Institute of Oceanology, Chinese Academy of Sciences
Article Information
- Muhammad Abu Bakar SIDDIK, Md Reaz CHAKLADER, Md Abu HANIF, Ashfaqun NAHAR, Ilham ILHAM, Anthony COLE, Ravi FOTEDAR
- Variation in the life-history traits of a Schilbid catfish, Clupisoma garua (Hamilton, 1822) in the coastal waters of southern Bangladesh
- Chinese Journal of Oceanology and Limnology, 35(5): 1189-1196
- http://dx.doi.org/10.1007/s00343-017-6008-6
Article History
- Received Jan. 28, 2016
- accepted in principle May. 12, 2016
- accepted for publication Jul. 4, 2016
2 Department of Marine Fisheries and Oceanography, Patuakhali Science and Technology University, Patuakhali-8602, Bangladesh;
3 Department of Aquatic Resources and Management, Jakarta Fisheries University, Jl. AUP Pasar Minggu Jakarta Selatan 12520 Indonesia;
4 Department of Environment and Agriculture, Curtin University, 1 Turner Avenue, Bentley, WA 6102, Australia
The southern coastal waters of Bangladesh are very rich in fishery resources and regular, intense interaction takes place between more saline and more turbid habitats, which provides juvenile fish with abundant food resources as well as some refuge from predation (Laegdsgaard and Johnson, 2001). Moreover, a large number of rivers and estuaries support mangrove forests. These make the southern coastal region of Bangladesh an ideal nursery and breeding ground for many offshore and estuarine shellfish and finfish (Mandal et al., 2013).
Clupisoma garua belongs to the order Siluriformes and is an important component of riverine and brackish water fisheries in Bangladesh. It is commonly known as Garua Bachcha which is preferred by all classes of consumers due to its taste and nutritive quality. This species was once extensively available in the coastal waters of Bangladesh and also occurs neighboring countries including India, Myanmar, Nepal and Pakistan (Talwar and Jhingran, 1991). But over the past decade, increasing anthropogenic and natural hazards have limited the species' geographical distribution across the region (Siddik et al., 2013; Chaklader et al., 2014; Hanif et al., 2015a; Sharker et al., 2015; Nahar et al., 2015) and has resulted in the species being categorized as critically endangered in Bangladesh (Hanif et al., 2015b; IUCN, 2015).
Studies on life history traits of any particular species are important for estimating how fish weight changes as a function of length, ascertaining the condition of the fish, comparing fish growth among habitats, and as a complement to species-specific reproduction and feeding studies (Le Cren, 1951; Ecoutin et al., 2005; Froese, 2006; Cicek et al., 2006; Sun et al., 2013; Chaklader et al., 2016a, b; Siddik et al., 2016c). Traits are also crucial for understanding fish biology, taxonomy, ecology as well as for sustainable management and conservation of fish (Chaklader et al., 2015; Siddik et al., 2015). Over the years morphometric characters, as a relatively cheap biological tool, have been used to study growth patterns and ontogenic changes in various species (Oscoz et al., 2005; Tarkan et al., 2006; Heydarnejad, 2009; Simon et al., 2010; Siddik et al., 2016a, b). Despite the available research on the life-history traits of different fish in southern Bangladesh, information about the critically endangered C. garua has not been investigated before. Therefore, this article describes length-frequency distribution (LFD), sex ratio (SR), length-weight relationships (LWRs), condition factors (CFs), relative growth (WR) and form factors (a3.0) in C. garua in the coastal waters of Bangladesh.
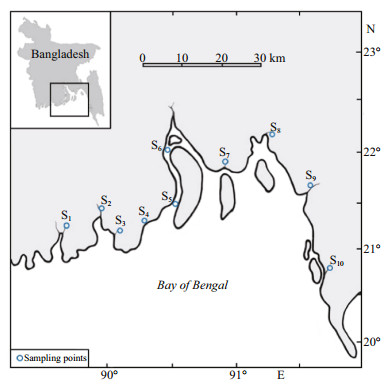
2 MATERIAL AND METHOD 2.1 Study area and samplingThe specimens of C. garua were collected occasionally from 10 stations along the entire coastal region of Bangladesh using traditional fishing gear during the daytime over a period of one year from August 2013 to July 2014 (Fig. 1). Samples were immediately preserved with ice in the fish landing areas and fixed with 5% formalin prior to being brought to the laboratory, Faculty of Fisheries, Patuakhali Science and Technology University, Bangladesh. Morphometric characters were measured using slide calipers and body weight (BW) was measured by a digital balance (Fig. 2).
|
| Figure 1 Sampling sites of C. garua in the waters of southern coastal region of Bangladesh |

|
| Figure 2 Schematic image of C.garua showing 15 morphometric attributes to infer morphological differences among the populations Total length (TL); fork length (FL); standard length (SL); head length (LH); head depth (HD↓); highest body depth (D2↓); lowest body depth (LBD↓); pre-dorsal length (LD1); post-dorsal length (D2S); height of dorsal fin (D↓); height of pectoral fin (P↓); height of ventral fin (V↓); height of anal fin (A↓); length of anal base (A1A2); maximum barbell length (MB). |
The LWR was calculated using the allometric equation W=aLb, where W is body weight (g), L is total length (cm), a is a coefficient of body form and b is an exponent of the growth type (Simon et al., 2010). The values of a and b were calculated by natural logarithms lnW=lna+blnL based on linear regression analysis. In addition, 95% confidence limits of b and the coefficient of determination R2 were estimated. Following Froese (2006) ln-ln plots of length and weight values were visually inspected for outliers prior to the regression analysis of ln BW on ln TL, with extremes being omitted from the regression analyses. Values of b are required to determine from the linear regressions when the allometric relationship varied from the isometric value (b=3).
2.3 Calculation of condition and form factorsFor the calculation of Fulton's condition factor (KF) (Fulton, 1904), the following equation KF=100×(W/L3) was used, where W and L are given in g and cm, respectively. Relative condition factor (KR) was calculated following the equation, KR=W/a×Lb (Le Cren, 1951). Allometric condition factor (KA) was calculated using the formula, KA=W/Lb (Tesch, 1971). Also, relative weight (WR) was estimated using the formula, WR=(W/WS)×100 (Froese, 2006). The form factor (a3.0) is used whether the body shape of a given population or species is significantly different from others (Froese, 2006).The equation that was used to calculate the form factor (a3.0) is: a3.0=10loga–S(b–3) (Froese, 2006), where a and b are regression parameters of the (LWR), S is the regression slope of log a vs b. A mean slope of S=-1.358 was used for estimating the form factor during this study due to lack of information on LWRs in this species.
2.4 Statistical analysisA χ2 goodness-of-fit test was applied to evaluate the sex-ratio from the expected value of 1:1 (male: female). The Kolmogorov-Smirnov test was applied to make comparisons between the sexes for lengthfrequency distributions. Following Anderson and Neumann (1996), the non-parametric Wilcoxon rank test was used to compare the mean relative weight of a population with 100 and Spearman rank test was used to correlate morphometric measurements such as TL, SL, and BW with Fulton's condition factor (K) and relative weight (WR). Also, the parameters (a) and (b) of the LWRs were compared by the analysis of covariance (ANCOVA). All statistical analyses were performed using SPSS v. 16.0 and STATISTICA version 8.0.
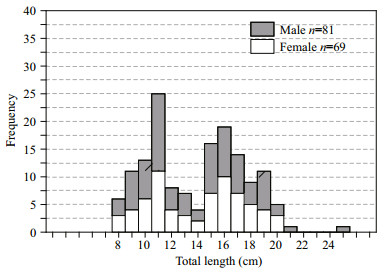
3 RESULT 3.1 Length-frequency distributionA total of 150 specimens of C. garua were collected from the entire southern coastal region of Bangladesh. The comparison of morphometric characters between males, females and pooled samples of C. garua is presented in Table 1. Univariate ANOVA revealed that females have higher mean values than that of males in all examined morphometric measurements except the head depth, highest body depth, lowest body depth, and height of dorsal fin. The total length varied from 8.60 to 25.20 cm (mean±SD=14.37±3.73) for males and 8.80 to 20.80 cm (mean±SD=14.57±3.44) for females of C. garua. Total length frequencies of males and females are shown in Fig. 3. The distribution of TL was non-normal (Kolmogorov-Smirnov test, P < 0.001), and showed two peaks at 11 and 16 cm for both males and females. Moreover, the BW varied between 4.26 and 128.80 g (mean±SD=23.99±19.79) in males and 4.66 and 61.40 g (mean±SD=23.42±15.71) in females of C. garua. The BW-frequency distribution also showed that the males and females of C. garua were not normally distributed (Kolmogorov-Smirnov test, P < 0.001).
|
| Figure 3 Distribution of the total length frequency of both sexes C. garua (Hamilton, 1822) in the waters of Bangladesh |
|
In the study, 54% were males and 46% were females among the 150 specimens (male=81; female=69; male:female=1: 0.85) of C. garua collected during the study. However, the overall sex ratio did not show significant difference from the expected value of 1:1 (df=1, χ2=0.96, P>0.05) (Table 2).

|
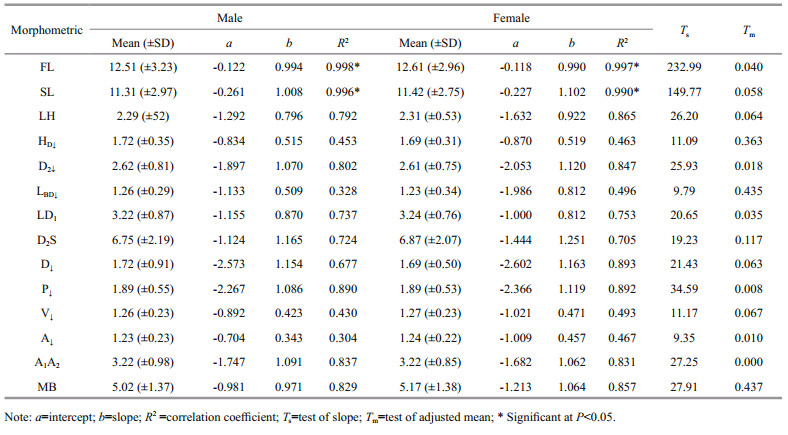
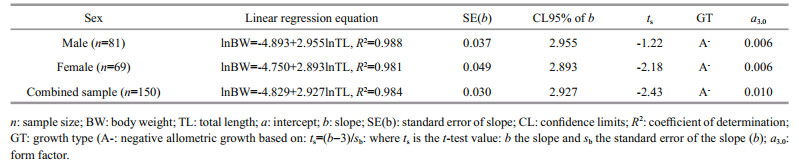
The statistics and parameters of the length-weight relationships (LWR), form factor (a3.0) and growth types of C. garua are shown in Figs. 4 and 5, and Table 3. The calculated b in the LWR for males, females and combined gender of C. garua were 2.955 (2.881 to 3.029), 2.893 (2.794 to 2.992) and 2.927 (2.867 to 2.987), respectively and indicated negative allometric growth as b was less than 3. However, there were no statistical differences in the intercepts and in the slopes between the sexes. The coefficient of determination (R2) estimated from the length-weight relationship for all male, female and combined was greater than 0.9 and was significant.

|
| Figure 4 Length-weight relationships of C. garua (Hamilton, 1822) of Bangladesh |

|
| Figure 5 Log-scale visual representation of the length– weight relationship of C. garua (Hamilton, 1822) of Bangladesh |
|
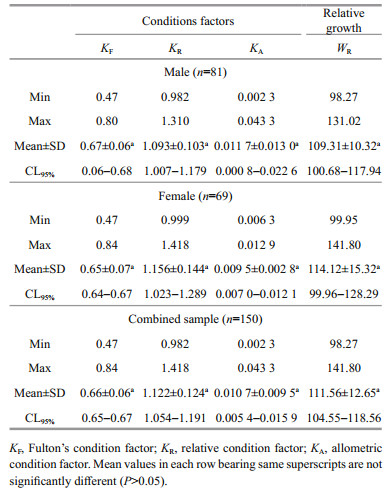
KF ranged from 0.47 to 0.80 (mean±SD=0.67±0.06) in males and from 0.47 to 0.84 (mean±SD=0.65±0.07) in females (Table 4). KR for males and females C. garua varied between 0.982 and 1.310 (mean±SD= 1.093±0.103) and between 0.999 and 1.418 (mean±SD=1.156±0.144), respectively. KA ranged from 0.002 3 to 0.043 3 (mean±SD=0.011 7± 0.013 0) for males and from 0.006 3 to 0.012 9 (mean±SD= 0.009 5±0.002 8) for females. WR for male and female C. garua ranged from 98.27 to 131.02 (mean±SD= 109.31±10.32) and from 99.95 to 141.80 (mean±SD= 114.12±15.32), respectively (Table 4). The calculated form factor was 0.006 and 0.006 for males and females, respectively (Table 3).
|
The results reveal that females have higher mean values in all morphometric dimensions than males except for the head depth, highest body depth, lowest body depth, and height of dorsal fin. The minimum size (TL) of C. garua was 8.60 cm and the maximum size was 25.20 cm TL, which is less than the maximum reported value of 36.6 cm in Betwa and Gomti rivers, Karnataka, Uttar Pradesh, India (Sani et al., 2010) and 33 cm in Gomti Rivers, India (Kumar et al., 2014). The maximum body weight of C. garua recorded in this study was 128.80 g. In this study, the absence of larger individuals in the sampling areas might be due to over exploitation in the coastal waters of Bangladesh (Hossain et al., 2012). Furthermore, local fishers used particular fishing gear with similar mesh size resulting in similar catch composition, unlike in previous studies, in which different fishing techniques caused biased estimation of the various population parameters including the maximum size (Hossain et al., 2012).
The male and female ratio may vary between 1:1 and 1:1.3 in a typical population reported by Dias et al. (2014) but in the study the ratio was 1:0.85 and it was not significantly different from 1:1. There are a variety of factors, including biased sex determination (Conover and Kynard, 1981), divergent sexual behavior, growth rate, varying climate and regional conditions, that could cause such variation (Dias et al., 2014).
In this study, the b values calculated from the TLBW relationship were 2.955 for males and 2.893 for females, indicating negative allometric growth of C. garua in the study i.e. the body does not increase in all dimensions in the same proportion to growth (Jobling, 2008) which were within the limit (2.5–3.5) reported by Oscoz et al. (2005), Esmaeili and Ebrahimi (2006) and by Arshad et al. (2008) in various fishes. Sani et al. (2010) stated that the b value of C. garua was 3.10 in Gomti Rivers, India, while in the same river Kumar et al. (2014) found the b value is 2.69. It is widely recognized that there are a number of factors that influence the length-weight relationship in fish, including growth phase, sex, season, food (quantity, quality and size), stage of maturity, and health and general fish condition, preservation techniques and differences in the observed length ranges of the specimen caught (Tesch, 1971; Hossain et al., 2012), all of which were not investigated in the present study. Moreover, the samples of C. garua were collected over an extended period irrespective of any particular season, so these data should be treated only as mean-annual values for comparative purposes.
Condition factors are indices reflecting interactions between biotic and abiotic factors in the physiology of fish, which reflects the well-being of their populations during various stages of the life cycle (Hossain et al., 2012). Several condition factors, including Fulton's condition factor (KF), the relative condition factor (KR) and the allometric condition factor (KA) were employed during this study in order to evaluate the general health and productivity of C. garua. These condition factors revealed no significant differences between sexes. Several studies conducted on population dynamics have revealed that high condition factor values indicate favorable environmental conditions such as habitat suitability and prey availability, while low values indicate more unfavorable environmental conditions (Blackwell et al., 2000). Table 4 shows Fulton's condition factor (KF) for males, females and pooled samples were 0.67, 0.65 and 0.66, respectively. The fluctuation of condition factor occurs due to interaction between feeding conditions, parasitic infections, and physiological factors (Le Cren, 1951). Differences in condition factor have been considered to indicate various biological features, such as fatness or suitability of the environment (Le Cren, 1951). The temporal decrease in KF between the sexes in the present study could be considered indicative of degradation in feeding conditions. However, no references dealing with the condition factors of the C. garua are available in the coastal waters of Bangladesh.
Relative weight (WR), used in this study, is an index frequently used to compare the condition of species. The estimated values of WR less than 100 for an individual, size group or population suggest problems such as low prey availability or high predator density, while values greater than 100 indicate a prey surplus or low predator density (Rypel and Richter, 2008). Recently, a number studies have used WR to provide assistance in the management and conservation of non-game fishes, especially those that are threatened or endangered (Richter, 2007; Muchlisin et al., 2010). During the present study, the relative weight (WR) showed no significant differences for either male (109.31±10.32) or female (114.12±15.32) C. garua, and WR values were close to 100 in this study indicating a habitat with a balance between food availability and the presence of predators (Anderson and Neumann, 1996). Moreover, it might suggest that the water quality of southern waters is still auspicious for these fisheries. However, C. garua is categorized as critically endangered in Bangladeshi waters by IUCN (2015) which might be due to various causes other than water quality (Rahman et al., 2012). Nevertheless, this information would aid in urgent detection of any long-term deterioration in condition that may occur. However, no research concerning the relative weight of C. garua is available in the literature thus preventing any comparison of this finding with previous results, although it will provide baseline information to compare with future investigations.
Only a few studies on form factor of fish are available in Bangladeshi waters (Hossain et al., 2012; Rahman et al., 2012). Froese 2006 reported that the estimated value of a3.0 is 0.01, indicating an elongate body shape of the fish. The form factor of C. garua was 0.006 and 0.006 for male and female, respectively indicating elongate body shape which is characteristic of many riverine fishes. Nonetheless, there is no information available concerning the form factor of this species in the literature, so this study is the first report on it in C. garua, which again will be helpful for future studies.
5 CONCLUSIONThis study provides basic information on the sex ratio, LFD, LWRs, and condition factors of C. garua in the waters of the southern coastal region of Bangladesh, which should be useful for the for fishery scientists and conservationists to initiate early management strategies and regulations for the sustainable conservation of the remaining stocks of this species. Furthermore, no length-weight, lengthlength data or condition-factor data currently exist in the FishBase for this species. So our results may contribute to this invaluable electronic database. Research in more detail is necessary to answer several questions about body-size range and the spawning periodicity of this fishery.
6 ACKNOWLEDGEMENTWe extend our sincere thanks to Dr. Sukham Munilkumar ICAR-Central Institute of Fisheries Education, Kolkata, India for his invaluable comments updating the manuscript.
| Anderson R O, Neumann R M. 1996. Length, weight, and associated structural indices. In: Murphy B R, Willis D W eds. Fisheries Techniques. 2nd edn. American Fisheries Society, Bethesda, Maryland. p. 447-482. |
| Arshad A, Jimmy A, Nurul Amin S M, Sidik B J, Harah Z M, 2008. Length-weight and length-length relationships of five fish species collected from seagrass beds of the Sungai Pulai estuary, Peninsular Malaysia. J. Appl.Ichthyol., 24(3): 328–329. Doi: 10.1111/j.1439-0426.2007.01026.x |
| Blackwell B G, Brown M L, Willis D W, 2000. Relative weight(Wr) status and current use in fisheries assessment and management. Reviews in Fisheries Science, 8(1): 1–44. Doi: 10.1080/10641260091129161 |
| Chaklader M R, Nahar A, Siddik M A B, Sharker R, 2014. Feeding habits and diet composition of Asian Catfish Mystus vittatus (Bloch, 1794) in shallow water of an impacted coastal habitat. World J. Fish Marine Sci., 6(6): 551–556. |
| Chaklader M R, Siddik M A B, Hanif M A, Nahar A, Mahmud S, Piria M, 2016a. Morphometric and meristic variation of endangered pabda catfish, Ompok pabda (HamiltonBuchanan, 1822) from southern coastal waters of Bangladesh. Pakistan J. Zool., 48(3): 681–687. |
| Chaklader M R, Siddik M A B, Nahar A, Hanif M A, Alam M J, Mahmud S, 2016b. Morphometric parameters and allometric growth in paradise threadfin Polynemus paradiseus (Linnaeus, 1758) from a coastal river of Bangladesh. J. Aquac. Res. Development, 7: 417. |
| Chaklader M R, Siddik M A B, Nahar A, 2015. Taxonomic diversity of paradise threadfin Polynemus paradiseus(Linnaeus, 1758) inhabiting southern coastal rivers in Bangladesh. Sains Malaysiana, 44(9): 1241–1248. Doi: 10.17576/jsm |
| Cicek E, Avsar D, Yeldan H, Ozutok M, 2006. Length-weight relationships for 31 teleost fishes caught by bottom trawl net in the Babadillimani Bight (Northeastern Mediterranean). J. Appl. Ichthyol., 22(4): 290–292. Doi: 10.1111/jai.2006.22.issue-4 |
| Conover D O, Kynard B O, 1981. Environmental sex determination:Interaction of temperature and genotype in a fish. Science, 213(4507): 577–579. Doi: 10.1126/science.213.4507.577 |
| Dias J, Fernandez W S, Schmidt T C S, 2014. Length weight relationship of 73 fish species caught in the southeastern inner continental shelf region of Brazil. Lat. Am. J. Aquat.Res., 42(1): 127–136. Doi: 10.3856/vol42-issue1-fulltext-10 |
| Ecoutin J M, Albaret J J, Trape S, 2005. Length-weight relationships for fish populations of a relatively undisturbed tropical estuary:the Gambia. Fisheries Res., 72(2-3): 347–351. Doi: 10.1016/j.fishres.2004.10.007 |
| Esmaeili H R, Ebrahimi M, 2006. Length-weight relationships of some freshwater fishes of Iran. J. Appl. Ichthyol., 22(4): 328–329. Doi: 10.1111/jai.2006.22.issue-4 |
| Froese R, 2006. Cube law, condition factor and weight-length relationships:history, meta-analysis and recommendations. J. Appl. Ichthyol., 22(4): 241–253. Doi: 10.1111/jai.2006.22.issue-4 |
| Fulton T W. 1904. The rate of growth of fishes. Twenty-second Annual Report. Part â…¢. Fisheries Board of Scotland, Edinburgh. p. 141-241. |
| Hanif M A, Siddik M A B, Chaklader M R, Mahmud S, Nahar A, Hoque M S, Munilkumar S, 2015a. Biodiversity and conservation of threatened freshwater fishes in Sandha River, South West Bangladesh. World Appl. Sci. J., 33(9): 1497–1510. |
| Hanif M A, Siddik M A B, Chaklader M R, Nahar A, Mahmud S, 2015b. Fish diversity in the southern coastal waters of Bangladesh:present status, threats and conservation perspectives. Croatian Journal of Fisheries, 73(4): 251–271. |
| Heydarnejad M S, 2009. Length-weight relationships for six freshwater fish species in Iran. Chinese Journal of Oceanology and Limnology, 27(1): 61–62. Doi: 10.1007/s00343-009-0061-8 |
| Hossain M Y, Rahman M M, Abdallah E M, 2012. Relationships between body size, weight, condition and fecundity of the threatened fish Puntius ticto (Hamilton, 1822) in the Ganges River, northwestern Bangladesh. Sains Malaysiana, 41(7): 803–814. |
| IUCN. 2015. The IUCN Red List of Threatened Species. Version 2015-4. http://www.iucnredlist.org/details/166588/0. Accessed on 2016-01-26. |
| Jobling M. 2008. Environmental factors and rates of development and growth. In: Hart P J, Reynolds J D eds. Handbook of Fish Biology and Fisheries, Vol. 1: Fish Biology. Blackwell Publishing Ltd. , Oxford. p. 97-122. |
| Kumar R, Yadav S S, Tripathi M, 2014. Studies on lengthweight relationship of seven commercially important freshwater fish species of Gomti River Lucknow (U.P.) India. International Journal of Fisheries and Aquatic Sciences, 1(3): 1–3. |
| Laegdsgaard P, Johnson C, 2001. Why do juvenile fish utilise mangrove habitats?. J. Exp. Mar. Biol. Ecol., 257(2): 229–253. Doi: 10.1016/S0022-0981(00)00331-2 |
| Le Cren E D, 1951. The length-weight relationship and seasonal cycle in gonad weight and condition in the perch (Perca fluviatilis). Journal of Animal Ecology, 20(2): 201–219. Doi: 10.2307/1540 |
| Mandal B, Mukherjee A, Banerjee S, 2013. A review on the ichthyofaunal diversity in mangrove based estuary of Sundarbans. Rev. Fish. Biol. Fisheries, 23(3): 365–374. Doi: 10.1007/s11160-012-9300-8 |
| Muchlisin Z A, Musman M, Azizah M N S, 2010. Lengthweight relationships and condition factors of two threatened fishes, Rasbora tawarensis and Poropuntius tawarensis, endemic to Lake Laut Tawar, Aceh Province, Indonesia. J. Appl. Ichthyol., 26(6): 949–953. Doi: 10.1111/jai.2010.26.issue-6 |
| Nahar A, Siddik M A B, Alam M A, Chaklader M R, 2015. Population genetic structure of paradise threadfin Polynemus paradiseus (Linnaeus, 1758) revealed by allozyme marker. Intl. J. Zool. Res., 11(2): 48–56. Doi: 10.3923/ijzr.2015.48.56 |
| Oscoz J, Campos F, Escala M C, 2005. Weight-length relationships of some fish species of the Iberian Peninsula. J. Appl. Ichthyol., 21(1): 73–74. Doi: 10.1111/jai.2005.21.issue-1 |
| Rahman M M, Hossain M Y, Jewel M A S, Rahman M M, Jasmine S, Abdallah E M, Ohtomi J, 2012. Population structure, length-weight and length-length relationships, condition-and form-factor of the pool barb Puntius sophore (Hamilton, 1822) (Cyprinidae) from the Chalan Beel, north-central Bangladesh. Sains Malaysiana, 41(7): 795–802. |
| Richter T J, 2007. Development and evaluation of standard weight equations for bridge-lip suckers and large-scale suckers. N. Am. J. Fish. Manage., 27(3): 936–939. Doi: 10.1577/M06-087.1 |
| Rypel A L, Richter T J, 2008. Empirical percentile standard weight equation for the blacktail redhorse. N. Am. J. Fish.Manage., 28(6): 1843–1846. Doi: 10.1577/M07-193.1 |
| Sani R, Gupta B K, Sarkar U K, Pandey A, Dubey V K, Singh Lakra W, 2010. Length-weight relationships of 14 Indian freshwater fish species from the Betwa (Yamuna River tributary) and Gomti (Ganga River tributary) rivers. J.Appl. Ichthyol., 26(3): 456–459. Doi: 10.1111/jai.2010.26.issue-3 |
| Sharker M R, Siddik M A B, Nahar A, Shahjahan M, Faroque A A, 2015. Genetic differentiation of wild and hatchery populations of Indian major carp Cirrhinus cirrhosis in Bangladesh. J. Environ. Biol., 36(5): 1223–1227. |
| Siddik M A B, Chaklader M R, Hanif M A M. Islam A. Fotedar R. 2016c. Length-weight relationships of four fish species from a coastal artisanal fishery, southern Bangladesh. J. Appl. Ichthyol. , http://dx.doi.org/10.1111/jai.13181. |
| Siddik M A B, Chaklader M R, Hanif M A, Islam M A, Sharker M R, Rahman M, 2016a. Stock identification of critically endangered olive barb, Puntius sarana (Hamilton, 1822) with emphasis on management implications. J. Aquac.Res. Development, 7(2): 411. |
| Siddik M A B, Hanif M A, Chaklader M R, Islam M A, Nahar A, Fotedar R, 2016b. A multivariate morphometric investigation to delineate stock structure of gangetic whiting, Sillaginopsis panijus (Teleostei:Sillaginidae). SpringerPlus, 5: 520. Doi: 10.1186/s40064-016-2143-3 |
| Siddik M A B, Hanif M A, Chaklader M R, Nahar A, Mahmud S, 2015. Fishery biology of gangetic whiting Sillaginopsis panijus (Hamilton, 1822) endemic to Ganges delta, Bangladesh. Egypt. J. Aquat. Res., 41(4): 307–313. Doi: 10.1016/j.ejar.2015.11.001 |
| Siddik M A B, Nahar A, Ahamed F, Masood Z, Hossain M Y, 2013. Conservation of critically endangered Olive Barb Puntius sarana (Hamilton, 1822) through artificial propagation. Our Nature, 11(2): 96–104. |
| Simon K D, Mazlan A G, Samat A, Zaidi C C, Aziz A, 2010. Size, growth and age of two congeneric archer fishes(Toxotes jaculatrix pallas, 1767 and Toxotes chatareus Hamilton, 1822) inhabiting Malaysian coastal waters. Sains Malaysiana, 39(5): 697–704. |
| Sun X X, Xu D D, Lou B, Zhang T, Xin J, Guo Y S, Ma S L, 2013. Genetic diversity and population structure of Eleutheronema rhadinum in the East and South China Seas revealed in mitochondrial COI sequences. Chinese Journal of Oceanology and Limnology, 31(6): 1276–1283. Doi: 10.1007/s00343-013-3005-2 |
| Talwar P K, Jhingran A G. 1991. Inland Fishes of India and Adjacent Countries, Vol. 2. Oxford & IBH Publishing Co. Pvt. Ltd. , New Delhi-Calcutta. p. 612-613. |
| Tarkan A S, Gaygusuz Ö, Acipinar H, Gürsoy Ç, Özuluğ M, 2006. Length-weight relationship of fishes from the Marmara region (NW-Turkey). J. Appl. Ichthyol., 22(4): 271–273. Doi: 10.1111/jai.2006.22.issue-4 |
| Tesch F W. 1971. Age and growth. In: Ricker W E ed. Methods for Assessment of Fish Production in Fresh Waters. Blackwell Scientific Publications, Oxford. p. 99-130. |
 2017, Vol. 35
2017, Vol. 35


