |
| Yang S C, Mu F H, Zhou H, Chen H Y, Wu S Y, 2009. Abundance and biomass of the meiofauna in Jiaozhou Bay and southern coastal waters of Shandong Peninsula in winter, 2006. Periodical of Ocean University of China, 39(sup.): 78–82. |
| Zeppilli D, Sarrazin J, Leduc D, Arbizu P M, Fontaneto D, Fontanier C, Gooday A J, Kristensen R M, Ivanenko V N, Sørensen M V, Vanreusel A, Thébault J, Mea M, Allio N, Andro T, Arvigo A, Castrec J, Danielo M, Foulon V, Fumeron R, Hermabessiere L, Hulot V, James T, Langonne-Augen R, Le Bot T, Long M, Mahabror D, Morel Q, Pantalos M, Pouplard E, Raimondeau L, RioCabello A, Seite S, Traisnel G, Urvoy K, van der Stegen T, Weyand M, Fernandes D, 2015. Is the meiofauna a good indicator for climate change and anthropogenic impacts? Mar. Biodiv., 45(3): 505–535. |
| Zhang Y, Lv Z B, Guan B, Liu Y J, Li F, Li S W, Ma Y Q, Yu J B, Li Y Z, 2013. Status of macrobenthic community and its relationships to trace metals and natural sediment characteristics. Clean-Soil, Air, Water, 41(10): 1027–1034. |
| Zhang Y, 2009. A study on seasonal variation of abundance and biomass of meiofauna at the typical station in Jiaozhou Bay. Chinese Agricultural Science Bulletin, 25(17): 296–301. |
| Zhang Z N, Zhou H, Yu Z S, Han J, 2001. Abundance and biomass of the benthic meiofauna in the northern soft bottom of the Jiaozhou Bay. Oceanologia et Limnologia Sinica, 32(3): 139–147. |
 2017, Vol. 35
2017, Vol. 35