Institute of Oceanology, Chinese Academy of Sciences
Article Information
- ZHAO Jiale(赵家乐), GAO Xiaojiang(高效江), YANG Jin(杨进)
- Influences of hydrological regime on heavy metal and salt ion concentrations in intertidal sediment from Chongming Dongtan, Changjiang River estuary, China
- Chinese Journal of Oceanology and Limnology, 35(6): 1329-1341
- http://dx.doi.org/10.1007/s00343-017-0191-3
Article History
- Received Jul. 18, 2016
- accepted in principle Sep. 26, 2016
- accepted for publication Oct. 20, 2016
In coastal areas, it has been a long history since tidal flats are converted into agricultural land because of their potential land resource (Healy and Hickey, 2002; Ellis and Atherton, 2003). China's rapid economic development and general shortage of arable land have encouraged the effective reclamation and cultivation of tidal flats, with a large area of presently cultivated farmland having been converted from former wetlands (Liu et al., 2005; Ma et al., 2015). During such reclamation, the salinity of sediment is usually reduced to meet the requirements of crop growth. For example, the salt ion concentrations in soils can be reduced by leaching with fresh water and irrigating them with water discharged from urban centers (Li et al., 2007; Norton-Brandão et al., 2013). A soil's moisture regime is thought to be one of the most important factors that control its physicochemical properties (e.g., pH and Eh), which indirectly affect its organic matter and CaCO3 content (Van Den Berg and Loch, 2000). Moreover, a soil's prevalent hydrological regime influences its redox potential, which is closely linked to the internal mobility of metals. Consequently, hydrological management often plays a crucial role in transforming tidal flats into farm land.
Pollution of alluvial sediments with heavy metals has been documented in many parts of the world (Du Laing et al., 2007; Li et al., 2007, 2016; Yap and Pang, 2011; Ong et al., 2013; Sheykhi and Moore, 2013), and such contaminants released in estuarine water can be subsequently transferred to coastal tidal flats (Williams et al., 1994). Certain tidal flats or alluvial plains along major river estuaries in China (e.g., the Changjiang (Yangtze) River and the Huanghe (Yellow) River) are severely contaminated with heavy metals (Li et al., 2007, 2014, 2015; Ma et al., 2015), which may pose a threat to soil quality if and when they are converted to farmland. The hydrological regime present during reclamation has a profound influence on the behavior of heavy metals in sediment. Some studies have shown that flooding minimizes the availability of Cu and Zn and enhances the immobilization effect of Cd (Speelmans et al., 2007; Zhu et al., 2012). Observations made under alternate moisture conditions, however, have shown that the lability of Pb in soils decreases with increasing incubation time, and that intermittent irrigation may be a promising means of reducing As input to paddy soils (Roberts et al., 2011; Khodaverdiloo et al., 2012). Soon (1994) and Zheng and Zhang (2011) demonstrated that heavy metals added to soil under field capacity conditions can be transferred over time from more labile fractions to less labile fractions. However, these studies have focused mainly on total metal concentrations and transformations of heavy metals in soils rather than on free metal contents in pore water. Ignoring the ion changes, including those of salt and metal ion concentrations in pore water, may lead to underestimation or overestimation of predicted bioavailability, which affects any evaluation of the ecological system. As such, in this work, we have directly investigated salt and metal ion concentrations in pore water during reclamation, using samples collected from Chongming Dongtan at the mouth of the Changjiang River estuary.
Chongming Island is the largest alluvial estuarine island of the Changjiang River estuary, and half of its present area has been obtained by reclamation of tidal flat land (Wu et al., 2005). With a long history of reclamation, Chongming Dongtan formed three zones of reclaimed land (the post-1998, 1990s, and 1960s zones) in addition to intertidal flats. Many studies have explored the heavy metals in the soils of Chongming and the sediments of Chongming Dongtan, such as studies of the spatial pattern of heavy metal accumulation on the intertidal flats (Zhang et al., 2001; Gorenc et al., 2004). Moreover, several other studies (e.g., Zhang et al., 2001; Cui et al., 2012) have studied the history of changes of heavy metals in the intertidal flats. However, those studies have not considered the importance of hydrological regime management in the process of reclamation of tidal flats in Chongming. Additionally, few studies have explored the concentrations of heavy metals and salt ions in pore water or the chemical speciation of metals under various moisture conditions of sediments, especially in the Changjiang estuary. This study, therefore, conducted a laboratory experiment that simulated the process of reclamation of Chongming Dongtan tidal flats with three different moisture regimes. This study aimed to assess the effects of the different hydrological regimes on the process of converting the tidal flats. The main purposes of the study were (1) to delineate significant differences of sediment properties, (2) to reveal the dynamic processes of heavy metal and salt ion concentrations in pore water of the sediment, and (3) to investigate the fractionation of heavy metals in the sediments under the different hydrological regimes.
2 MATERIAL AND METHOD 2.1 Collection and preparation of sedimentsSediments for a greenhouse experiment were collected in August 2014 from the flat intertidal zone (31.46°N, 121.94°E) of Chongming Dongtan, which is located at the mouth of the Changjiang River estuary. Eight subsamples of surface sediments were randomly collected from a zone approximately 20 m×20 m in size. The uppermost 20 cm of sediments from each subsample weighed approximately 6 kg. All samples were returned for laboratory analysis in large polyethylene bags and, upon arrival, were mixed thoroughly in order to produce a homogenized, composite sample. The sediments were then naturally dried at room temperature and then passed through a 4-mm mesh sieve. Cylindrical plastic containers with bottom diameters of 8 cm and heights of 19 cm were then each filled with sediments to a height of 10 cm. Before being decanted, the mixed sample was averaged into several portions, and 200 g of dried sediment was collected from each portion in order to check their homogeneities. The containers were each drilled with a drainage hole at their base that was 3 mm in diameter, and which were covered by geotextiles in order to minimize the loss of sediments from each vessel. All containers were then stored under one of three different hydrological regimes in a greenhouse, which was kept at about 20℃ and received 16 h of light per day.
2.2 Simulation experiment with different hydrological regimesThe above-described containers were each subjected one of the following three hydrological regimes for the duration of the experiments.
Regime 1 (R1): permanently flooded. The sediment in each container was flooded by placing each container in a larger box (50 cm×35 cm×25 cm) filled with deionized water. The water level was kept at 5 cm above the sediment surface during the flooded periods
Regime 2 (R2): alternating five-day-long periods of flooding and emergence. The sediments in each container were repeatedly inserted and removed from a larger box (50 cm×35 cm×25 cm) on a five-day cycle to simulate an alternating hydrological regime. Deionized water within each sediment's container was allowed to percolate for five days after each period of flooding, with fresh deionized water prepared for each new period of submergence. Each empty large box was prepared for a new five-day submergence period by cleaning it with deionized water.
Regime 3 (R3): Continuous field capacity. A field capacity condition was achieved by regularly adding deionized water (when necessary) until the water tended to percolate.
Each sediment container was fitted with two 10-cm Rhizon MOM pore water samplers (Eijkelkamp, Giesbeek, NL) to extract the sediment solution in vacuum tubes. The two Rhizon samplers were installed horizontally at a depth of 5 cm below the sediment surface.
Three parallel experiments were set up for each hydrological regime. All treatments were continuously monitored intensively for 100 days. Pore water samples were taken every five days and sediments were taken after completion of the whole experiment. In addition, the simulation experiment with different hydrological are limited to laboratory conditions.
2.3 AnalysesThe moisture content of the sediment was determined by sequentially comparing the weights before and after heating at 110℃ until a constant weight was obtained. The sediment pH value was measured in sediment slurries at a 1:2.5 (w/v) soil/ water ratio using a pH meter. Sediment Eh was measured during incubation using an OPR combination Pt-band electrode (ORP431; Shanghai Dapu Instrument, China). Soil organic matter (SOM) content was measured using the dichromate oxidation method (Walkley and Black, 1934). Sediment particle size was determined using a particle size analyzer (MS-2000, UK) (Murray, 2002). The carbonate content was determined by back-titration (with 0.5 mol/L NaOH) of an excess 0.25 mol/L H2SO4 added to 1 g of sediment (Nelson, 1982). Acid volatile sulfide (AVS) contents were determined on fresh sediment samples by transformation of sulfide into H2S and absorption in a Zn-acetate solution, followed by a back titration (Tack et al., 1997). Sediment salinity was measured by analyzing the total contents of dissolved base ions (Ca2+, Mg2+, Na+, K+, CO32-, HCO3-, Cl−, and SO42-) in carbon dioxide-free water suspension (1:5, w/v) (Page and Miller, 1991). The concentrations of metals in the sediment (including Fe, Mn, Cu, Cr, Ni, Zn, and Pb) were determined by firstly digesting samples in Teflon tubes using a triacid mixture (HNO3–HF–HClO4), and subsequently analyzing these solutions on an inductively coupled plasma optical emission spectrometer (HITACHI P-4010, Japan). The sediment samples before and after experiment were used to analyze the chemical speciation (acid extractable soluble, reducible, oxidizable and residual fractions) of heavy metals with BCR sequential extraction (Ure et al., 1993).
The amount of water used for each of the three different hydrological regimes (R1–R3) was identical. Quality assurance and quality control testing was conducted by using duplicate analyses and the standard reference sample GBW07309. Analytical precision was within 10% variability. The ranges of recoveries for the heavy metals Cr, Cu, Zn, Pb, and Ni were 96.57%–104.6%, 95.89%–104.5%, 97.25%– 103.6%, 97.25%–104.8%, and 96.10%–101.1%, respectively. Extracted pore water was analyzed for Ca2+, Mg2+, Na+, K+, CO32-, HCO3-, Cl−, and SO42- following the methods of Page and Miller (1991). Concentrations of the metals Cr, Cu, Zn, Ni, Pb, Fe, and Mn were determined using inductively coupled plasma mass spectrometry (XSERIES 2, USA).
2.4 StatisticsStatistical analyses were performed using the software of SPSS v. 19.0. Pearson's correlation coefficients were used to examine the degree to which relationships existed between heavy metal and salt ion concentrations in pore waters collected under each hydrological regime (R1–R3). The 100-day experiment duration was divided into four periods, each of which contained about 25 days. These aligned with the five-day periodicity employed in R2, which allowed differences in the concentrations of species in pore water to be compared between each of the three simulated hydrological regimes. No comparison was made of data collected in the first 25-day period for different regimes due to water-sediment transitional effects that occur. Mean concentrations of heavy metals and salt ions recorded for the remaining three periods (25–50, 51–75, and 76–100 days) were compared between themselves and between regimes. Significance differences between mean concentrations were analyzed using the DUNCAN multiple comparison method. The results of fractionation of heavy metals in sediments before and after incubation were analyzed by analysis of variance (ANOVA) and least-significant difference (LSD) tests.
3 RESULT 3.1 Sediment characteristicsThe metal contents and physicochemical properties of the sediments before and after the experiment are presented in Table 1. The low standard deviations of the control indicate that the sediment was sufficiently mixed prior to filling containers for each of the different hydrological regimes. The sediment used in the experiment consisted of 10% clay, 56% silt and 34% sand. The pH of the sediment before incubation was 8.22±0.02, whereas it decreased in experiments R1, R2, and R3 after incubation to 7.96, 7.84, and 8.10, respectively. There were no significant pH differences between the pre-and post-incubation control. In all three treatment periods, the concentrations of heavy metals Cr, Cu, Pb, Zn, Fe, and Mn in soils in R2 decreased significantly (P < 0.05) compared to their initial values. The reductions in R2 were 2.78% for Cr, 10.5% for Cu, 11.3% for Pb, 5.77% for Zn, 11.2% for Fe, and 3.78% for Mn; however, the total concentrations of each of these elements in R1 and R3 sediments did not change significantly over the same period. Notably, Ni concentration did not decrease under any of the treatments. The contents of CaCO3, SOM, and AVS in the sediment before incubation were 4.60%, 10.7 g/kg, and 0.83 mmol/kg, respectively, and none showed any significant changes pre-and post-incubation under any regime. Nonetheless, the salinity in sediments decreased sharply (P < 0.05) from an initial value of 0.33% to 0.08% in R1 and 0.03% in R2.

|
There was an obvious difference (P < 0.05) between the period of day 0–25 and the period from day 25 to the end of the experiment for R1, R2, and R3. In addition, after the first 25 days, a significant difference in redox potential (P < 0.05) also manifested between the three hydrological regimes at equivalent times, with the absolute values of the redox potential being R3 > R2 > R1 (Fig. 1).
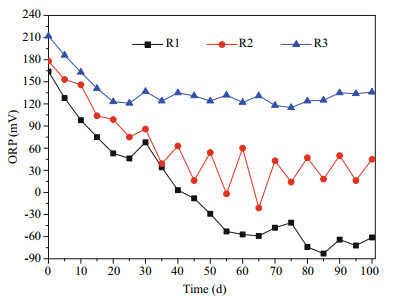
|
| Figure 1 Sediment redox potential (mV) of the three treatments |
For R1, the redox potential decreased at the beginning of the 25-day period; however, after being subjected to longer periods of flooding, it decreased continually to around -72 mV at the end of the experiment. The redox potential in treatment R2 initially decreased during days 0–25, but subsequently showed a periodic pattern ranging between -18 mV and 58 mV, which matched the regime's alternating flooding and emergence periods. For R3, the average value of the redox potential after the first 25 days was around 140 mV.
3.3 Heavy metal and salt ion concentrations in the pore waterSalt ion concentrations in the pore water of the sediments in each regime were recorded as R3 > R1 > R2 (Fig. 2). During days 0–25, the concentrations of salt ions in R2 showed a greater decrease than those in R1; however, these concentrations increased with time in R3 over the same period. During days 26–50, K+, Na+, Ca2+, Mg2+, Cl−, and SO42- concentrations decreased significantly in R1. The concentration of Ca2+ showed a slight increase during days 51–100, while the continuous flooding in R1 caused SO42- concentrations in the pore water to show a greater decrease than Cl− concentrations. No significant change in salt ion concentrations was observed in R2, with all noted to have remained consistently low during days 15–100. For R3, the K+, Ca2+, Na+, and Mg2+ cationic concentrations in pore water showed a decreasing trend after the first 25 days, while the anionic Cl− concentration fluctuated randomly.

|
| Figure 2 Ca2+, Mg2+, K+, Na+, Cl- and SO42- concentrations in the pore water |
The measured concentrations of Fe, Mn, Cr, Cu, Pb, and Zn in pore water are shown in Fig. 3. The temporal changes in the pore water concentrations of Mn and Fe were very similar. More reducing conditions were established in regimes with long periods of submersion, which caused the pore water concentrations of Fe and Mn to increase significantly; especially for R1, which was continuously flooded. In R2, which had a five-day flooding period, Fe and Mn concentrations in the pore water also increased while the sediment was submerged. However, the field capacity regime (R3) was characterized by a much higher redox potential, which resulted in significantly lower concentrations of Fe and Mn in pore water. In general, the concentrations of Cr in pore water increased to around 12 μg/Lafter 50 days, while those of Cu, Pb, and Zn decreased with time to less than 10 μg/L. After 25 days, the lowest Cu, Pb, and Zn concentrations in the pore water occurred in R2 (below 5 μg/L), though these cations showed a gradually decreasing concentration trend in R1.
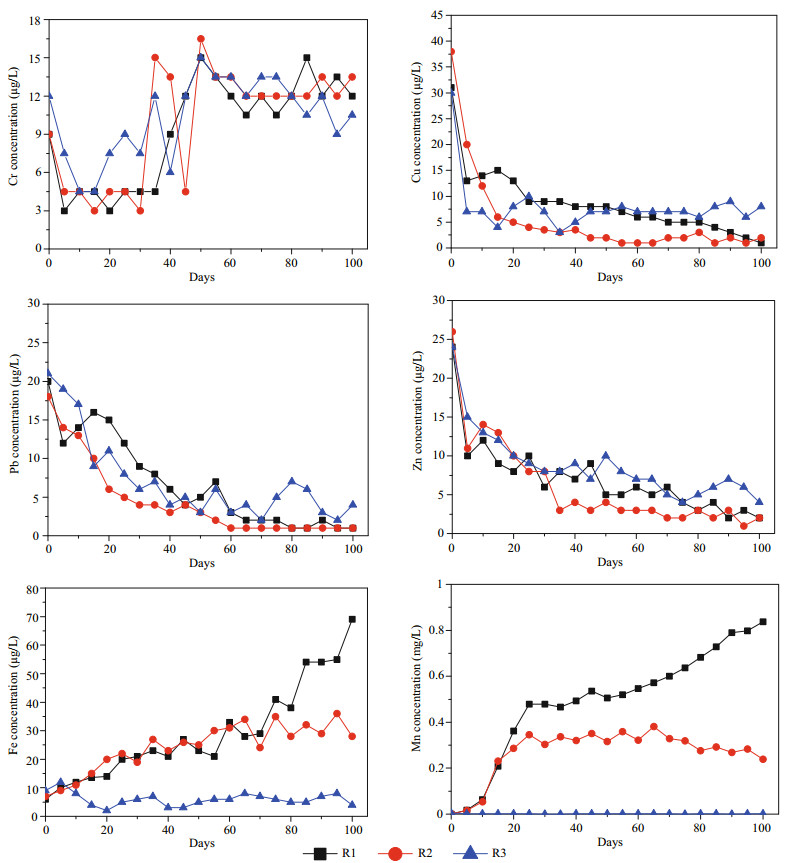
|
| Figure 3 Cr, Cu, Pb, Zn, Fe and Mn concentrations in the pore water |
The average heavy metal and salt ion concentrations over three periods (days 25–50, 51–75, and 76–100) are summarized in Table 2. Mean value accompanied by different letters (a–e) are significant different within columns for each element at the same correlation level (P < 0.05). Significant differences between the three hydrological conditions were also recorded. Salt ion concentrations in the pore water of the sediments showed significant variation under the different regimes (Fig. 2 and Table 2), demonstrating that they impart notably different controls on salt ions. In R1, salt ion concentrations in the pore water showed a clear difference between days 0–25 and days 26–100 (P < 0.05); however, no obvious changes in concentrations were identified between different periods in R2. In R3, large differences in the concentrations of Ca2+, K+, Mg2+, Na+, and SO42- in the pore water were observed between days 26–75 and days 76–100, but there were no clear changes in Cl− concentration over this period. For heavy metals, significant differences between the three regimes were observed for both Mn and Fe (Table 2). For Cu, Pb, and Zn, R1 and R2 showed no notable differences of concentration in the pore water after 50 days (P < 0.05). Additionally, there were almost no differences between three regimes and periods for Cr pore water concentrations.

|
The measured distributions of heavy metals (Ni, Cr, Cu, Zn, and Pb) in untreated sediment before incubation are presented in Table 3. Measured Ni, Cr, Zn, and Pb concentrations were ordered residual > oxidizable > reducible > acid extractable fraction, whereas the concentration of Cu in each fraction was generally ordered residual > reducible > oxidizable > acid extractable fraction. Heavy metals were mostly present in the residual fraction, which accounted for about 44%–84% of the total. The oxidizable fractions of Ni, Cr, Zn, and Pb represented the second largest proportion, about 13%–32%, in the sediments. The proportion of the reducible fractions for all heavy metals varied from 2.5% to 22%, and the proportion of the acid extractable fraction varied from 0.28% to 5.4%, making it the least abundant of all heavy metals.
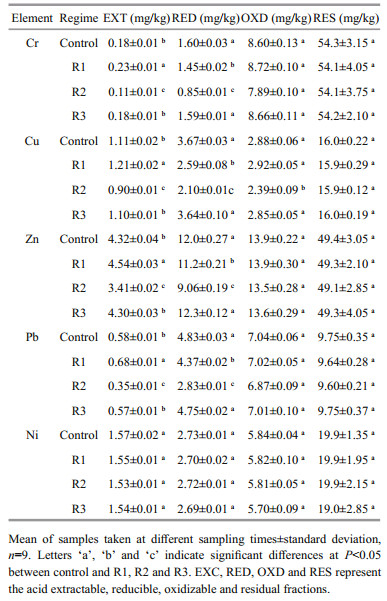
|
Compared to the sediments before incubation, the chemical fractions of Cr, Cu, Zn, and Pb showed significant variation under R1 and R2, but no large changes were observed under R3 (Table 3). Additionally, neither the fraction distributions of Ni nor the concentrations of the residual fraction of all metals changed significantly among the regimes. The reducible fractions of four metals (Cr, Cu, Zn, and Pb) under permanent flooding (R1) were significantly lower (P < 0.05), whereas their acid extractable fractions were significantly higher (P < 0.05). However, the acid extractable fractions and reducible fractions of Cr, Cu, Zn, and Pb, together with the oxidizable fraction of Cu, decreased significantly (P < 0.05) under R2.
4 DISCUSSION 4.1 Relationships among heavy metal and salt ion concentrations in the pore water of the sedimentsThe relationships between heavy metal and salt ion concentrations in the pore water were evaluated by correlation analysis (Table 4). There were no significant correlations at the level of R < 0.01 in R3, although Cu, Pb, and Zn showed positive correlations with salt ions (R < 0.01) in the R1 and R2 treatments. High salinity in the intertidal flat may promote heavy metal mobility through complexation with salt anions, and ion exchange between the cations and metal ions (Du Laing et al., 2008). In addition, Cu probably forms stronger complexes with organic ligands in sediment solution. The distribution of Cu between ionic and complexed forms in water is complex, although Cu-Cl complexes should generally decrease in abundance as salinity decreases (Verslycke et al., 2003). The sodium cation might also have an influence on metal extraction through ion exchange in high ionic strength solutions (Nedwed and Clifford, 2000). In addition, when Ca2+ and Mg2+ are abundant in the sediment solution, metals could be mobilized from particles because of competition between these divalent cations and the metal ions (Paalman et al., 1994).
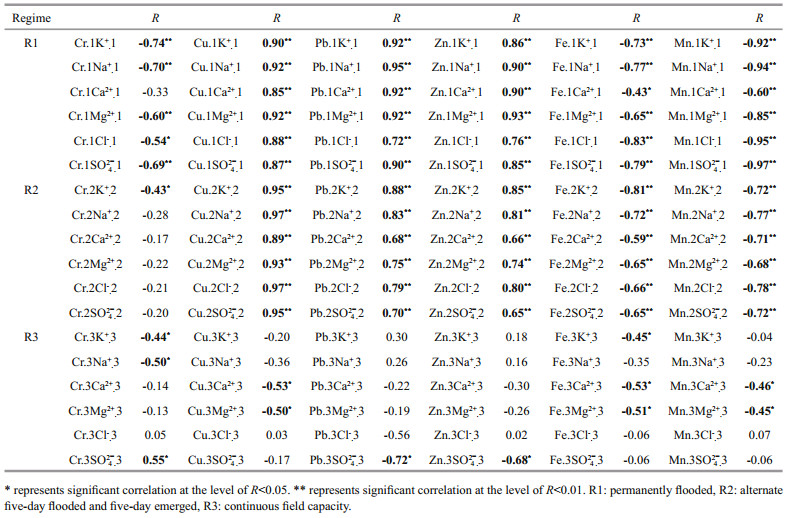
|
Both Fe and Mn showed negative correlation with salt ions under R1 and R2 conditions. An increased dissolution of Fe and Mn oxides was recorded under the reductive redox potential of treatments R1 and R2, which led to a relative increase of the "free" ion concentrations in the pore water (Balint et al., 2013). However, salt ions were not affected by the redox potential. Moreover, flooding and leaching by fresh water is known to reduce the salt ion concentrations in pore water in the tidal flat sediments (Li et al., 2007; Du Laing et al., 2008).
4.2 Effect of redox potential on pore water concentrations of Fe, Mn, Cr, Cu, Pb and ZnThe concentrations of the heavy metals in pore water can be regarded as one of the most important parameters to characterize metal mobility. The concentrations of Fe and Mn under three different hydrological regimes showed a variety of dynamic and/or periodic trends. During a flooding period, the increases in the pore water concentrations of Fe and Mn were ascribed to reduction of Fe and Mn oxides by microorganism (Wahid and Kamalam, 1993; Gambrell, 1994; Balint et al., 2013). Indeed, there was a particularly remarkable reduction of Fe and Mn oxides under R1 and R2 conditions (Fig. 3). According to Okbah et al. (2008), Fe oxides are reduced at a lower redox potential than Mn oxides, which was illustrated that Mn appearing to a faster response than Fe especially in treatment R1 and R2. Both Fe and Mn showed a periodic trend of increasing and decreasing concentrations in pore water that corresponded with alternating five-day cycles in R2. Therefore, it can be concluded that the responses of Fe and Mn concentrations in pore water are mainly determined by the durations of emergences and flooding periods.
Chromium showed no significant relationship between experiment duration and different hydrological conditions, nor was there a significant temporal effect (Fig. 3 and Table 2). This stability may mainly be a consequence of the complicated and flexible valences that the Cr ion can exhibit. According to Masscheleyn et al. (1992), Cr chemistry and solubility under more reduced soil redox ( < +100 mV) levels is controlled by the chemical reduction of Cr(Ⅵ) by soluble ferrous iron. Moreover, Stefánsson et al. (2015) found that Cr(Ⅵ) becomes an increasingly dominant form of dissolved Cr in water with pH values above 7–8. However, Masscheleyn also reported that Cr(Ⅲ) is readily oxidized to Cr(Ⅵ) in soil and water due to the catalyzing effect of Fe and/ or Mn oxides. Soluble complexes may also form with organic components in the presence of Cr(Ⅲ) in solution, and this Cr(Ⅲ) can easily precipitate as Cr(OH)3. In turn, sediments could also absorb these soluble Cr(Ⅲ)-DOC complexes, which would lead to the removal of Cr(Ⅲ) from solution. Under both oxidized and reduced conditions, the mobilization of Cr could be explained by these countering reactions (Guo et al., 1997), and so the relatively minor variation in Cr concentration identified herein may be due to these factors operating under each of the different hydrological regimes.
Kashem and Singh (2001) reported that sulfate reduction could initiate around -100 mV, especially in solutions where Fe and Mn oxide reduction has already taken place. Although the AVS increased indistinctively under the R1 (-70 mV) in this study, the behavior of the elements (especially trace metals) under the reducing conditions should presumably be affected by sulfides. In particular, Cu, Pb, and Zn are expected to occur as CuS, PbS, ZnS in estuarine anoxic sediments, as sulfate is readily available in these environments (Du Laing et al., 2009). Consequently, the concentrations of Cu, Pb and Zn in the pore water were very low, and steadily decreased under R1 and R2 conditions.
4.3 Fractionation of heavy metals in sediments under the different hydrological regimesDuring flooding (e.g., R1), biological and microbiological activities—combined with limited oxygen diffusion—cause oxygen depletion and thus establish reducing conditions (Lagomarsino et al., 2016). The reducible fraction theoretically represents the contents of each metal bound to Fe and/or Mn oxides, and which would be released if the sediment were subjected to more reducing conditions (Panda et al., 1995; Ma and Dong, 2004). Therefore, a marked transformation of Fe-Mn oxides to extractable fractions occurred when the redox potential decreased to around -115 mV. The concentrations of Cr, Cu, Pb, and Zn in the acid extractable fraction thus increased (P < 0.05), and the reducible fractions of the four metals in the flooded soil decreased (P < 0.05) (Table 3).
During R2, the redox potential of the sediment became reduced during the flooding stage, which led to a proportion of the trace metals bound to Fe/Mn oxides being released into the pore water. Moreover, during leaching process with deionized water, the decreased Ca2+ concentration in the soil solution directly lead that the portion of the carbonate in saline soil would be dissolved (Li et al., 2011). This process might have led to the decreased contents of acid extractable Cr, Cu, Pb, and Zn. Cu bound to the oxidizable fractions was the most significant (P < 0.05) among all of the heavy metals. This is in agreement with the results of other studies (Chartier et al., 2001; Du Laing et al., 2009), which predicted that Cu concentration is strongly related to organic matter. Although no significant changes (P < 0.05) in SOM were observed after the experiment, copper can complex with dissolved organic matter (DOM) (Li et al., 2011). The complexing capacity of Cu is weaker with larger dissolved organic molecules than with small organic molecules, based on the latter having notably more binding sites (Jiang et al., 2001). Moreover, the greater abundance of acidic ligands in small organic molecules may have an influence on copper's binding capacity (Soler-Rovira et al., 2010). Therefore, some dissolved organic matter (mainly small organic molecules) were likely leached out during R2 and probably resulted in the decreased concentration of the oxidizable fraction of Cu.
In General, the acid extractable fraction is considered to be readily mobile and easily bioavailable, whereas the residual fraction is considered to involve a crystalline lattice and be more inactive. Under the alternating hydrological regime (R2), the proportions of the acid extractable fraction for heavy metals (Cr, Cu, Zn, and Pb) decreased significantly (P < 0.05) and those of the residual fraction increased sigificantly (P < 0.05) compared to the proportions of fractionation of the heavy metals in the sediment before incubation (Table 3). Therefore, it seems that the hydrological regimes affected the heavy metal fractionation and that the bioavailability of heavy metals in sediments was reduced under the alternating regime.
5 CONCLUSIONThe hydrological regime in a tidal flat sedimentation zone has a pronounced influence on the heavy metal and salt ion concentrations in the uppermost sediment layer. In terms of heavy metal concentrations in the sediments, Ni did not decrease under any of the R1– R3 treatments. Similarly, the total concentrations of Cr, Cu, Pb, Zn, Fe, and Mn in sediments did not change significantly under either flooded (R1) or field capacity (R3) conditions. However, the concentrations of Cr, Cu, Pb, Zn, Fe, and Mn in soils decreased significantly under an alternating moisture regime (R2) when compared to the initial heavy metal values, which is attributed to the effects of leaching.
Salt ions and heavy metal concentrations in the pore water were also analyzed. Under flooding regime, Fe, Mn, and Cr concentrations in the pore water increased, and Cu, Pb, and Zn concentrations decreased. Salt ion concentrations decreased during the initial 50 days of the experiment, after which Ca2+ increased slightly, while SO42- decreased significantly. The alternating hydrological condition (R2) caused the concentration of Fe, Mn, and Cr in the pore water to fluctuate and increase, wherase Cu, Pb and Zn decreased over the same periods. Salt ion concentrations under R2 conditions decreased sharply during the first 25 days and remained at a consistently low level until the end of the experiment. The regime of keeping the soil at field capacity (R3) produced low concentrations of Fe, Mn, Cu, Pb, Zn, and slightly increased concentrations of Cr in pore water. Salt cation (K+, Ca2+, Na+, and Mg2+) concentrations increased at the beginning of R3, and then decreased until the end of the treatment, while Cl- and SO42- concentrations fluctuated.
The fractionation of heavy metals was also investigated. The fractionation of Ni and the concentration of all metals bound to the residual fraction did not show any significant changes among the regimes. Chemical fractionations of Cr, Cu, Zn, and Pb were redistributed under R1 and R2 conditions, whereas the reducible fractions of these four metals decreased under R1, and their acid extractable fractions increased significantly. However, the amounts of the acid extractable and the reducible fractions of Cr, Cu, Zn, and Pb, together with the oxidizable fraction of Cu, decreased significantly under R2. Our findings suggest that an alternating hydrological regime can reduce the availability of heavy metals in the sediment or soil whilst also reducing sediment salinity.
| Balint R, Orbeci C, Nechifor G, Plesca M, Ajmone-Marsan F, 2013. Effect of redox conditions on the Crystallinity of Fe oxides in soil. Revista de Chimie, 64(11): 1218–1223. |
| Chartier M, Mercier G, Blais J F, 2001. Partitioning of trace metals before and after biological removal of metals from sediments. Water Research, 35(6): 1435–1444. Doi: 10.1016/s0043-1354(00)00404-8 |
| Cui J, Liu C, Li Z L, Wang L, Chen X F, Ye Z Z, Fang C M, 2012. Long-term changes in topsoil chemical properties under centuries of cultivation after reclamation of coastal wetlands in the Yangtze Estuary, China. Soil and Tillage Research, 123: 50–60. Doi: 10.1016/j.still.2012.03.009 |
| Du Laing G, De Vos R, Vandecasteele B, Lesage E, Tack F M G, Verloo M G, 2008. Effect of salinity on heavy metal mobility and availability in intertidal sediments of the Scheldt estuary. Estuarine, Coastal and Shelf Science, 77(4): 589–602. Doi: 10.1016/j.ecss.2007.10.017 |
| Du Laing G, Rinklebe J, Vandecasteele B, Meers E, Tack F M G, 2009. Trace metal behaviour in estuarine and riverine floodplain soils and sediments:a review. Science of the Total Environment, 407(13): 3972–3985. Doi: 10.1016/j.scitotenv.2008.07.025 |
| Du Laing G, Vanthuyne D R J, Vandecasteele B, Tack F M G, Verloo M G, 2007. Influence of hydrological regime on pore water metal concentrations in a contaminated sediment-derived soil. Environmental Pollution, 147(3): 615–625. Doi: 10.1016/j.envpol.2006.10.004 |
| Ellis S, Atherton J K, 2003. Properties and development of soils on reclaimed alluvial sediments of the Humber estuary, eastern England. Catena, 52(2): 129–147. Doi: 10.1016/s0341-8162(02)00179-0 |
| Gambrell R P, 1994. Trace and toxic metals in wetlands-a review. Journal of Environmental Quality, 23(5): 883–891. |
| Gorenc S, Kostaschuk R, Chen Z, 2004. Spatial variations in heavy metals on tidal flats in the Yangtze Estuary, China. Environmental Geology, 45(8): 1101–1108. Doi: 10.1007/s00254-004-0968-5 |
| Guo T Z, DeLaune R D, Patrick Jr W H, 1997. The influence of sediment redox chemistry on chemically active forms of arsenic, cadmium, chromium, and zinc in estuarine sediment. Environment International, 23(3): 305–316. Doi: 10.1016/S0160-4120(97)00033-0 |
| Healy M, Hickey K R, 2002. Historic land reclamation in the intertidal wetlands of the Shannon estuary, western Ireland. Journal of Coastal Research(S36): 365–373. |
| Jiang J, Wang G, Fang L, 2001. Complexation between soil water-soluble organic matter and heavy metal. Soil and Environmental Sciences, 10(1): 67–71. |
| Kashem M A, Singh B R, 2001. Metal availability in contaminated soils:Ⅰ. Effects of flooding and organic matter on changes in Eh, pH and solubility of Cd, Ni andZn. Nutrient Cycling in Agroecosystems, 61(3): 247–255. Doi: 10.1023/a:1013762204510 |
| Khodaverdiloo H, Rahmanian M, Rezapour S, Dashtaki S G, Hadi H, Han F X, 2012. Effect of wetting-drying cycles on redistribution of lead in some semi-arid zone soils spiked with a lead salt. Pedosphere, 22(3): 304–313. Doi: 10.1016/S1002-0160(12)60017-4 |
| Lagomarsino A, Agnelli A E, Linquist B, Adviento-Borbe M A, Agnelli A, Gavina G, Ravaglia S, Ferrara R M, 2016. Alternate wetting and drying of rice reduced CH4 Emissions but triggered N2O peaks in a clayey soil of central Italy. Pedosphere, 26(4): 533–548. Doi: 10.1016/S1002-0160(15)60063-7 |
| Li P Y, Qian H, Howard K W F, Wu J H, Lyu X, 2014. Anthropogenic pollution and variability of manganese in alluvial sediments of the Yellow River, Ningxia, northwest China. Environmental Monitoring and Assessment, 186(3): 1385–1398. Doi: 10.1007/s10661-013-3461-3 |
| Li P Y, Qian H, Howard K W F, Wu J H, 2015. Heavy metal contamination of Yellow River alluvial sediments, northwest China. Environmental Earth Sciences, 73(7): 3403–3415. Doi: 10.1007/s12665-014-3628-4 |
| Li P Y, Wu J H, Qian H, Zhou W F, 2016. Distribution, enrichment and sources of trace metals in the topsoil in the vicinity of a steel wire plant along the Silk Road economic belt, northwest China. Environmental Earth Sciences, 75(10): 909. Doi: 10.1007/s12665-016-5719-x |
| Li Q S, Liu Y N, Du Y F, Cui Z H, Shi L, Wang L L, Li H J, 2011. The behavior of heavy metals in tidal flat sediments during fresh water leaching. Chemosphere, 82(6): 834–838. Doi: 10.1016/j.chemosphere.2010.11.026 |
| Li Q S, Wu Z F, Chu B, Zhang N, Cai S S, Fang J H, 2007. Heavy metals in coastal wetland sediments of the Pearl River Estuary, China. Environmental Pollution, 149(2): 158–164. Doi: 10.1016/j.envpol.2007.01.006 |
| Liu J Y, Liu M L, Tian H Q, Zhuang D F, Zhang Z X, Zhang W, Tang X M, Deng X Z, 2005. Spatial and temporal patterns of China's cropland during 1990-2000:an analysis based on Landsat TM data. Remote Sensing of Environment, 98(4): 442–456. Doi: 10.1016/j.rse.2005.08.012 |
| Ma C, Zheng R, Zhao J L, Han X M, Wang L, Gao X J, Zhang C S, 2015. Relationships between heavy metal concentrations in soils and reclamation history in the reclaimed coastal area of Chongming Dongtan of the Yangtze River Estuary, China. Journal of Soils and Sediments, 15(1): 139–152. Doi: 10.1007/s11368-014-0976-3 |
| Ma L Q, Dong Y, 2004. Effects of incubation on solubility and mobility of trace metals in two contaminated soils. Environmental Pollution, 130(3): 301–307. Doi: 10.1016/j.envpol.2004.01.007 |
| Masscheleyn P H, Pardue J H, DeLaune R D, Patrick Jr W H, 1992. Chromium redox Chemistry in a lower Mississippi Valley bottomland hardwood wetland. Environmental Science & Technology, 26(6): 1217–1226. Doi: 10.1021/es50002a611 |
| Murray M R, 2002. Is laser particle size determination possible for carbonate-rich lake sediments. Journal of Paleolimnology, 27(2): 173–183. Doi: 10.1023/a:1014281412035 |
| Nedwed T, Clifford D A, 2000. Feasibility of extracting lead from lead battery recycling site soil using highconcentration chloride solutions. Environmental Progress, 19(3): 197–206. Doi: 10.1002/ep.670190312 |
| Nelson R E. 1982. Carbonate and gypsum. In: Page A L eds. Methods of Soil Analysis, Part 2. 2nd edn. ASA, Madison, WI, USA. p. 181-197. |
| Norton-Brandão D, Scherrenberg S M, van Lier J B, 2013. Reclamation of used urban waters for irrigation purposes-A review of treatment technologies. Journal of Environmental Management, 122: 85–98. Doi: 10.1016/j.jenvman.2013.03.012 |
| Okbah M A, Nasr S M, Kasem S M, 2008. Heavy metals availability (Fe, Mn, Zn, Cu and Cr) in Aden Gulf sediments under aerobic and anaerobic conditions. Chemistry and Ecology, 24(2): 109–117. Doi: 10.1080/02757540801919305 |
| Ong G H, Yap C K, Maziah M, Suhaimi H, Tan S G, 2013. An investigation of arsenic contamination in Peninsular Malaysia based on Centella asiatica and soil samples. Environmental Monitoring and Assessment, 185(4): 3243–3254. Doi: 10.1007/s10661-012-2787-6 |
| Paalman M A A, Van Der Weijden C H, Loch J P G, 1994. Sorption of cadmium on suspended matter under estuarine conditions; competition and complexation with major sea-water ions. Water, Air, and Soil Pollution, 73(1): 49–60. Doi: 10.1007/bf00477975 |
| Page A L, Miller R H. 1991. The Method of Soil Analysis. Min J K, Hao X R, Yan H J, Xie C T, Trans. China Agriculture and Technology Press, Beijing, China. p. 93-112. (in Chinese) |
| Panda D, Subramanian V, Panigrahy R C, 1995. Geochemical fractionation of heavy metals in Chilka lake (east coast of India)-a tropical coastal lagoon. Environmental Geology, 26(4): 199–210. Doi: 10.1007/BF00770470 |
| Roberts L C, Hug S J, Voegelin A, Dittmar J, Kretzschmar R, Wehrli B, Saha G C, Badruzzaman A B M, Ali M A, 2011. Arsenic dynamics in porewater of an intermittently irrigated paddy field in bangladesh. Environmental Science & Technology, 45(3): 971–976. Doi: 10.1021/es102882q |
| Sheykhi V, Moore F, 2013. Evaluation of potentially toxic metals pollution in the sediments of the Kor river, southwest Iran. Environmental Monitoring and Assessment, 185(4): 3219–3232. Doi: 10.1007/s10661-012-2785-8 |
| Soler-Rovira P, Madejón E, Madejón P, Plaza C, 2010. In situ remediation of metal-contaminated soils with organic amendments:role of humic acids in copper bioavailability. Chemosphere, 79(8): 844–849. Doi: 10.1016/j.chemosphere.2010.02.054 |
| Soon Y K, 1994. Effect of long term cropping on availability of Cu, Mn and Zn in soil following clearing of a boreal forest. Plant and Soil, 160(1): 157–160. Doi: 10.1007/bf00150358 |
| Speelmans M, Vanthuyne D R J, Lock K, Hendrickx F, Du L G, Tack F M G, Janssen C R, 2007. Influence of flooding, salinity and inundation time on the bioavailability of metals in wetlands. Science of the Total Environment, 380(1-3): 144–153. Doi: 10.1016/j.scitotenv.2006.07.041 |
| Stefánsson A, Gunnarsson I, Kaasalainen H, Arnórsson S, 2015. Chromium geochemistry and speciation in natural waters, Iceland. Applied Geochemistry, 62: 200–206. Doi: 10.1016/j.apgeochem.2014.07.007 |
| Tack F M, Lapauw F, Verloo M G, 1997. Determination and fractionation of sulphur in a contaminated dredged sediment. Talanta, 44(12): 2185–2192. Doi: 10.1016/S0039-9140(97)00035-0 |
| Ure A M, Quevauviller P, Muntau H, Griepink B, 1993. Speciation of heavy metals in soils and sediments.An account of the improvement and harmonization of extraction techniques undertaken under the auspices of the BCR of the commission of the European communities. International Journal of Environmental Analytical Chemistry, 51(1-4): 135–151. Doi: 10.1080/03067319308027619 |
| Van Den Berg G A, Loch J P G, 2000. Decalcification of soils subject to periodic waterlogging. European Journal of Soil Science, 51(1): 27–33. Doi: 10.1046/j.1365-2389.2000.00279.x |
| Verslycke T, Vangheluwe M, Heijerick D, De Schamphelaere K, Van Sprang P, Janssen C R, 2003. The toxicity of metal mixtures to the estuarine mysid Neomysis integer(Crustacea:Mysidacea) under changing salinity. Aquatic Toxicology, 64(3): 307–315. Doi: 10.1016/s0166-445x(03)00061-4 |
| Wahid P A, Kamalam N V, 1993. Reductive dissolution of crystalline and amorphous Fe(Ⅲ) oxides by microorganisms in submerged soil. Biology and Fertility of Soils, 15(2): 144–148. Doi: 10.1007/bf00336433 |
| Walkley A, Black I A, 1934. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Science, 37(1): 29–38. Doi: 10.1097/00010694-193401000-00003 |
| Williams T P, Bubb J M, Lester J N, 1994. Metal accumulation within salt marsh environments:a review. Marine Pollution Bulletin, 28(5): 277–290. Doi: 10.1016/0025-326x(94)90152-x |
| Wu J H, Fu C Z, Lu F, Chen J K, 2005. Changes in free-living nematode community structure in relation to progressive land reclamation at an intertidal marsh. Applied Soil Ecology, 29(1): 47–58. Doi: 10.1016/j.apsoil.2004.09.003 |
| Yap C K, Pang B H, 2011. Assessment of Cu, Pb and Zn contamination in sediment of north western Peninsular Malaysia by using sediment quality values and different geochemical indices. Environmental Monitoring and Assessment, 183(1-4): 23–39. Doi: 10.1007/s10661-011-1903-3 |
| Zhang W, Yu L, Hutchinson S M, Xu S, Chen Z, Gao X, 2001. China's Yangtze Estuary:ⅠGeomorphic influence on heavy metal accumulation in intertidal sediments. Geomorphology, 41(2-3): 195–205. Doi: 10.1016/S0169-555X(01)00116-7 |
| Zheng S N, Zhang M K, 2011. Effect of moisture regime on the redistribution of heavy metals in paddy soil. Journal of Environmental Sciences, 23(3): 434–443. Doi: 10.1016/s1001-0742(10)60428-7 |
| Zhu Q H, Huang D Y, Liu S L, Zhou B, Luo Z C, Zhu H H, 2012. Flooding-enhanced immobilization effect of sepiolite on cadmium in paddy soil. Journal of Soils and Sediments, 12(2): 169–177. Doi: 10.1007/s11368-011-0444-2 |
 2017, Vol. 35
2017, Vol. 35


