Institute of Oceanology, Chinese Academy of Sciences
Article Information
- LI Zhe(李哲), XIAO Yan(肖艳), YANG Jixiang(杨吉祥), LI Chao(李超), GAO Xia(高遐), GUO Jinsong(郭劲松)
- Response of cellular stoichiometry and phosphorus storage of the cyanobacteria Aphanizomenon flos-aquae to smallscale turbulence
- Chinese Journal of Oceanology and Limnology, 35(6): 1409-1416
- http://dx.doi.org/10.1007/s00343-017-6178-2
Article History
- Received Jun. 28, 2016
- accepted in principle Aug. 23, 2016
- accepted for publication Sep. 27, 2016
2 State Key Laboratory of Bioreactor Engineering, College of Biotechnology, East China University of Science and Technology, Shanghai 200237, China
Microalgae are planktonic photoautotrophs and major producers in aquatic systems. They can inhabit aggressively mixed, fastly dissipating turbulent fields, and are smaller, by one or more orders of magnitude, than the smallest eddy sizes (Reynolds, 2006). Changes in turbulent mixing play a critical role in the development of microalgal communities.
Turbulent mixing has various effects on microalgae, acting at scales from 1 to 1 000 m or more. Mixing changes the position and distribution of microalgae in the water column, minimizes sinking of individuals (Thomas et al., 1995), brings nutrient-rich water into contact with plankton cells, regulates light accessibility and photosynthesis intensities (Ebert et al., 2001; Huisman et al., 2002), and affects the encounter possibility between microalgae and predators (Rothschild and Osborn, 1988). The indirect effects of small-scale turbulence on planktonic habitats have been widely reported. However, the direct effects of small-scale turbulence on the physiology of microalgae has only been understood in the past two decades (Thomas and Gibson, 1990; Thomas et al., 1995; Gallardo Rodríguez et al., 2009; Hondzo and Wüest, 2009). An early review by Thomas and Gibson (1990) summarized the direct physiological effects of small-scale turbulence on microalgae. When assessed in terms of dissipation rate, viscous stress, or shear stress, small-scale turbulence mainly impacts cellular nutrient uptake through changes in the sublayer thickness (Estrada and Berdalet, 1997; Hondzo and Lyn, 1999; Warnaars and Hondzo, 2006), and results in direct damage to microalgal cells (Michels et al., 2010).
Microalgae have various functional traits, based on physiological adaptation strategies and compositional homeostasis (Litchman and Klausmeier, 2008; Montechiaro and Giordano, 2010). Under certain environmental stressors, e.g., variations or limitations of light or nutrients, microalgae adapt to their habitat through metabolic adjustment, while keeping cellular carbon, nitrogen, and phosphorus within an acceptable range (Sterner and Elser, 2002; Klausmeier et al., 2008). For example, increases in light intensity and elevated environmental CO2 might potentially regulate the rate and activities of photosynthesis, which would result in changes in the cellular C/N and C/P ratios (Dickman et al., 2006; Ayata et al., 2014). Under phosphorus limitation, most microalgae species have the capability for luxury uptake of phosphorus, storing surplus phosphorus as polyphosphate (PolyP) in the cellular phosphorus pool to relieve the stress of extracellular phosphorus deficiency (Eixler et al., 2006). Recent research has indicated that increases in temperature and exposure to lower light might have positive effects on microalgae, enhancing their luxury P uptake (Powell et al., 2008, 2009). However, the effect of turbulent mixing fields on the plasticity of microalgae adaptation, in terms of their phosphorus storage, has not been well reported.
Aphanizomenon flos-aquae, a bloom-forming harmful species of cyanobacteria is frequently detected in lakes and reservoirs (Padisák et al., 1999). To control A. flos-aquae blooms and prevent the potential of releasing cyan-toxins, increase in turbulence mixing of the water column was normally used as a feasible approach to break thermal stratification and to dilute algal biomass and nutrient concentrations. However, evidence showed regulating flushing rates might not prevent A. flos-aquae development, although total algal biomass (or biovolume) would decrease by flushing as reported by Padisák et al. (1999). In addition, such phenomenon on other species of cyanobacteria has also been reported in recent years (Elliott, 2010). The lack of information on the eco-physiological response of A. flos-aquae to small scale turbulence seemed to be a major barrier on evaluating the effectiveness to control A. flos-aquae blooms by flushing. In the present study, we investigated the effect of smallscale turbulence on phosphorus storage and stoichiometry of A. flos-aquae. Batch experiments were carried out to investigate changes in cellular PolyP and total cellular C, N, and P of A. flos-aquae under different turbulence mixing. Adaptation strategies of A. flos-aquae and their mechanisms under small-scale turbulence are then discussed.
2 MATERIAL AND METHOD 2.1 Cultivation and experimental conditionsA. flos-aquae (FACHB-1209), initially isolated from Lake Dianchi in Yunnan Province in China, was obtained from the Freshwater Algae Culture Collection at the Institute of Hydrobiology, Chinese Academy of Science. It was grown and enriched in BG-11 medium with a phosphate concentration of 2 mg/L at room temperature (22±1℃), and at an irradiance (photosynthetically available radiation, PAR) of 25 μmol/(m2·s), supplied by cool-white fluorescent light from a horizontal direction with a dark-light ratio of 12 h:12 h. The PAR was measured by a Li-190SA sensor (LI-COR, Lincoln, NE, USA). Although a filamentous microorganism frequently detected in natural water bodies, A. flos-aquae (FACHB-1209) was unicellular in the present study due to isolation and long-term laboratory culturing. Morphological change of A. flos-aquae in responding to turbulence is not discussed in the present study. After enrichment, A. flos-aquae were then incubated in P free BG-11 medium for a period of 10 days before the growing experiment.
A 1-L beaker was used as the reactor in the experiment. A self-designed impeller was located at the center of the beaker. Geometric dimensions are shown in Fig. 1. The liquid height was 113 mm. Continuous stirring was performed at the start of the growing experiment at rotation speeds of 100, 200, 300, and 400 r/min. An additional treatment without a stirrer and in a still condition (stagnant water) was used as the control.
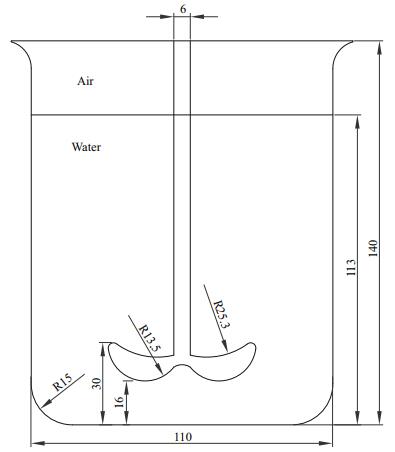
|
| Figure 1 Geometric dimensions of the reactor (unit: mm) |
A two-phase flow Computational Fluid Dynamics (CFD) model was used to simulate hydrodynamics in the reactor under different rotation speed. The whole reactor was considered as the computational domain and was discretized using unstructured grids, where finer grids were performed in the impeller and shaft regions (maximum size=1 mm). About 1 000 000 total computational grids (maximum size=2 mm) were created using the tetra mesh option of ANSYS ICEM CFD (ANSYS Inc.) in order to get the grid independent solution for the flow. The model was validated by a commercial 2D particle image velocimetry (PIV) system (ILA, German). Detail information on CFD modeling and its validation is reported in supplementary material. Table 1 shows the integrated results of turbulence mixing rate and Kolmogorov length microscale in different levels of rotation speed.
The growing experiment in the reactors was inoculated with pre-treated A. flos-aquae. A haemocytometer was used to count cells. Sampling was undertaken in the middle of the day during the experiment. The initial cell abundance in the reactor was 7.90×105 cells/mL. The experiment was carried out with BG-11 medium within a period of 7 days after inoculation. The initial concentration level of soluble reactive phosphorus (SRP) in the medium was set to 2 mg/L. At the end of this 7-day experiment, the SRP was greater than 0.4 mg/L in all treatments, which supported the inference that algae was not limited by P during the experiment. We compared cellular stoichiometry and PolyP in response to different levels of turbulent mixing when A. flosaquae was in the exponential growth phase (the 3rd day). As algal cells could settle down and accumulate at the bottom of the reactor in the control treatment, a sudden but mild manual shake was provided prior to taking samples. The test was run in triplicate for two weeks under the conditions described above, and sampled every 2 days for the determination of varying parameters, described as below.
2.2 Biological and chemical analysisCells in the reactor were counted every day under a microscope (Olympus CX 43, Japan) using appropriate magnification and a hemocytometer. Growth rate was measured in the exponential growth phase, according to Guillard (1973): μ=ln (x2/x1)/(t2– t1), where x2 and x1 are the cell densities at sampling day of t2 and t1, respectively. In vivo chlorophyll fluorescence was measured by PHYTO-PAM (Walz®, Effeltrich, Germany). The maximum effective quantum yield of PSII was evaluated by Fv/Fm=(Fm-F0)/Fm (Maxwell and Johnson, 2000), where Fv is the difference between F m and F0, which are the maximum and minimum fluorescence of the dark adapted stage of PSII.
To determine cellular C, N, and P, samples were passed through dried filters (GF/F, pre-combusted at 450℃, 4 h, Waterman, London, UK). Each filter was split for analysis of C, N, and total cellular phosphorus (TCP). Cellular C and N were measured with a CNHOS-elemental analyzer (Elementar® vario EL cube, Hanau, Germany). Samples for TCP were diluted with distilled water, and the TCP was determined according to Parkinson and Allen (1975) and further developed by O'Halloran and CadeMenun (2008). Cellular PolyP was determined according to the method developed by Eixler et al. (2005). Samples for the measurement of chlorophyll a were extracted from samples by 95% (v/v) ethanol for 24 h, and determined by spectrophotometer (Wintermans and De Mots, 1965).
2.3 Data analysisData were logged into SPSS (IBM, Armonk, NY, USA) or Origin (OriginLab, Northampton, MA, USA) software for statistical analysis, e.g., analysis of variance (ANOVA). Data in this study are presented as means±standard deviation.
3 RESULT 3.1 Growth rate and photosynthetic activityThere was a significant effect of turbulent mixing on growth rates of A. flos-aquae during the exponential growth phase (ANOVA, P≤0.05). Minimum growth rates were recorded in the control (non-turbulent) treatment, when compared with all other turbulent mixing treatments. A significant increase in algal growth rate was evident in response to increased turbulent mixing, up to a certain level. At a turbulent dissipation rate of 0.001 51 m2/s3, the growth rate of A. flos-aquae was approximately 2.8 times greater than the control. However, continuous increase in turbulent mixing did not significantly affect the growth rate of A. flos-aquae when the turbulent dissipation rate was at the range from 0.001 51 m2/s3 to 0.006 63 m2/s3 (ANOVA, P > 0.05). At a turbulent mixing rate of 0.022 59 m2/s3, there was a slight decrease in growth rates, which was followed by a significant decrease when the turbulent dissipation rate was increased to 0.050 58 m2/s3 (ANOVA, P≤0.05) (Fig. 2a).
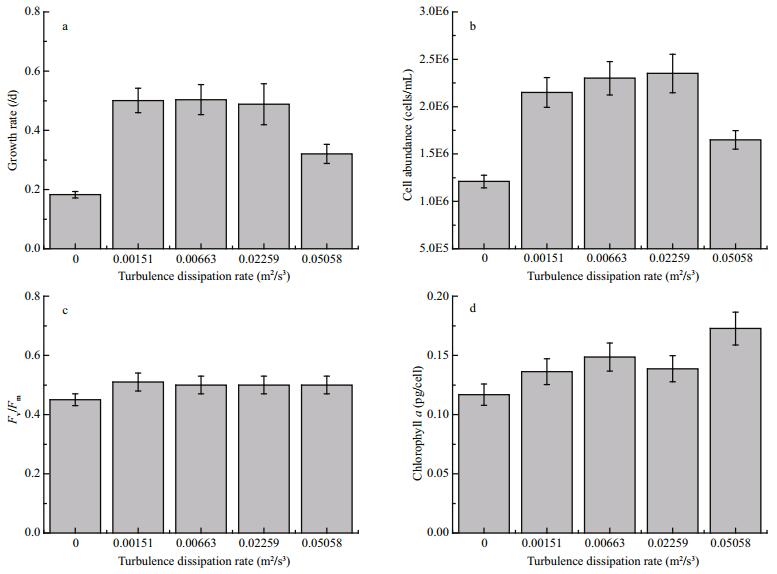
|
| Figure 2 Changes in growth rate (a), cell abundance (b), ratio of Fv/Fm (c), cellular chlorophyll a (d), and under different turbulent dissipation rates during the exponential growth phase |
Similar to growth rates, there were significant variations in cell abundance under different turbulent dissipation rates. The maximum cell abundance in the reactors was (2.35±0.20)×106 cells/mL at a turbulent dissipation rate of 0.022 59 m2/s3. However, biomass parameters, i.e., growth rate and cell abundance, did not differ significantly between the turbulent dissipation rates of 0.006 63 m2/s3 and 0.022 59 m2/s3 (ANOVA, P > 0.05) (Fig. 2b). Increasing the turbulent dissipation rate from 0.022 59 m2/s3 to 0.050 58 m2/s3, led to an apparent change in the biomass parameters during the exponential growth phase of A. flos-aquae.
The ratio of Fv/Fm did not change significantly among different levels of turbulent mixing (ANOVA, P > 0.05) (Fig. 2c), except for the relatively low value of Fv/Fm recorded in the control treatment (ANOVA, P≤0.05). Cellular chlorophyll a was relatively high at higher turbulent dissipation rates, probably owing to the reduction in cell abundance at the highest turbulent dissipation rates (Fig. 2d). Furthermore, the time series data of Fv/Fm during the whole experiment did not change significantly among different levels of turbulent mixing intensity (not shown in the present study). This partially supports the inference that turbulent mixing might not affect the maximum effective quantum yield of PSII.
3.2 Cellular stoichiometry and PolyPChanges in cellular C, N, and P in response to different turbulent dissipation rates in the exponential growth phase are shown in Fig. 3. There were significant differences in cellular C, N, and P among different levels of turbulent mixing (ANOVA, P≤0.05). There was no significant difference in cellular C between the control treatment and the turbulent dissipation rate of 0.001 51 m2/s3 (t-test, P > 0.05). In contrast, there was a 33.8% increase in N and 48.8% decrease in P between the control and aforementioned turbulent dissipation rate.
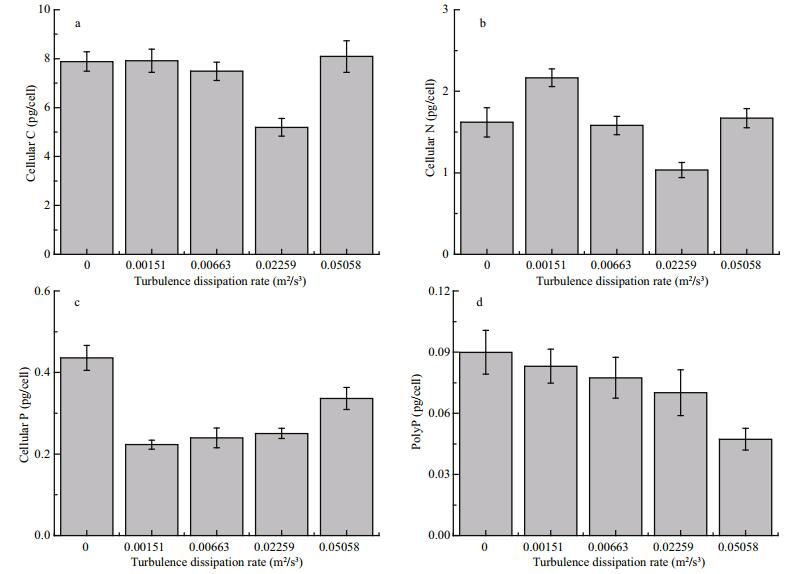
|
| Figure 3 Cellular C (a), N (b), P (c), and PolyP (d) in Aphanizomenon flos-aquae under different turbulent dissipation rates |
Surprisingly, we found that cellular C (-30.6%) and N (-34.5%) were significantly lower at a turbulent dissipation rate of 0.022 59 m2/s3 compared with 0.006 63 m2/s3 or higher. As the turbulent dissipation rate increased to 0.050 58 m2/s3, there were significant increases in the cellular C (+55.7%) and N (+61.4%).
Changes in molar ratios of C/N, N/P, and C/P, and the mass ratio of Chl a/C and PolyP/P are shown in Table 2. Results from our experiment showed plasticity of A. flos-aquae in response to small-scale turbulence. There was an increase in both the N/P and C/P ratios as turbulent mixing increased, compared with the control treatment. However, the C/N ratio was higher in the control treatment than at low levels of turbulent mixing. Variations in the N/P and C/P ratios were much larger (Fig. 3) than the C/N ratio, in response to different levels of turbulent mixing. Reductions in cellular C and N resulted in decreases in N/P and C/P ratios at a turbulent dissipation rate of 0.022 59 m2/s3, compared with the control. Variations in the Chl a/C ratio were similar to the C/N ratio. Maximum C/N and Chl a/C ratios were recorded at the turbulent dissipation rate of 0.022 59 m2/s3. Furthermore, a continuous decrease in PolyP with an increase in turbulent dissipation rate was also recorded.

|
Over the past two decades, the effects of smallscale turbulence on the physiology of microalgae have been confirmed, although different species of microalgae may exhibit different physiological response. Turbulence mixing keep microalgae suspended in the upper layer of the water column, which benefits their light requirements (Reynolds, 2006). For example, diatoms require turbulence to remain at the surface of the water column, which can then trigger blooms (Reynolds, 2006). However, higher intensities of turbulent mixing, e.g., shear stress between 1 pa and 1.3 pa (Michels et al., 2010), cause adverse effects on the viability of microalgal cells (Hondzo and Lyn, 1999).
In our study, turbulent mixing on the Kolmogorov length microscale was at the higher range of turbulence in natural aquatic ecosystems, where the normal range of the Kolmogorov length microscale is approximately 0.6–3.0 mm (Reynolds, 2006). At this range of turbulent mixing, the changes in maximum effective quantum yield of PSII (measured by the ratio of Fv /Fm) and cellular chlorophyll a in the exponential phase support the hypothesis that the photosynthetic activity of A. flos-aquae significantly increases when compared with stagnant conditions. But in the gradient of turbulence mixing, change in maximum effective quantum yield of PSII and cellular chlorophyll a differed with growth rate of A. flosaquae. Similar results were also discussed by Thomas et al. (1995), whose research indicated that at saturating irradiances, photosynthesis was less sensitive than growth to changes in turbulence mixing. The photosynthetic apparatus of microalgae showed little or no disruption in response to turbulence (Thomas et al., 1995).
As the maximum effective quantum yield of PSII and formation of chlorophyll a in cells are primarily driven by light intensity, which, according to the light nutrient hypothesis, accesses every single cell in the system in a nutrient-rich environment (Sterner and Elser, 2002; Dickman et al., 2006), our study results (see Section 3.1) further provides evidence that there were no significant differences in light accessibility among different treatments in the experiment. The potential indirect effects of light regime under the gradient of turbulent mixing, e.g. self-shading effect due to increase in population in the reactors, could be ignored with caution. Nevertheless, stronger evidence is required for further study.
As a product of photosynthesis, cellular C decreased at a turbulent dissipation rate of 0.022 59 m2/s3 and significantly increased at higher turbulent dissipation rates. This led to a unimodal response of Chl a/C, indicating that the capability of C synthesis was lowest at the turbulent dissipation rate of 0.022 59 m2/s3. Photosynthetic efficiency in terms of Chl a/C ratio (Thomas et al., 1995; Halsey et al., 2014) might be recovered at higher intensities of turbulent mixing. Research by Thomas et al. (1995) showed both photosynthetic efficiency and respiration rates increased with turbulence, which partially supports our results. However, recent research by Leupold et al. (2013) showed that increase in the shear stress under a certain range had a stimulating effect on photosynthesis activity within microalgae cells. To some extent, we may inference that the different results might be partially due to the range of turbulent mixing intensities served to different species of microalgae in different studies. Further evidence is required.
Nutrient uptake is lowest in stagnant condition in a green alga, but increases with turbulent mixing (Warnaars and Hondzo, 2006). In our study, under turbulent mixing, cellular N and C of A. flos-aquae showed approximately the same responses except for N at 0.001 51 m2/s3 which increased whereas C did not. Unlike cellular C and N, P uptake and assimilation in cells did not show a unimodal response. Formation and storage of PolyP is commonly regarded as a nutritional strategy of microalgae when encountering environmental stress (Eixler et al., 2006). It is interesting that cellular P increased with an increase in turbulent mixing relative to 0.001 51 m2/s3, but concentrations of PolyP and the relative abundance of cellular P showed a continuously decreasing trend. However, due to the batch culture, the phosphorus concentration in the medium decreased rapidly in the turbulence treatment, and it seems that A. flos-aquae was under P-limitation conditions in the mid and late stages of the experiment. In comparison, the P concentration in the control treatment (stagant water) was sufficient even at the end of the test (data were not shown). We inferred that extracellular Pideficiency may result in a rapid decline of cellular P storage, which was consistent with the result of Tripathi et al. (2013). Therefore, the cellular P of A. flos-aquae with turbulence treatment may lower than the control. On the other hand, as the respiration rates of microalgae might increase with turbulence (Thomas et al., 1995), the low cellular P under turbulence treatment at least partially involved decreased metabolic costs of phosphorus, so a possible explanation for this was that PolyP was used by the cells for synthesis of cellular constituents at a rate that exceeded replenishment (Powell et al., 2008). As the respiration rate was hypothesized to be increased with the increase of turbulence mixing (Thomas et al., 1995), we further inferred that reallocation of cellular P could be the response to the increased respiration rates (Halsey and Jones, 2015).
Previous studies (Karp-Boss et al., 1996; Warnaars and Hondzo, 2006; Hondzo and Wüest, 2009) support the hypothesis that turbulent mixing regulates the thickness of the cellular surface diffusive sublayer, affecting cellular nutrient fluxes and finally the physiology of microalgae. From this point of view, it could be further inferred that the direct physical impacts of small-scale turbulent mixing might be unbiased in the acquisition of nutrients, e.g., C, N, and P, as their diffusive coefficients through the diffusive sublayer are uniformly driven by physical forces (e.g., shear stress, from small-scale turbulence). If the hypothesis is well tested and verfied, the cellular stoichiometry of C, N, and P might not vary in response to small-scale turbulent mixing. Although the limit of our current study cannot provide further evidence to fight the above hypothesis, the asynchronous nature of cell stoichiometry and the relative stability of photosynthetic activity under turbulent mixing provide us with a different scenario of the physiological adapation of microalgae under turbulent conditions.
5 CONCLUSIONWithin a certain range, turbulent mixing stimulates the growth of Aphanizomenon flos-aquae, with an inhibitory effect evident at higher mixing intensities. The photosynthetic activity of A. flos-aquae was not affected by turbulent mixing, whereas both the growth rate and the efficiency of photosynthesis showed a unimodal response to increased turbulence. Increases in turbulent mixing might stimulate respiration rates, which might lead to the use of PolyP for the synthesis of cellular constituents. More research is required to test and verify the hypothesis that turbulent mixing changes the diffusive sublayer, regulating the nutrient flux of cells.
| Ayata S D, Lévy M, Aumont O, Resplandy L, Tagliabue A, Sciandra A, Bernard O, 2014. Phytoplankton plasticity drives large variability in carbon fixation efficiency. Geophys. Res. Lett., 41(24): 8994–9000. Doi: 10.1002/2014GL062249 |
| Dickman E M, Vanni M J, Horgan M J, 2006. Interactive effects of light and nutrients on phytoplankton stoichiometry. Oecologia, 149(4): 676–689. Doi: 10.1007/s00442-006-0473-5 |
| Ebert U, Arrayás M, Temme N, Sommeijer B, Huisman J, 2001. Critical conditions for phytoplankton blooms. Bull.Math. Biol., 63(6): 1095–1124. Doi: 10.1006/bulm.2001.0261 |
| Eixler S, Karsten U, Selig U, 2006. Phosphorus storage in Chlorella vulgaris (Trebouxiophyceae, Chlorophyta) cells and its dependence on phosphate supply. Phycologia, 45(1): 53–60. Doi: 10.2216/04-79.1 |
| Eixler S, Selig U, Karsten U, 2005. Extraction and detection methods for polyphosphate storage in autotrophic planktonic organisms. Hydrobiologia, 533(1-3): 135–143. Doi: 10.1007/s10750-004-2406-9 |
| Elliott J A, 2010. The seasonal sensitivity of Cyanobacteria and other phytoplankton to changes in flushing rate and water temperature. Global Change Biol., 16(2): 864–876. Doi: 10.1111/gcb.2010.16.issue-2 |
| Estrada M, Berdalet E, 1997. Phytoplankton in a turbulent world. Scientia Marina, 61(S1): 125–140. |
| Gallardo Rodríguez J J, Sánchez Mirón A, García Camacho F, Cerón García M C, Belarbi E H, Chisti Y, Molina Grima E, 2009. Causes of shear sensitivity of the toxic dinoflagellate Protoceratium reticulatum. Biotechnology Progress, 25(3): 792–800. Doi: 10.1002/btpr.v25:3 |
| Guillard R R L. 1973. Division rates. In: Stein J R ed. Handbook of Phycological Methods. I. Culture Methods and Growth Measurements. Cambridge University Press, Cambridge. p. 289-312. |
| Halsey K H, Jones B M, 2015. Phytoplankton strategies for photosynthetic energy allocation. Annu. Rev. Mar. Sci., 7(1): 265–297. Doi: 10.1146/annurev-marine-010814-015813 |
| Halsey K H, Milligan A J, Behrenfeld M J, 2014. Contrasting strategies of photosynthetic energy utilization drive lifestyle strategies in ecologically important picoeukaryotes. Metabolites, 4(2): 260–280. Doi: 10.3390/metabo4020260 |
| Hondzo M, Lyn D, 1999. Quantified small-scale turbulence inhibits the growth of a green alga. Freshwater Biology, 41(1): 51–61. Doi: 10.1046/j.1365-2427.1999.00389.x |
| Hondzo M, Wüest A, 2009. Do Microscopic organisms feel turbulent flows? Environ. Sci. Technol., 43(3): 764–768. Doi: 10.1021/es801655p |
| Huisman J, Arrayás M, Ebert U, Sommeijer B, 2002. How do sinking phytoplankton species manage to persist? Am. Nat., 159(3): 245–254. Doi: 10.1086/338511 |
| Karp-Boss L, Boss E, Jumars P A, 1996. Nutrient fluxes to planktonic osmotrophs in the presence of fluid motion. Oceanogr. Mar. Biol. Annu. Rev, 34: 71–107. |
| Klausmeier C A, Litchman E, Daufresne T, Levin S A, 2008. Phytoplankton stoichiometry. Ecol. Res., 23(3): 479–485. Doi: 10.1007/s11284-008-0470-8 |
| Leupold M, Hindersin S, Gust G, Kerner M, Hanelt D, 2013. Influence of mixing and shear stress on Chlorella vulgaris, Scenedesmus obliquus, and Chlamydomonas reinhardtii. J. Appl. Phycol., 25(2): 485–495. Doi: 10.1007/s10811-012-9882-5 |
| Litchman E, Klausmeier C A, 2008. Trait-based community ecology of phytoplankton. Ann. Rev. Ecol. Evol. Syst., 39(1): 615–639. Doi: 10.1146/annurev.ecolsys.39.110707.173549 |
| Maxwell K, Johnson G N, 2000. Chlorophyll fluorescence-a practical guide. J. Exp. Bot., 51(345): 659–668. Doi: 10.1093/jexbot/51.345.659 |
| Michels M H A, van der Goot A J, Norsker N H, Wijffels R H, 2010. Effects of shear stress on the microalgae Chaetoceros muelleri. Bioproc. Biosyst. Eng., 33(8): 921–927. Doi: 10.1007/s00449-010-0415-9 |
| Montechiaro F, Giordano M, 2010. Compositional homeostasis of the dinoflagellate Protoceratium reticulatum grown at three different pCO2. J. Plant Physiol., 167(2): 110–113. Doi: 10.1016/j.jplph.2009.07.013 |
| O'Halloran I P, Cade-Menun B J. 2008. Total and organic phosphorus. In: Carter M R, Gregorich E G eds. Soil Sampling and Methods of Analysis. 2nd edn. CRC Press, Boca Raton. |
| Padisák J, Köhler J, Hoeg S. 1999. Effect of changing flushing rates on development of late summer Aphanizomenon and Microcystis populations in a shallow lake, Müggelsee, Berlin, Germany. In: Tundisi J G, Straškraba M eds. Theoretical Reservoir Ecology and Its Applications. Backhuys Publishers, Leiden. p. 411-424. |
| Parkinson J A, Allen S E, 1975. A wet oxidation procedure suitable for the determination of nitrogen and mineral nutrients in biological material. Commun. Soil Sci. Plant Anal., 6(1): 1–11. Doi: 10.1080/00103627509366539 |
| Powell N, Shilton A N, Pratt S, Chisti Y, 2008. Factors influencing luxury uptake of phosphorus by microalgae in waste stabilization ponds. Environ. Sci. Technol., 42(16): 5958–5962. Doi: 10.1021/es703118s |
| Powell N, Shilton A, Chisti Y, Pratt S, 2009. Towards a luxury uptake process via microalgae-defining the polyphosphate dynamics. Water Res., 43(17): 4207–4213. Doi: 10.1016/j.watres.2009.06.011 |
| Reynolds C S, 2006. The Ecology of Phytoplankton. Cambridge University Press, Cambridge. |
| Rothschild B J, Osborn T R, 1988. Small-scale turbulence and plankton contact rates. J. Plankton Res., 10(3): 465–474. Doi: 10.1093/plankt/10.3.465 |
| Sterner R W, Elser J J, 2002. Ecological Stoichiometry:The Biology of Elements from Molecules to the Biosphere. Princeton University Press, Princeton. |
| Thomas W H, Gibson C H, 1990. Effects of small-scale turbulence on microalgae. J. Appl. Phycol., 2(1): 71–77. Doi: 10.1007/BF02179771 |
| Thomas W H, Vernet M, Gibson C H, 1995. Effects of smallscale turbulence on photosynthesis, pigmentation, cell division, and cell size in the marine dinoflagellate Gomaulax polyedra (Dinophyceae). J. Phycol., 31(1): 50–59. Doi: 10.1111/j.0022-3646.1995.00050.x |
| Tripathi K, Sharma N K, Kageyama H, Takabe T, Rai A K, 2013. Physiological, biochemical and molecular responses of the halophilic cyanobacterium Aphanothece halophytica to Pi-deficiency. Eur. J. Phycol., 48(4): 461–473. Doi: 10.1080/09670262.2013.859303 |
| Warnaars T A, Hondzo M, 2006. Small-scale fluid motion mediates growth and nutrient uptake of Selenastrum capricornutum. Freshwater Biology, 51(6): 999–1. Doi: 10.1111/fwb.2006.51.issue-6 |
| Wintermans J F G M, De Mots A, 1965. Spectrophotometric characteristics of chlorophylls a and b and their phenophytins in ethanol. Biochimica et Biophysica Acta(BBA)-Biophysics including Photosynthesis, 109(2): 448–453. Doi: 10.1016/0926-6585(65)90170-6 |
 2017, Vol. 35
2017, Vol. 35



