Institute of Oceanology, Chinese Academy of Sciences
Article Information
- ABATE Rediat(ABATERediat), GAO Yahui(高亚辉), CHEN Changping(陈长平), LIANG Junrong(梁君荣), MU Wenhua(穆文华), KIFILE Demeke(KIFILEDemeke), CHEN Yanghang(陈杨航)
- Decadal variations in diatoms and dinoflagellates on the inner shelf of the East China Sea, China
- Chinese Journal of Oceanology and Limnology, 35(6): 1374-1386
- http://dx.doi.org/10.1007/s00343-017-6029-1
Article History
- Received Jan. 29, 2016
- accepted in principle Sep. 7, 2016
- accepted for publication Nov. 3, 2016
2 Key Laboratory of the Ministry of Education for Coastal and Wetland Ecosystem, College of the Environment & Ecology, Xiamen University, Xiamen 361102, China;
3 College of Natural Science, Arba Minch University, Arba Minch PO Box 21, Ethiopia;
4 Department of Zoological Sciences, Addis Ababa University, Addis Ababa, PO Box 1176, Ethiopia
Diatoms and dinoflagellates dominate phytoplankton in coastal and upwelling areas of the ocean, and play a variety of roles in ecosystems (Klais et al., 2011; Hinder et al., 2012). These two phytoplankton groups exhibit unique, distinct, and often contrasting adaptive ecologies, enabling them to maintain their niche partitioning worldwide despite the turbulence-nutrient matrix of habitats and onshore-offshore gradients (Smayda, 2002). In marine systems, the water column thermo-physical dynamics are intrinsically associated with the production and distribution of pelagic and benthic populations, which in turn are strongly coupled with the distribution of algal remains in marine sediments (Jones and Birks, 2004). Consequently, the relationship between the algal remains and the water column physico-chemical condition has provided a strong basis for using algal remains as biological indicators of changes in algal production and environmental conditions. Thus, sediment-preserved remains, such as phytoplankton pigment, and skeletal structures, such as diatom frustules, provide a useful basis for biogeochemical studies, and can be used to gain an improved understanding of long-term plankton abundance and compositional changes (Battarbee et al., 2002; Finkelstein and Gajewski, 2007; Schüller and Savage, 2011).
The East China Sea (ECS) is a marginal sea; it is considered one of the most dynamic sea systems in the world because of the range of eutrophic conditions and environmental changes it experiences (Huh and Su, 1999; Su and Huh, 2002; Zhou et al., 2008). In recent years, changes have been reported in biological production and phytoplankton species composition in several coastal water systems and seas in response to long-term changes in climate (Sugimoto and Tadokoro, 1997; Chiba and Saino, 2003; Leterme et al. 2005; Zhang and Gong, 2005; Kuwae et al., 2006; Beaugrand, 2009; Montes-Hugo et al., 2009; Klais et al., 2011; Hinder et al. 2012). Similarly, Zhou et al. (2008) reported a decreasing trend in the contribution of diatoms to the entire phytoplankton community in the Changjiang (Yangtze) River estuary (CRE) and adjacent coastal ECS since the 1960s. The area that entails the Changjiang River Basin and the coastal region of the ECS is well-known for its intensive industrial and agricultural activities and numerous densely populated urban areas. These activities have contributed to ongoing increases in fertilizer use and disposal of waste materials that ultimately find their way into the CRE and the ECS. Consequently, increasing trends in dissolved nitrogen, phosphorus, and chlorophyll-a (Chl-a) (Chai et al., 2006) concentrations; increased frequency of red tides (Zhou et al., 2008); decreases in dissolved silica; and changes in the composition of bloom formingphytoplankton (Li et al., 2007) have been recorded in the estuary and adjacent coastal areas of the ECS over the past five decades.
Just as nutrients loads have changed in response to increases in artificial fertilizer use, sediment loads are subject to substantial changes because of dam construction (Song et al., 2008; Wang et al., 2011). However, there is a lack of scientifically robust information about the responses of diatoms and dinoflagellates to changes in nutrients, sediment, and sediment-related conditions in the coastal zone of the ECS, from either long-term sampling or sediment cores. The aim of this study therefore was to bridge this knowledge gap by 1) analysing the long-term variation in total phytoplankton production, 2) analysing the long-term variations in diatom and dinoflagellate production using phytoplankton-group specific marker pigments, and 3) comparing diatom production using the diatom valve count and pigment signature as proxies. To achieve these aims, a sediment core sample was collected from the coastal zone of the ECS and long-term changes in diatom and dinoflagellate production were investigated and compared.
2 MATERIAL AND METHOD 2.1 Study areaThe East China Sea is the largest continental marginal sea in the Western North Pacific (Huh and Su, 1999; Chiang et al., 2004) and is one of the most developed shelf areas in the world. Primary production on its wide shallow continental shelf is supported by frequent replenishment of nutrients to the euphotic zone from a deeper layer (Furuya et al., 2003). Vast quantities of terrigenous and anthropogenic materials flow into the ECS from different river basins and, as a result, it supports high biological production (Furuya et al., 2003). Our study site was located at 28°26.159 1′N and 122°11.074 5′E, close to the CRE, in the inner shelf area of the ECS. The sampling area was characterized by fine-grained sediments, dispersed from the Changjiang's plume (McKee et al., 1983). Cheng et al. (2014) reported that centric diatoms and one species of silicoflagellate (Dictyocha fibula) were the dominant species in the area from the CRE to the ECS.
Sedimentation rates in the ECS vary considerably and have been estimated to be between ~0.02 and 2 cm/a, with sedimentation decreasing towards the south along the inner shelf and towards the east in an offshore direction (Huh and Su, 1999). The ECS borders mainland China, the Kuroshio Current (KC), Taiwan and the Taiwan Strait, and the Yellow Sea to the west, east, south, and north, respectively (Huh and Su, 1999) (Fig. 1). During the winter season, the majority of the runoff from the Changjiang River flows southeastward along the coast of Zhejiang Province. During the summer season, the increased runoff from the Changjiang River turns eastward or northeastward towards Cheju Island, Korea (Zhou et al., 2008). The ECS is a dynamic system that results from the interactions of different currents. The warm and oligotrophic KC and the Taiwan Warm Current (TWC), both with high temperatures and salinities (Huh and Su, 1999), dominate the circulation and flow northward along the edge of the ECS shelf. The Changjiang Cold Water (CJCW) has low salinity and temperatures and flows southward along the bottom of the coast of mainland China (Milliman et al., 1989). Because of these major currents, the ECS experiences high tidal currents, extremely high sediment loads, frequent and intense storm events, and consequent resuspension of sediments. These physical features and processes ensure that the ECS is one of the most dynamic marginal seas in the world (Huh and Su, 1999).
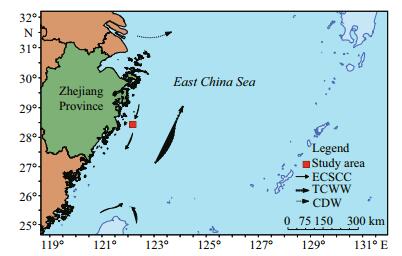
|
| Figure 1 Location of the study area showing the sampling site (red), and regional water circulation and current regimes, including the Changjiang River Diluted Water (CDW), the East China Sea Coastal Current (ECSCC), and the Taiwan Current Warm Water (TCWW) |
A sediment core sample, 47-cm long, was collected at a water depth of 43 m from 28°26.159 1′N and 122°11.074 5′E. After collection, the sediment core was sliced into 2-cm intervals and approximately 0.5 cm of the outer rim of each sediment slice was trimmed off to minimize contamination between layers. Sediment samples were then placed in a freezer at -20℃ until laboratory analysis. Sediments were dated with the α-spectrometry 210Pb (via 210Po) method (Huh and Su, 1999; Su and Huh, 2002), and 210Pb dates were calculated using the method outlined by Appleby and Oldfield (1978). Semi-log plots were used to derive 210Pb-based deposition rates. From the slope (m) of the exponential downcore decrease in 210Pb (decay), and assuming a constant initial radionuclide concentration (despite variable sedimentation rates), sediment accumulation rates (SPb-210) were calculated using SPb-210=-λ/m, where λ was the decay constant of 210Pb.
2.3 Pigment and diatom valve analysesSediment samples were processed in a dark room. Homogeneously mixed freeze-dried sub-samples (about 5 g) were transferred into 50-mL centrifuge tubes and covered with aluminium foil to prevent light exposure. The pigments were then extracted with 10 mL of N, N-dimethyl ammonium naphthalene acetic acid (DMF) by shaking for 2 h at 100 r/min on a rotating mixer (Scieniz HS-3). Extracts were centrifuged (3 000 r/min, 15 min) and filtered through a 0.2-μm GF/F membrane (Whatman). Extracted filtrates (approximately 600 μL) were poured into HPLC tubes and mixed with ammonium acetate (1 mol/L) at a volume ratio of 1:1. All pigments were quantitatively analyzed by Daa HPLC (Ultimate 3000, Thermo Fisher). For chromatographic separation, samples of approximately 100 μL were injected through a C8 column (Agilent, Germany) for reverse phase chromatography using 1 mol/L ammonium acetate and methanol (20:80 v), and 100% methanol. Pigments were then identified and calibrated according to their peak patterns and retention times (Zapatal et al., 2000; Romero-Viana et al., 2009). The dry weight of the sediment was also determined and wet concentrations were converted to dry weight, and the pigment concentration per gram of dry weight (μg/g dw) calculated. Some pigments were selected as biomarker signatures; for example, chlorophyll-a, peridinin, fucoxanthin, and diatoxanthin and diadinoxanthin were chosen as indicator pigments for total phytoplankton production, dinoflagellates, diatoms, and for both dinoflagellates and diatoms, respectively (Higgins and Mackey, 2000; Verleyen et al., 2004; Schüller and Savage, 2011; Aneeshkumar and Sujatha, 2012). Since the relative abundance of specific carotenoid pigments was selectively affected during deposition, pigments were examined individually relative to historical down-core maxima and minima (Leavitt, 1993).
Prior to the sediment diatom analysis, wet sediment (1 g) was weighed and HCl and H2O2 were added to remove carbonates and organic matter (Renberg, 1990). To identify diatom species and count valves, well-mixed acid-treated sediment suspensions containing diatom valves were mounted on microscope slides using Naphrax. Prepared slides were observed under a fluorescence microscope (Olympus BX51TRF) at magnifications of 400 or 1 000. Subsamples from the top, middle, and bottom of the sediment core were observed under a scanning electron microscope (JSM6390). Broken valves were counted using the method of Schrader and Gersonde (1978). The dry weight of the sediment was also determined and the wet valve count was converted to dry weight, following which the number of diatom valves per gram of dry weight (v/g dw) was then calculated. Taxa were identified to species and subspecies level using appropriate identification keys (Guo and Qian, 1984; Jin et al., 1985, 1992; Round et al., 1990; Cheng et al., 2012a; 2013).
2.4 Statistics and software applicationsThe Shannon diversity index was used as a measure of diatom species diversity and was calculated using a multivariate statistical package (MVSP 3.2). We categorized the diatoms and pigment ratios into their closest groups (time periods, in this case) to facilitate comparison of long-term changes using TWINSPAN 2.3 (two-way indicator for species analysis (Hill et al., 1975; Hill and Šmilauer, 2005)). Biostratographic analysis was carried out with C2 (version 1.7.5). The relative production of planktonic diatoms relative to total diatom abundance (planktonic diatom:total diatom abundance; Plan:Tabn) was calculated. To show decadal variations in diatom assemblage, the percentile compositions of dominant diatom species were analysed. Dominant species were defined as those that contributed more than 3% and occurred in more than 3 different subsamples.
The ratio of accessory pigments to Chl-a has been used previously as a more conservative proxy to calculate the contribution of different groups of algae (Goericke and Montoya, 1998 and references therein). We calculated the ratios between different pigment signatures to highlight the decadal variation in diatom and dinoflagellate occurrences. The fucoxanthin/ TChl-a (fuc/TChl-a) ratio was calculated to estimate relative diatom production; to ensure there was no bias from dinoflagellates, diatoxanthin and diadinoxanthin, these were not incorporated in the equation. The ratio of fucoxanthin/peridinin (fuc/per) was calculated to support calculation of decadal trends in ecological interactions between diatoms and dinoflagellates. Since water column irradiance and physico-chemical variables are intrinsically associated with phytoplankton species composition and their pigment composition, we calculated the relative production of planktonic diatoms relative to the total diatom abundance (planktonic diatom:total diatom abundance; Plan:Tabn). The irradiance conditions are also considered important for diatoms. We calculated the diatom mean irradiance by adding diadinoxanthin (DD) and diatoxanthin (D) concentrations and dividing by total chlorophyll-a (TChl-a is the sum of chlorophyll-a and its derivatives and bacteriochlorphylls) (Verleyen et al., 2004).
3 RESULT 3.1 Sediment ageResults from 210Pb dating of the sediment core showed that the average sedimentation rate was 0.94 cm2/a, which shows that each centimetre represents about one year's sediment accumulation. The excess 210Pb, calculated by subtracting the 226Ra activity from the total 210Pb activity, is illustrated in Fig. 2. Based on the calculated sedimentation rate, the 47-cm-long sediment core is about 50 years old, and covers the period from 1961 to 2011 AD. The core showed no evidence that sediments had been either reworked or disturbed by burrowing or fragments of animal remains. Furthermore, the inverse relationship between 210Pb activity and core depth shows that the sediment was not substantially mixed or reworked. Considering the dynamic character of the ECS, Su and Huh (2002) and Huh and Su (1999) suggested that 210Pb profiles should be supported by other sediment profiles such as 137Cs and 239, 240Pu. Comparison of the results from this study agreed well with sedimentation rates of between 0.6 to 0.9 cm/a calculated by Su and Huh (2002) and Huh and Su (1999) from 137Cs and 239, 240Pu sediment profiles of the inner shelf of ECS, close to our study area. Recently, Cheng et al. (2014) reported an average sedimentation rate of 1.57 cm/a. The difference between the sedimentation rates for the two studies reflects the characteristics of the study sites; that is, the study area of Cheng et al. (2014) was situated close to the coastal area of the CRE, which receives more sediments from the Changjiang River.
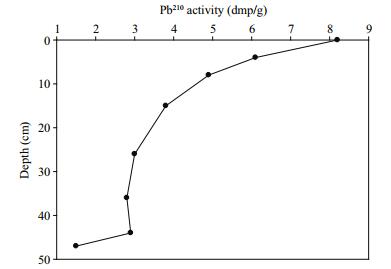
|
| Figure 2 Core profile of excess Pb210 concentrations for the age model |
The results from TWINSPAN 2.3 analysis show that the time span of the sediment core was divided into three periods, namely period Ⅰ from 1961 to 1982, period Ⅱ from 1982 to 2001, and period Ⅲ from 2001 to 2011. A total of 135 diatom taxa belonging to 37 genera were identified, and 23% of the species were from the centric diatom genus Coscinodiscus. After Coscinodiscus, the three pennate diatom genera, Pleurosigma, Trachyneis, and Nitzschia, contributed 8%, 7%, and 6%, respectively. Diatom absolute abundance varied with depth and ranged from 29 152 to 177 501 v/g dw and averaged 72 137 v/g dw (Fig. 3). Generally, the concentrations of sediment diatom valves were moderate. The Shannon species diversity index ranged from 2.917 to 3.413 and averaged 3.22, and species richness varied from 43 to 75 species. Benthic diatom species were dominated by species such as Actinoptychus senarius, Planktoneilla blanda, Thalassiothrix longissima, Thalassionema nitzschioides, and Tryblioptychus cocconeiformis.
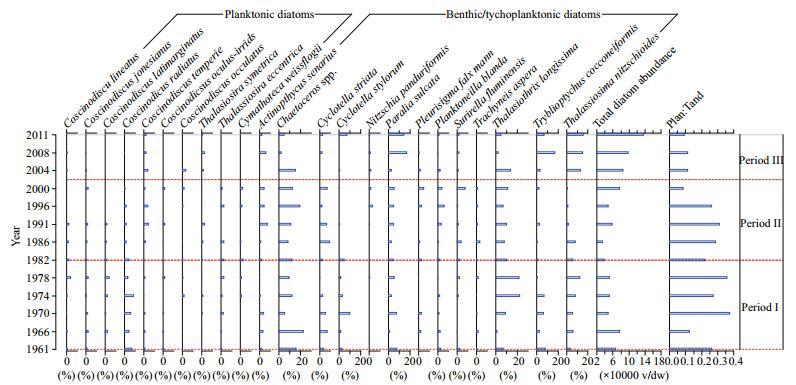
|
| Figure 3 Decadal variations in diatom species composition, total diatom absolute abundance, and the ratio of planktonic diatom:total diatom abundance (Plan:Tabn) |
Diatom abundance increased after 2001, which coincides with the TGD construction in 2003. There was a clear pattern in the decadal values of the planktonic diatom:total diatom abundance ratio (Plan:Tabn ratio); in general, the planktonic diatom species abundance increased and dominated from 1961 to 2001, whereas benthic diatoms dominated from 2001 to 2011 (Fig. 3). The mean values of the Plan:Tabn ratio were 0.25, 0.27, and 1.6 for periods Ⅲ, Ⅱ, and Ⅰ, respectively.
3.3 Long-term trends in pigment concentrationsChl-a was the main pigment in the sediment core followed by pheophorbide-a. With the exception of pheophorbide-a, the lowest and highest mean concentrations of all pigments were recorded in period Ⅰ and period Ⅲ, respectively (Fig. 4). In period Ⅰ the concentrations of fucoxanthin and peridinin were relatively low. The concentration of all pigments increased noticeably in period Ⅲ, with maximum values observed between 2001 and 2007. The changes in the decadal concentrations of pheophorbide-a in the three periods were minimal compared with the variation observed in other pigments, such as fucoxanthin, peridinin, diatoxanthin, and diadinoxanthin. Relative to the concentration in period Ⅰ, the concentration of fucoxanthin increased by 48.7% in period Ⅱ (1982–2001) and increased by 191% in period Ⅲ relative to period Ⅱ. Peridinin increased by only 34.9% and 51.5% in periods Ⅱ and Ⅲ relative to the concentrations in the preceding periods. The positive correlation between fucoxanthin and Chl-a (R=0.94, P < 0.05) was stronger than that between peridinin and Chl-a (R=0.71, P < 0.05). Diatom valve abundance was not correlated with diatom marker pigments.
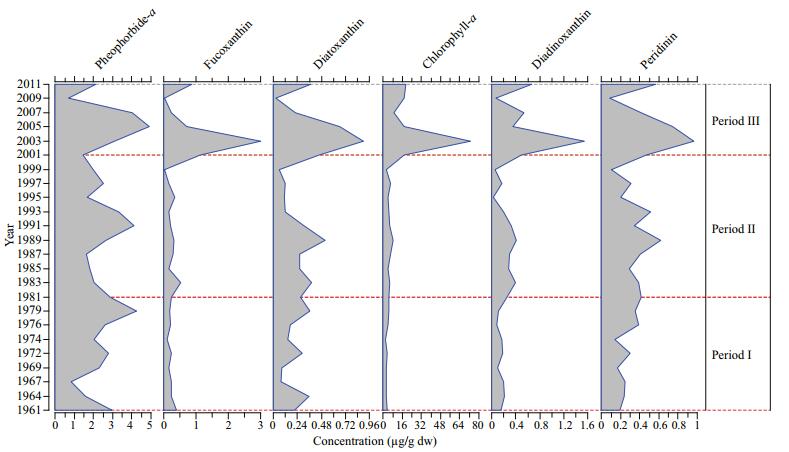
|
| Figure 4 Decadal variations in pigment concentrations (μg/g) in period Ⅰ (1961–1982), period Ⅱ (1982–2001), and period Ⅲ (2001–2011) |
The ratios of fucoxanthin/TChl-a, diatom mean irradiance (diatoxanthin+diadinoxanthin/TChl-a), and fucoxanthin/peridinin have gradually decreased over the past 50 years. Trends in the ratios also changed after 2001 (Fig. 5). The fucoxanthin/TChl-a ratio gradually decreased until 2001 and then rapidly increased after 2001. The peridinin/TChl-a ratio increased from mid-1970 to mid-1980, decreased randomly until 2003, and then increased slightly from 2003 to 2007. The fucoxanthin/peridinin ratio was relatively low from 1961 to 2001 and then dramatically increased after 2001. The fucoxanthin/peridinin ratio decreased by 3.1% in period Ⅱ relative to the ratio in period Ⅰ, and increased by 45.3% in period Ⅲ relative to the ratio in period Ⅱ. There was no clear pattern in the diatom mean irradiance from 1961 until the late 1990s, after which it decreased noticeably. The ratio of TChl-a/fucoxanthin was 163.6% higher in period Ⅱ than in period Ⅰ and was 420% higher in period Ⅲ than in period Ⅱ, and coincided with increases of 93.5% and 305.5% in the Chl-a concentration in periods Ⅱ and Ⅲ, respectively.
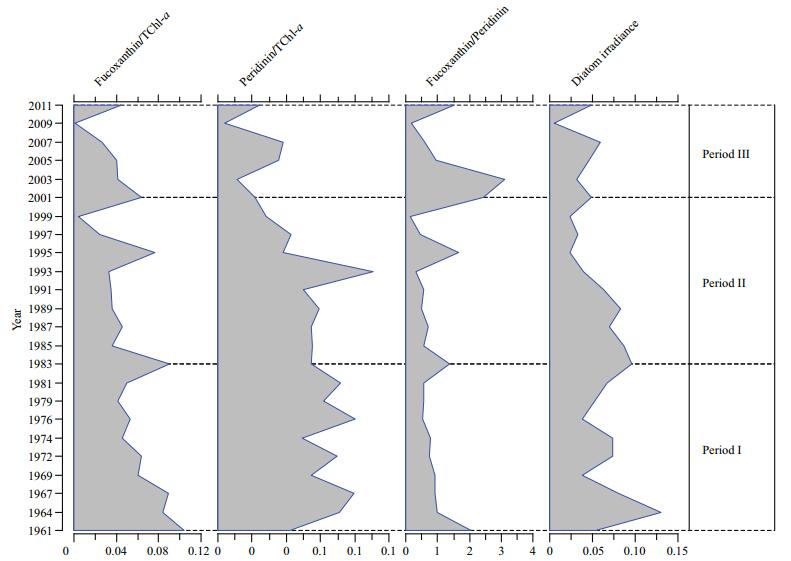
|
| Figure 5 Decadal patterns in the relative production of diatoms (fucoxanthin/TChl-a), relative production of dinoflagellates (Peridinin/TChl-a), diatom production relative to dinoflagellate production (fucoxanthin/peridinin), and diatom mean irradiance (diatoxanthin+diadinoxanthin/ TChl-a, D+DD/TChl-a) in the three periods |
The East China Sea is considered one of the most productive systems in the world (Gong et al., 2006). However, the observed moderate sediment pigment concentrations and the low diatom valve abundance indicate that phytoplankton remains were poorly preserved in sediments from the ECS. The concentrations of sediment-preserved signatures may be low because picoplankton are the major primary producers in the ECS and also account for the majority of surface water Chl-a (Yuh-Ling and Chen, 2000; Song et al., 2008). Generally, picoplankton are efficiently grazed by microzooplankton (Zhang et al., 2006), which results in the production of faecal pellets that are readily lost through photodegradation or reingestion (Welschmeyer and Lorenzen, 1985), or grazing of microzooplankton by mesozooplankton and incorporation into higher trophic biomass (Zhou et al., 2011) before being deposited into sediment. In line with this, Chl-b and other pigments mainly contributed by picophytoplankton species would not be expected in the ECS. The low diatom valve concentrations may be the result of differential dissolution of weakly silicified species like Skeletonema costatum (Schüller and Savage, 2011) and current wash (Furuya et al., 2003). As the ECS is exposed to frequent mixing processes and has large shallow areas (Su and Huh, 2002), the phytoplankton remains spend more time in the water column, resulting in increased physico-chemical and biological degradation and loss of the phytoplankton remains. Furthermore, large amounts of terrestrial sediment discharged into the ECS by the Changjiang River (Furuya et al., 2003) dilute the proxy, leading to reduced concentrations of phytoplankton remains in the sediment.
The absence of any correlation between diatom valve counts and pigment markers indicates that there was a discrepancy between diatom production and the preservation of its remaining materials. The same result has been reported for Doubtful Sound, New Zealand (Schüller and Savage, 2011), where weakly silicified diatom species like Skeletonema costatum disappeared because of selective dissolution, resulting in discrepancies between diatom abundance and pigment data. However, Verleyen et al. (2004) found a positive correlation between diatom pigments (diadinoxanthin, diatoxanthin, fucoxanthin) and diatom biovolume in the marine sediment. The general long-term trend in diatom marker pigments was comparable to the diatom valve abundance, except during period Ⅱ, when the abundance of diatom valves decreased and pigment marker concentrations increased. This increase was probably related to diatom marker pigments contributed by other phytoplankton groups. Other researchers reported that there were no correlations between peridinin and dinoflagellate abundance (Leavetti and Findlay, 1994) or fucoxanthin and diatom abundance (Schüller and Savage, 2011) in lake and coastal sediments, respectively.
The Chl-a concentration indicates the presence of autotrophic benthic organisms, undegraded phytoplankton detrital material, and faecal pellets, and is well-accepted as an indicator of primary production (fucoxanthin/peridinin), and diatom mean irradiance (diatoxanthin+diadinoxanthin/ TChl-a, D+DD/TChl-a) in the three periods production. In general, the decreases and increases in Chl-a coincided closely with increases and decreases in both peridinin and fucoxanthin. The strong positive correlations between Chl-a and both peridinin and fucoxanthin indicate the dominance and cooccurrence of these two phylogenic groups (mainly diatoms and dinoflagellates) in the phytoplankton composition of the ECS. Some of the dinoflagellate groups may also contribute fucoxanthin. Hansen and Josefson (2003) reported a strong positive correlation between fucoxanthin and Chl-a in the transition zone of the North and Baltic Seas. The fact that the correlation between Chl-a and fucoxanthin was stronger than that between Chl-a and peridinin implies that diatoms are the dominant phytoplankton group and represent the greatest proportion of total phytoplankton production in the coastal ECS.
4.2 Temporal variation in diatom and dinoflagellate productionThe changes in the fucoxanthin/peridinin ratio indicate that the relative productions of diatoms and dinoflagellates have changed. In particular, diatom production has been gradually decreasing in the past 50 years up to 2001. This decrease can be explained by regional climate fluctuations (Lin and Lu, 2009; Lee et al., 2012) and nutrient changes (Chai et al., 2006; Li et al., 2011) observed around the Changjiang River Basin and in the coastal ECS since the late 1970s. In the ECS, the general trend in Chl-a production indicates the influence of climate change and anthropogenic impacts and has been coupled with a major local-regional climate shift, the TGD construction, and anthropogenic nutrient inputs.
4.2.1 Period ⅠIn this period, human influences on the ECS and its surroundings were not significant, and the western North Pacific regional climate was characterized by cool weather and decreased rainfall (Chai et al., 2006; Song et al., 2008; Lin and Lu, 2009; Haiyan et al., 2010; Wang et al., 2011; Lee et al., 2012). Data from this study indicate that diatom primary production was moderate in this period, which is consistent with the result recorded by Haiyan et al. (2010), who used BSi and Chl-a as proxies of paleo-productivity.
4.2.2 Period ⅡThis period is recognized by a shift in the localregional climate to warm and wet conditions (Lin and Lu, 2009; Lee et al., 2012) and increased inputs of nitrogen-containing fertilizers (Chai et al., 2006; Li et al., 2011). Coincidently, dinoflagellate production started to increase from the late 1970s. The fucoxanthin/peridinin ratio declined in this period, indicating that the increased production of dinoflagellates came at the expense of diatom production. The rise in dinoflagellate production was associated with the increase in artificial nitrogen inputs in the area surrounding the ECS, which shifted the balance of the N:P ratio in coastal systems. Considering the evolutionary advantages of dinoflagellates and their ability to bloom under nutrient-depleted conditions (Rivkin and Swift, 1985), the observed increase in dinoflagellate production over the past three decades is not unexpected. It is also consistent with previous studies of the ECS; for example, Kui (2007) reported a declining pattern in the contribution of diatoms to total phytoplankton abundance, with average contributions of 90%, 85%, and 64% in the early 1980s, mid-1980s, and in 1997, respectively. Shifts in the patterns of diatom and dinoflagellate dominance because of climatic variations have been reported from the central Baltic and the North Sea (Alheit et al., 2005) and elsewhere (deYoung et al., 2004; Wooster and Zhang, 2004). Leavetti and Findlay (1994) reported phytoplankton pigment compositional change because of nitrogen fertilization in an experimental lake, while Klais et al. (2011) also reported dissolved silica depletion that coincided with a significant increase in dinoflagellate abundance in the semi-enclosed Gulf of Riga.
Fucoxanthin, a pigment largely from diatoms and other phytoplankton, such as prymnesiophytes, crysophytes, and some dinoflagellates (Higgins and Mackey, 2000) showed a considerable (48.7%) increase during the period from 1982 to 2001. The rise of fucoxanthin in this period was probably associated with the increased production of dinoflagellates, as some dinoflagellate species contain this pigment (Jeffrey et al., 1997). This idea is supported by the fact that 1) peridinin made a considerable contribution to the total pigment concentration and peridinin was strongly correlated with fucoxanthin, and 2) other fucoxanthin-containing microalgae, such as crysophytes (19′-butanoyloxyfucoxanthin) and prymnesiophycae (19′-hexfucoxanthin) (Higgins and Mackey, 2000), were absent from sediment samples. However, in contrast to the increased fucoxanthin concentrations observed in this period, diatom valve abundance decreased in this period. Similarly, Haiyan et al. (2010) also reported decreased diatom production from 1980 to 1997. This therefore suggests that the increase in the Chl-a concentration during this period was the result of the increased production of dinoflagellates and other algal groups. In the same way, Haiyan et al. (2010) also suggested increases in the production of algae other than diatoms for the period from 1980 to 1997.
4.2.3 Period ⅢDuring this period, the pigment concentrations and diatom valve abundances increased drastically, which indicates that dinoflagellate, diatom, and total phytoplankton production increased noticeably after 2001. The highest pigment concentration was recorded between 2001 and 2007, and reflects the impact of the TGD construction. Furthermore, relative to the concentrations between 2004 and 2006, pigment concentrations decreased sharply between 2008 and 2010, but were still considerably higher than those recorded before construction of the TGD. This further indicates the pronounced impact of the TGD construction on the coastal zone of the ECS. The impact of the TGD construction is probably related to various complex physico-chemical and biological interactions. For example, the TGD construction reduced the volume of freshwater entering the ECS at least during water filling periods, which reduced stratification by density in the coastal ECS and promoted phytoplankton production. The TGD construction also resulted in a decrease in the terrestrial sediment loads discharged to the coastal part of the ECS (Jiao et al., 2007), which led to reduced light transparency and reduced phytoplankton production. Thus, the TGD construction reduced sediment inputs from the Changjiang River (Yang et al., 2006), which resulted in increased light availability in the coastal ECS. The effects of the TGD construction on the ecology of Changjiang estuary and in the surrounding ECS have been reported by many researchers (Gong et al., 2006; Jiao et al., 2007; Song et al., 2008; Wang et al., 2011). Furthermore, the number of tropical cyclones increased after 2000 (Lee et al., 2012), which further favoured diatoms because of increased mixing; in the absence of stratification, upwelling promotes the occurrence of diatom blooms in continental shelf areas (Yoder et al., 1983). Humborg et al. (1997) also observed a rapid decline in the Si:N ratio and consquent changes in phytoplanton composition and cell density in the Black Sea as result of dam construction in the Danube River, and reported that the cocolithphore group became the dominant phytoplankton community.
4.3 Changes in diatom species composition and light conditionsDecadal variations in diatom mean irradiance indicate that phytoplankton light availability has fluctuated in the ECS over the past 50 years. Laboratory experiments showed that the concentrations of protective pigments decreased and the concentrations of light harvesting pigments increased at low irradiance density, and that the concentrations of light harvesting pigments decreased and protective pigments increased at high irradiance density (Van De Poll et al., 2005). Generally, low values of the Plan:Tabn ratio are considered to reflect dominance of benthic diatoms, and are related to mixing processes and improved light conditions in the water column, whereas lower diatom mean irradiance is an indication of decreased irradiance in the water column.
The lower ratios of diatom mean irradiance before period Ⅲ seem to indicate increased availability of light that would have induced diatom production. However, the high values of the Plan:Tabn ratios in these periods indicate that planktonic diatom species dominated the diatom community, which further implies that benthic diatoms were limited by light. The dominance of benthic diatoms after 2001 clearly indicates increased availability of light. After 2001, the Plan:Tabn ratio decreased noticeably, implying greater production of benthic diatoms than planktonic diatoms. In highly turbid waters the relative abundance of planktonic diatoms increases at the expense of benthic diatoms (Weckström and Juggins, 2005); however, in this study, benthic diatom abundance overwhelmingly increased when the light conditions improved. Phytoplankton production in coastal parts of the ECS, and particularly in areas close to the Changjiang Estuary, frequently experienced light limitations (Zhu et al., 2009), which further suggests that increases in incoming irradiance in the water column may trigger high primary production of benthic diatoms.
Suspended inorganic particles attenuate the light that penetrates into the deep water column and interferes with phytoplankton photosynthesis (Kirk, 1983). Recently, Liu et al. (2013) reported that the cell density and growth rates of Phaeodactylum tricornutum Bohlin and Gymnodinium sp. decreased as a result of increased suspended particles. On the other hand, Guenther and Bozelli (2004) showed that phytoplankton density decreased because of light limitations caused by bauxite tailings and inorganic turbidity in Amazonia Lake, Brazil. Furthermore, Yoder (1985) discussed limitations of photosynthesis because of high turbidity in coastal waters across the southeastern continental shelf of the South Atlantic Bight. High primary production was limited to the upper part of water column in the coastal zone of the central Georgia embayment, and high turbidity in this area was the result of suspended particles (Oertel and Dunstan, 1981). Similarly, Randall and Day Jr (1987) reported light-limited primary production across the Louisiana estuary because of high turbidity. Thus, the dominance of benthic or neritic diatoms such as Paralia salcata, Actinoptychus senarius and Thalassionema nitzschioides during the past 10 years is most probably associated with the decreased sediment influxes from the Changjiang River that have resulted from the construction of the TGD (Wang et al., 2011) and consequent increases in light availability.
The increased concentrations of diatom irradiance protective pigments (diatoxanthin+diadinoxanthin) and Chl-a between 2001 and 2011 were the result of increased irradiance and a consequent increase in primary production. Regardless of the fact that the D+DD/TChl-a ratio should increase when irradiance increases in the water column, as indicated by the Plan:Tabn ratios, the increases in the fucoxanthin and Chl-a concentrations from 2001 to 2011 coincided with decreased D+DD/Chl-a ratios. This resulted from a very dramatic increase in the TChl-a concentration (420%), which in turn was probably related to an increase in the relative abundance of benthic diatoms associated with the increased intensity of the available irradiance. Furthermore, this decrease in diatom irradiance probably resulted from high Chl-a production by other algal groups.
5 CONCLUSIONThe long-term trends in diatom and dinoflagellate marker signatures indicate that there was variation in the decadal productions of both of these phylogenic groups. Diatoms dominated in the periods before 1982 and after 2001; inputs of nitrogen fertilizer were low before 1982, while after 2001, the TGD construction impacted on the coastal ECS. Dinoflagellate production increased between 1982 and 2001 and coincided with increased nitrogen fertilizer inputs and a warm-wet phase. However, on the decadal time scale, the production of dinoflagellates has been increasing up to the present. The dramatic change in diatom abundance and most pigment signatures from 2001 to 2007 implies that there have been substantial biogeochemical changes in the coastal area of the ECS as a result of the construction of the TGD. Further studies of dinoflagellate cell counts and species composition in the inner, mid, and outer shelf of the ECS and the NW Pacific Ocean will contribute to an improved understanding of these two major phytoplankton groups and their decadal-scale dynamics.
| Alheit J, Möllmann C, Dutz J, Kornilovs G, Loewe P, Mohrholz V, Wasmund N, 2005. Synchronous ecological regime shifts in the central Baltic and the North Sea in the late 1980s. ICES Journal of Marine Science, 62(7): 1 205–1 215. Doi: 10.1016/j.icesjms.2005.04.024 |
| Aneeshkumar N, Sujatha C H, 2012. Biomarker pigment signatures in Cochin back water system-A tropical estuary south west coast of India. Estuarine, Coastal and Shelf Science, 99: 182–190. Doi: 10.1016/j.ecss.2011.12.029 |
| Appleby P G, Oldfield F, 1978. The calculation of lead-210 dates assuming a constant rate of supply of unsupported 210Pb to the sediment. Catena, 5(1): 1–8. Doi: 10.1016/S0341-8162(78)80002-2 |
| Battarbee R, Grytnes J A, Thompson R, Appleby P G, Catalan J, Korhola A, Birks H J B, Heegaard E, Lami A, 2002. Comparing palaeolimnological and instrumental evidence of climate change for remote mountain lakes over the last 200 years. Journal of Paleolimnology, 28(1): 161–179. Doi: 10.1023/A:1020384204940 |
| Beaugrand G, 2009. Decadal changes in climate and ecosystems in the North Atlantic Ocean and adjacent seas. Deep Sea Research Part Ⅱ:Topical Studies in Oceanography, 56(8-10): 656–673. Doi: 10.1016/j.dsr2.2008.12.022 |
| Chai C, Yu Z M, Song X X, Cao X H, 2006. The status and characteristics of eutrophication in the Yangtze River(Changjiang) estuary and the adjacent East China Sea, China. Hydrobiologia, 563(1): 313–328. Doi: 10.1007/s10750-006-0021-7 |
| Chen Y L L, 2000. Comparisons of primary productivity and phytoplankton size structure in the marginal regions of southern East China Sea. Continental Shelf Research, 20(4-5): 437–458. Doi: 10.1016/S0278-4343(99)00080-1 |
| Cheng F, Song X, Yu Z, Liu D, 2012b. Historical records of eutrophication in Changjiang (Yangtze) River estuary and its adjacent East China Sea. Biogeosciences, 9(6): 6 261–6 291. Doi: 10.5194/bgd-9-6261-2012 |
| Cheng F, Yu Z, Song X, 2014. Long-term changes in sedimentary diatom assemblages and their environmental implications in the Changjiang (Yangtze) River estuary, China. Chinese Journal of Oceanology and Limnology, 32(1): 155–161. Doi: 10.1007/s00343-014-3133-3 |
| Cheng Z D, Gao Y H, Liu S C, Wang D Z, Chen C P, Liang J R, Li Y, Qi Y Z, 2013. Flora Algarum Marinarum Sinicarum:Tomus V. Bacillariophyta, NO. Ⅲ Pennatae Ⅱ, Naviculales, Naviculaceae, Cymbellaceae, Auriculaceae, Gomphonemaceae.. Science Press, Beijing183p. |
| Cheng Z D, Gao Y H, Liu S C, Wang D Z, Chen C P, Liang J R, Qi Y Z, 2012a. Flora Algarum Marinarum Sinicarum:Tomus V. Bacillariophyta, No. Ⅱ Pennatae I, Diatomales, Achnanthales, Phaeodactylales, Eunotiales. Science Press, Beijing137p. |
| Chiang K P, Chou Y H, Chang J, Gong G C, 2004. Winter distribution of diatom assemblages in the East China Sea. Journal of Oceanography, 60(6): 1 053–1 062. Doi: 10.1007/s10872-005-0013-7 |
| Chiba S, Saino T, 2003. Variation in mesozooplankton community structure in the Japan/East Sea (1991-1999) with possible influence of the ENSO scale climatic variability. Progress in Oceanography, 57(3-4): 317–339. Doi: 10.1016/S0079-6611(03)00104-6 |
| deYoung B, Harris R, Alheit J, Beaugrand G, Mantua N, Shannon L, 2004. Detecting regime shifts in the ocean:data considerations. Progress in Oceanography, 60(2-4): 143–164. Doi: 10.1016/j.pocean.2004.02.017 |
| Finkelstein S A, Gajewski K, 2007. A palaeolimnological record of diatom-community dynamics and late-Holocene climatic changes from Prescott Island, Nunavut, central Canadian Arctic. The Holocene, 17(6): 803–812. Doi: 10.1177/0959683607080521 |
| Furuya K, Hayashi M, Yabushita Y, Ishikawa A, 2003. Phytoplankton dynamics in the East China Sea in spring and summer as revealed by HPLC-derived pigment signatures. Deep Sea Research Part Ⅱ:Topical Studies in Oceanography, 50(2): 367–387. Doi: 10.1016/S0967-0645(02)00460-5 |
| Goericke R, Montoya J P, 1998. Estimating the contribution of microalgal taxa to chlorophyll a in the field-variations of pigment ratios under nutrient-and light-limited growth. Marine Ecology Progress Series, 169: 97–112. Doi: 10.3354/meps169097 |
| Gong G C, Chang J, Chiang K P, Hsiung T M, Hung C C, Duan S W, Codispoti L A, 2006. Reduction of primary production and changing of nutrient ratio in the East China Sea:effect of the three gorges dam. Geophysical Research Letters, 33(7): L07610. |
| Guenther M, Bozelli R, 2004. Effects of inorganic turbidity on the phytoplankton of an amazonian lake impacted by bauxite tailings. Hydrobiologia, 511(1-3): 151–159. |
| Guo Y J, Qian S B, 1984. Flora Algarum Marinarum Sinicarum:Tomus. V. Bacillariophyta. Science Press, Beijing. |
| Hansen J L S, Josefson A B, 2003. Accumulation of algal pigments and live planktonic diatoms in aphotic sediments during the spring bloom in the transition zone of the North and Baltic Seas. Marine Ecology Progress Series, 248: 41–54. Doi: 10.3354/meps248041 |
| Higgins H W, Mackey D J, 2000. Algal class abundances, estimated from chlorophyll and carotenoid pigments, in the western Equatorial Pacific under El Niño and non-El Niño conditions. Deep Sea Research Part Ⅰ:Oceanographic Research Papers, 47(8): 1 461–1 483. Doi: 10.1016/S0967-0637(99)00114-4 |
| Hill M O, Bunce R G H, Shaw M W, 1975. Indicator species analysis, a divisive polythetic method of classification, and its application to a survey of native pinewoods in scotland. The Journal of Ecology, 63(2): 597–613. Doi: 10.2307/2258738 |
| Hill M O, Šmilauer P. 2005. TWINSPAN for Windows Version 2. 3. Software and User Guide. Centre for Ecology and Hydrology & University of South Bohemia, Huntingdon, Ceske Budejovice. |
| Hinder S L, Hays G C, Edwards M, Roberts E C, Walne A W, Gravenor M B, 2012. Changes in marine dinoflagellate and diatom abundance under climate change. Nature Climate Change, 2(4): 271–275. Doi: 10.1038/nclimate1388 |
| Huh C A, Su C C, 1999. Sedimentation dynamics in the East China Sea elucidated from 210Pb, 137Cs and 239, 240Pu. Marine Geology, 160(1-2): 183–196. Doi: 10.1016/S0025-3227(99)00020-1 |
| Humborg C, Ittekkot V, Cociasu A, Bodungen B V, 1997. Effect of Danube River dam on Black Sea biogeochemistry and ecosystem structure. Nature, 386(6623): 385–388. Doi: 10.1038/386385a0 |
| Jeffrey S W, Mantoura R F C, Wright S W. 1997. Phytoplankton Pigments in Oceanography: Guidelines to Modern Methods. UNESCO Publishing, Paris. http://ci.nii.ac.jp/ncid/BA29767891 |
| Jiao N Z, Zhang Y, Zeng Y H, Gardner W D, Mishonov A V, Richardson M J, Hong N, Pan D L, Yan X H, Jo Y H, Chen C T A, Wang P X, Chen Y Y, Hong H S, Bai Y, Chen X H, Huang B Q, Deng H, Shi Y, Yang D C, 2007. Ecological anomalies in the East China Sea:impacts of the Three Gorges Dam. Water Research, 41(6): 1 287–1 293. Doi: 10.1016/j.watres.2006.11.053 |
| Jin D X, Cheng Z D, Lin J M, Liu S C. 1985. The Marine Benthic Diatoms in China. China Ocean Press, SpringerVerlag, Beijng, Berlin Heidelberg. 313p. |
| Jin D X, Cheng Z D, Lin J M, Ma J H. 1992. The Marine Benthic Diatoms in China. China Ocean Press, Beijng. 437p. (in Chinese) |
| Jin H Y, Chen J F, Weng H X, et al, 2010. Variations in paleoproductivity and the environmental implications over the past six decades in the Changjiang Estuary. Acta Oceanologica Sinica, 29(3): 38–45. Doi: 10.1007/s13131-010-0035-x |
| Jones V J, Birks H J B, 2004. Lake-sediment records of recent environmental change on Svalbard:results of diatom analysis. Journal of Paleolimnology, 31(4): 445–466. Doi: 10.1023/B:JOPL.0000022544.35526.11 |
| Kirk J T O, 1983. Light and Photosynthesis in Aquatic Ecosystems. Cambridge University Press, Cambridge. |
| Klais R, Tamminen T, Kremp A, Spilling K, Olli K, 2011. Decadal-scale changes of dinoflagellates and diatoms in the anomalous Baltic sea spring bloom. PLoS One, 6(6): e21567. Doi: 10.1371/journal.pone.0021567 |
| Kuwae M, Yamashita A, Hayami Y, Kaneda A, Sugimoto T, Inouchi Y, Amano A, Takeoka H, 2006. Sedimentary records of multidecadal-scale variability of diatom productivity in the Bungo Channel, Japan, associated with the Pacific Decadal Oscillation. Journal of Oceanography, 62(5): 657–666. Doi: 10.1007/s10872-006-0084-0 |
| Leavetti P R, Findlay D l, 1994. Comparison of fossil pigments with 20 years of phytoplankton data from eutrophic lake 227, experimental lakes area, ontario. Canadian Journal of Fisheries and Aquatic Sciences, 51(10): 2 286–2 299. Doi: 10.1139/f94-232 |
| Leavitt P R, 1993. A review of factors that regulate carotenoid and chlorophyll deposition and fossil pigment abundance. Journal of Paleolimnology, 9(2): 109–127. Doi: 10.1007/BF00677513 |
| Lee H S, Yamashita T, Mishima T, 2012. Multi-decadal variations of ENSO, the Pacific Decadal Oscillation and tropical cyclones in the western North Pacific. Progress in Oceanography, 105: 67–80. Doi: 10.1016/j.pocean.2012.04.009 |
| Leterme S C, Edwards M, Seuront L, Attrill M J, Reid P C, John A W G, 2005. Decadal basin-scale changes in diatoms, dinoflagellates, and phytoplankton color across the North Atlantic. Limnology and Oceanography, 50(4): 1 244–1 253. Doi: 10.4319/lo.2005.50.4.1244 |
| Li M, Xu K, Watanabe M, Chen Z, 2007. Long-term variations in dissolved silicate, nitrogen, and phosphorus flux from the Yangtze River into the East China Sea and impacts on estuarine ecosystem. Estuarine, Coastal and Shelf Science, 71(1-2): 3–12. Doi: 10.1016/j.ecss.2006.08.013 |
| Li X X, Bianchi T S, Yang Z S, Osterman L E, Allison M A, DiMarco S F, Yang G P, 2011. Historical trends of hypoxia in Changjiang River estuary:applications of chemical biomarkers and microfossils. Journal of Marine Systems, 86(3-4): 57–68. Doi: 10.1016/j.jmarsys.2011.02.003 |
| Lin Z D, Lu R Y, 2009. The ENSO's effect on eastern China rainfall in the following early summer. Advances in Atmospheric Sciences, 26(2): 333–342. Doi: 10.1007/s00376-009-0333-4 |
| Liu C G, Wang J L, Feng J F, Peng S T, 2013. Effects of suspended particles on the growth of two dominant phytoplankton species of Bohai Bay, China. Marine Pollution Bulletin, 74(1): 220–224. Doi: 10.1016/j.marpolbul.2013.06.054 |
| McKee B A, Nittrouer C A, DeMaster D J, 1983. Concepts of sediment deposition and accumulation applied to the continental shelf near the mouth of the Yangtze River. Geology, 11(11): 631–633. Doi: 10.1130/0091-7613(1983)11<631:COSDAA>2.0.CO;2 |
| Milliman J D, Qin Y P, Park Y A. 1989. Sediment and sedimentary processes in the Yellow and East China Seas. In: Taira A, Masunda A eds. Sedimentary Facies in the Active Plate Margin. Terra Scientific Publishing Company, Tokyo. p. 233-249. |
| Montes-Hugo M, Doney S C, Ducklow H W, Fraser W, Martinson D, Stammerjohn S E, Schofield O, 2009. Recent changes in phytoplankton communities associated with rapid regional climate change along the Western Antarctic Peninsula. Science, 323(5920): 1 470–1 473. Doi: 10.1126/science.1164533 |
| Oertel G F, Dunstan W M, 1981. Suspended-sediment distribution and certain aspects of phytoplankton production off Georgia, U.S.A. Marine Geology, 40(1-2): 171–197. Doi: 10.1016/0025-3227(81)90049-9 |
| Randall J M, Day Jr J W, 1987. Effects of river discharge and vertical circulation on aquatic primary production in a turbid Louisiana (USA) estuary. Netherlands Journal of Sea Research, 21(3): 231–242. Doi: 10.1016/0077-7579(87)90015-9 |
| Renberg I, 1990. A procedure for preparing large sets of diatom slides from sediment cores. Journal of Paleolimnology, 4(1): 87–90. |
| Rivkin R B, Swift E, 1985. Phosphorus metabolism of oceanic dinoflagellates:phosphate uptake, chemical composition and growth of Pyrocystis noctiluca. Marine Biology, 88(2): 189–198. Doi: 10.1007/BF00397166 |
| Romero-Viana L, Keely B J, Camacho A, Vicente E, Rosa Miracle M, 2009. Photoautotrophic community changes in Lagunillo del Tejo (Spain) in response to lake level fluctuation:two centuries of sedimentary pigment records. Organic Geochemistry, 40(3): 376–386. Doi: 10.1016/j.orggeochem.2008.11.010 |
| Round F E, Crawford R M, Mann D G, 1990. The Diatoms:Biology & Morphology of the Genera. Cambridge University Press, Cambridge. |
| Schrader H J, Gersonde R. 1978. Diatoms and silicoflagellates. In: Zachariasse W J ed. Micropaleontological Counting Methods and Techniques-An Exercise of an Eight Metres Section of the Lower Pliocene of Cap Rossello. Utrecht Micropaleontology Bulletin, Sicily. p. 129-176. |
| Schüller S E, Savage C, 2011. Spatial distribution of diatom and pigment sedimentary records in surface sediments in Doubtful Sound, Fiordland, New Zealand. New Zealand Journal of Marine and Freshwater Research, 45(4): 591–608. Doi: 10.1080/00288330.2011.561865 |
| Smayda T J, 2002. Adaptive ecology, growth strategies and the global bloom expansion of dinoflagellates. Journal of Oceanography, 58(2): 281–294. Doi: 10.1023/A:1015861725470 |
| Song S Q, Sun J, Luan Q S, Shen Z L, 2008. Size-fractionated phytoplankton biomass in autumn of the Changjiang(Yangtze) River Estuary and its adjacent waters after the Three Gorges Dam construction. Chinese Journal of Oceanology and Limnology, 26(3): 268–275. Doi: 10.1007/s00343-008-0268-0 |
| Su C C, Huh C A, 2002. 210Pb, 137Cs and 239, 240Pu in East China Sea sediments:sources, pathways and budgets of sediments and radionuclides. Marine Geology, 183(1-4): 163–178. Doi: 10.1016/S0025-3227(02)00165-2 |
| Sugimoto T, Tadokoro K, 1997. Interannual-interdecadal variations in zooplankton biomass, chlorophyll concentration and physical environment in the subarctic Pacific and Bering Sea. Fisheries Oceanography, 6(2): 74–93. Doi: 10.1046/j.1365-2419.1997.00031.x |
| Van De Poll W H, Van Leeuwe M A, Roggeveld J, Buma A G J, 2005. Nutrient limitation and high irradiance acclimation reduce par and uv-induced viability loss in the antarctic diatomchaetocerosbrevis(bacillariophyceae). Journal of Phycology, 41(4): 840–850. Doi: 10.1111/jpy.2005.41.issue-4 |
| Verleyen E, Hodgson D A, Leavitt P R, Sabbe K, Vyverman W, 2004. Quantifying habitat-specific diatom production:a critical assessment using morphological and biogeochemical markers in Antarctic marine and lake sediments. Limnology and Oceanography, 49(4): 1 528–1 539. |
| Wang H J, Saito Y, Zhang Y, Bi N S, Sun X X, Yang Z S, 2011. Recent changes of sediment flux to the western Pacific Ocean from major rivers in East and Southeast Asia. Earth-Science Reviews, 108(1-2): 80–100. Doi: 10.1016/j.earscirev.2011.06.003 |
| Wang K. 2007. The distribution of nutrients, nutritional support of nitrogen and its eutrophication effects in Changjiang Estuary and adjacent area. The Second Institute of Oceanography, Soa, Hangzhou. 45p. (in Chinese with English abstract) |
| Weckström K, Juggins S, 2006. Coastal diatom-environment relationships from the Gulf of Finland, Baltic Sea. Journal of Phycology, 42(1): 21–35. Doi: 10.1111/jpy.2006.42.issue-1 |
| Welschmeyer N A, Lorenzen C J, 1985. Role of herbivory in controlling phytoplankton abundance:annual pigment budget for a temperate marine fjord. Marine Biology, 90(1): 75–86. Doi: 10.1007/BF00428217 |
| Wooster W S, Zhang C I, 2004. Regime shifts in the North Pacific:early indications of the 1976-1977 event. Progress in Oceanography, 60(2-4): 183–200. Doi: 10.1016/j.pocean.2004.02.005 |
| Yang Z, Wang H, Saito Y, Milliman J D, Xu K, Qiao S, Shi G, 2006. Dam impacts on the Changjiang (Yangtze) River sediment discharge to the sea:the past 55 years and after the Three Gorges Dam. Water Resources Research, 42(4): W04407. |
| Yoder J A, Atkinson L P, Stephen Bishop S, Hofmann E E, Lee T N, 1983. Effect of upwelling on phytoplankton productivity of the outer southeastern United States continental shelf. Continental Shelf Research, 1(4): 385–404. Doi: 10.1016/0278-4343(83)90004-3 |
| Yoder J. 1985. Environmental control of phytoplankton production on the southeastern U. S. continental shelf. In: Atkinson L P, Menzel D W, Bush K A eds. Oceanography of the Southeastern U. S. Continental Shelf. American Geophysical Union, Washington. p. 93-103. |
| Zapatal M, Rodríguezl F, Garrido J L, 2000. Separation of chlorophylls and carotenoids from marine phytoplankton:a new HPLC method using a reversed phase C8 column and pyridine-containing mobile phases. Marine Ecology Progress Series, 195: 29–45. Doi: 10.3354/meps195029 |
| Zhang C I, Gong Y, 2005. Effect of ocean climate changes on the Korean stock of Pacific saury, Cololabis saira(Brevoort). Journal of Oceanography, 61(2): 313–325. Doi: 10.1007/s10872-005-0042-2 |
| Zhang W C, Li H B, Xiao T, Zhang J, Li C L, Sun S, 2006. Impact of microzooplankton and copepods on the growth of phytoplankton in the Yellow Sea and East China Sea. Hydrobiologia, 553(1): 357–366. Doi: 10.1007/s10750-005-0857-2 |
| Zhou L B, Tan Y H, Huang L M, Huang J M, Huang J R, Liu H X, Lian X P, 2011. Phytoplankton growth and microzooplankton grazing in the continental shelf area of northeastern South China Sea after Typhoon Fengshen. Continental Shelf Research, 31(16): 1 663–1 671. Doi: 10.1016/j.csr.2011.06.017 |
| Zhou M, Shen Z L, Yu R C, 2008. Responses of a coastal phytoplankton community to increased nutrient input from the Changjiang (Yangtze) River. Continental Shelf Research, 28(12): 1 483–1 489. Doi: 10.1016/j.csr.2007.02.009 |
| Zhu Z Y, Ng W M, Liu S M, Zhang J, Chen J C, Wu Y, 2009. Estuarine phytoplankton dynamics and shift of limiting factors:a study in the Changjiang (Yangtze River) Estuary and adjacent area. Estuarine, Coastal and Shelf Science, 84(3): 393–401. Doi: 10.1016/j.ecss.2009.07.005 |
 2017, Vol. 35
2017, Vol. 35


