Institute of Oceanology, Chinese Academy of Sciences
Article Information
- WU Xiaodong(吴晓东), LI Wenchao(李文朝), PAN Jizheng(潘继征), MA Shuzhan(马书占), CHEN Bingfa(陈丙法), HE Shangwei(何尚卫)
- Restoration in northern Lake Gehu, a eutrophic lake in China
- Chinese Journal of Oceanology and Limnology, 35(6): 1417-1431
- http://dx.doi.org/10.1007/s00343-017-6107-4
Article History
- Received Apr. 11, 2016
- accepted in principle May. 27, 2016
- accepted for publication Sep. 12, 2016
2 University of Chinese Academy of Sciences, Beijing 100049, China;
3 School of Environmental Science and Engineering, Suzhou University of Science and Technology, Suzhou 215011, China;
4 Yancheng Academy of Environmental Protection Technology and Engineering, Nanjing University, Yancheng 224000, China
Lake eutrophication is a global environmental issue (Dokulil et al., 2011). As increasing amounts of nutrients enter shallow lakes, transparency decreases, and submerged macrophytes die out. As a result, cyanobacteria blooms occur more frequently, which leads to a series of environmental issues, such as deterioration of water quality and ecological degradation (Chen et al., 2009, 2013). Submerged macrophytes are an important part of shallow lake ecosystems, and they play a key role in maintaining the clarity of lake water and biodiversity, as well as other ecological functions (Scheffer et al., 1993; Jeppesen et al., 1998; Van Zuidam and Peeters, 2015). The restoration of a eutrophic lake involves the transformation of its ecosystem from algae-to macrophyte-stable state by taking certain measures (Scheffer et al., 2001; Qin et al., 2014). To achieve this goal, many restoration measures have been widely developed and applied for lakes (Sas, 1989; Cook et al., 1993, 2005; Phillips et al., 1994; Jeppesen et al., 2005, 2012; Søndergaard et al., 2008; Qin et al., 2011; Chen et al., 2013).
Most shallow lakes along the middle and lower reaches of the Changjiang (Yangtze) River have been eutrophic or are eutrophying (Qin, 2002). Since the 1980s, many efforts have been made to restore submerged macrophytes and ecosystems. They have been carried out within local regions, and most have been implemented within enclosures. Ecological engineering projects have also been carried out to improve water quality, which include wave bands, enclosures and recovery zones of the aquatic plant, e.g., Lake Wuli (Chen et al., 2009) and the Meiliang Gulf in Lake Taihu (Pu, 1993; Qin et al., 2013). These small scale restoration tests on aquatic plants have been adopted to improve water quality to varying degrees because some aquatic plants have been successfully restored during restoration projects. However, because the enclosure in a test site is withdrawn after the project has finished, the restored aquatic plant soon disappears and water quality degrades (Qin et al., 2013). In addition to nutrient concentrations, submerged macrophyte restoration is affected by many factors, such as light intensity, temperature, sediment, water depth, wind wave, fish, and other factors, among which light intensity is the most important (Dennison et al., 1993; Scheffer, 1998; Bornette and Puijalon, 2011). Compared with small shallow lakes, large shallow lakes tend to have heavy wind wave, which makes restoration difficult (Gulati et al., 2008). Overall, the restoration of large shallow lakes in the subtropical zone is still a problem for lake managers. Moreover, whether or not the restoration of local areas within large shallow lakes should be a priority requires further discussion.
Lake Gehu is a major shallow lake in southeast China. In the last 20 years, with the development of industrialization and urbanization, the Lake Gehu ecosystem has been damaged. Since 2009, many measures have been carried out in northern Lake Gehu by local governments, including isolating the rivers that flow into the lake, dredging, terrain restoration, aquatic plant restoration, interception of algae by enclosures, mechanical removal of algae, and biomanipulation, among others. This research mainly discusses the feasibility of restoration for local areas of Lake Gehu, which can serve as a reference for the restoration of the entire lake and similar shallow lakes in the subtropical zone.
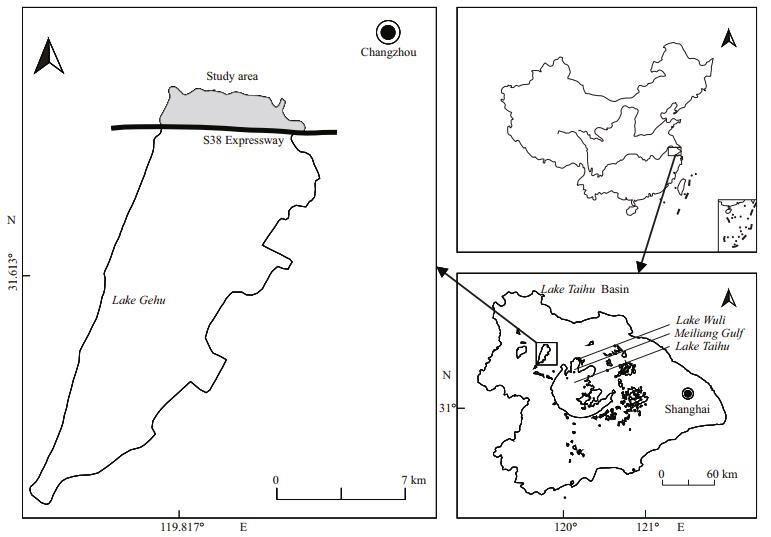
2 MATERIAL AND METHOD 2.1 Study areaLake Gehu is the second largest lake in the Lake Taihu Basin (Fig. 1). It plays a key role in protecting the water quality of Lake Taihu, forming the most important natural defense line for Lake Taihu (Xu et al., 2013). With an average depth of only 1.2 m and an overall length of 30 km from north to south, the lake covers a total area of 164 km2. Located in the upper reaches of Lake Taihu, Lake Gehu plays an important role in flood regulation and storage, water supply, and ecological regulation (Xu et al., 2013). In recent years, Lake Gehu's water quality has deteriorated. Not only has the self-purification capacity of its water body been diminished, but its aquatic plants have died out. However, submerged macrophytes have historically been widely distributed in Lake Gehu. Prior to 1998, submerged macrophyte coverage in Lake Gehu was very high, accounting for more than 80% of the total area. In 1986, coverage was 87.5%; in 1990, coverage was approximately 100%; in 1993, coverage was 93%; and in 1994, coverage was 95%. From 1999 to 2004, submerged macrophyte coverage decreased at a rate of 10% per year because it was highly affected by rapid algal reproduction. According to a survey in 2004, submerged macrophyte coverage was approximately 14%. In 2006, the submerged macrophytes in the lake region along the east coast were distributed in sheets, and coverage was less than 2%. By October of 2007, patches of submerged macrophytes had disappeared, and only small numbers were distributed in spots. Lake Gehu had transformed from a clear water state into a turbid water state (Yang et al., 2005). The study area mentioned in this paper is located in the north of the expressway, covering an area of 15.26 km2. The Biandan and Xiaxi Rivers flow into the lake. The total nitrogen (TN) imported by the two rivers accounts for 56.9% of the total input, and the total phosphorus (TP) imported by the two rivers accounts for 68.8% of the total input. Contamination is worst in the northern Lake Gehu, and this is where submerged macrophyte degeneration first began.
|
| Figure 1 Regional map of Lake Gehu and its location in the Taihu Lake Basin The region north of the expressway is regarded as the restoration region (hereafter referred to as Zone A), and the region south of the expressway is regarded as the control region (hereafter referred to as Zone B). |
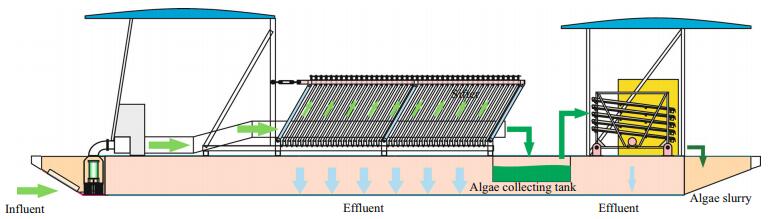
In 2008, sediment was used to build a weir to construct the expressway (S38), dividing Lake Gehu in two. There are only three exits within the northern and southern regions, all located under the bridge with 115 m, 30 m and 100 m in width separately. From 2009 to 2011, the rivers flowing into the lake were dredged for 13.6 km, and sediment in Zone A was also dredged. After drained, Zone A was removed of heavily polluted sediment on its surface. From 2010 to 2011, by reconstructing subaqueous relief, several artificial islands were built. In 2011, an ecological revetment, 14.5 km in length, was built in Zone A. Ecological revetment is covered with wetland plants and herbs. In 2012, because the rivers emptying into the lake were diverted, a large volume of water from the Xiaxi and Biandan Rivers flowed into the region south of the expressway, whereas the water flowing into Zone A was reduced. In the spring of 2012, emergent plants were planted in these artificial shallows (water depth are less than 1 m), including Phragmites australis, Zizania latifolia and Typha orientalis. In the winter of 2012, Potamogeton crispus L. turions were introduced by broadcast sowing in artificial shallows near S38 Expressway. In 2012, a pre-reservoir system was built on the lake inlet of Zone A. In July 2012, enclosures were set at the three exits between Zones A and B to block algae in the region south of the expressway. In May–October 2013 and 2014, large mechanical equipment for cyanobacteria removal was used to refloat phytoplankton (Fig. 2). For physical filtration interception, large mechanical equipment for cyanobacteria removal was used to bring algae-laden surface water into a sifter from the upper end of the filter, and the phytoplankton was held at the sifter and entered an algae collecting tank. Five sets of cyanobacteria removal equipment were used to remove blue-green algae in Zone A from May to October each year, with a water handling capacity of 500 m3 per hour. From 2012 to 2014, 10 million silver carp (Hypophthalmichthys molitrix) and bighead carp (H. nobilis) were stocked in Zone A.
|
| Figure 2 Diagram of large mechanical equipment for cyanobacteria removal |
Water samples were collected monthly from 2007 to 2014. There were only two sampling points (1, 6) before 2010 in Zone A, and three in Zone B. After 2011, four sampling points (2, 3, 4, and 5) were added in Zone A (Fig. 3). The water chemistry and phytoplankton samples were collected at a 0.5-m depth below the water surface. Nutrient analysis was carried out on the water chemistry samples according to the Standard Method (APHA, 1980). The Secchi depth (SD) was measured using a Secchi disk with a diameter of 30 cm. Total suspended solids (TSS), inorganic suspended solids (ISS), and organic suspended solids (OSS) were measured by the gravimetric method (State Environmental Protection Administration, 2002). Pigments samples were extracted with 90% acetone at 4℃ in the dark for 24 h to measure chlorophyll a (chl a). The supernatant was centrifuged and light absorbance was measured by spectrophotometry against 90% acetone at 630, 645, 663, and 750 nm. The equations from Jeffrey and Humphrey (1975) were applied to calculate the chl a concentrations.
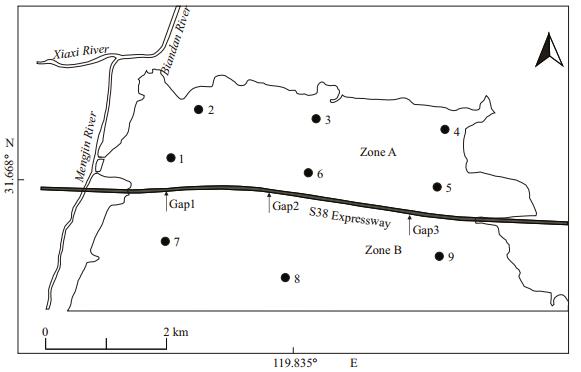
|
| Figure 3 Research site and sampling stations |
Photosynthetically Active Radiation (PAR) was measured and continuously recorded with an XR-620CTD (RBR Ltd., Canada) (Lin et al., 2009), the data of which was collected monthly from June in 2009 to August in 2010 and January in 2013 to December in 2014. Sampling locations are shown in Fig. 3. The reduction law of PAR in water with homogeneous optical properties conforms to the following (Keith et al., 2002; Yu et al., 2008; Lin et al., 2009):
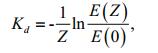 (1)
(1)where, Kd is the attenuation coefficient, Z is the depth between the lake surface and the measurement point, and E(Z) and E(0) are PAR intensity at point Z and at point 0 m, respectively. Kd was calculated by regression of the PAR intensity at different depths under the water surface (R2≥0.90, n≥5).
The euphotic depth (Zeu) is generally referred to as the depth at which the light intensity is 1% of the irradiancy of the water surface, and it can be estimated by the following equation (Zhang et al., 2005):
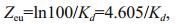 (2)
(2)where, Zeu=euphotic depth and Kd=attenuation coefficient. Phytoplankton samples were collected in 1 000-mL plastic bottles. Phytoplankton samples were fixed in acetic Lugol's solution and preserved with 5% formalin after sedimentation for 48 h. For determination of phytoplankton, the supernatant was removed and approximately 50 mL residue was collected (Chen et al., 2009). The cells, or thrums, in 1 mL of the concentrated samples were dispersed by ultrasonication. The phytoplankton in the concentrated samples were identified according to taxonomic identification keys based on cell size and formation (Hu and Wei, 2006) under a phase contrast microscope (Nikon, Japan). The TN concentrations in sediments were measured with semi-micro method, and TP with Mo-Sb colorimetry. A survey of the coverage and water depth distributions of aquatic plants was carried out in summer and winter from 2008 to 2014.
2.4 Statistical analysisSignificant differences between Zone A and Zone B, in terms of the average values in the time variations, were determined by independent-sample t-tests at P < 0.05. All statistical tests were carried out in SPSS 13.0 for Windows.
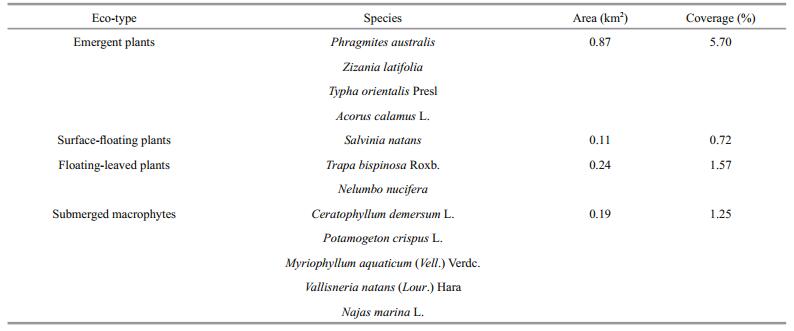
3 RESULT 3.1 Restoration of aquatic macrophytesAfter restoration, aquatic plant coverage in Zone A increased year on year, as did the number of aquatic plant species. According to the July 2013 survey, there were only four species of emergent plants in Zone A (P. australis and Z. latifolia were dominant), surface-floating plants including mainly Salvinia natans, floating-leaved plants including Trapa bispinosa Roxb. and Nelumbo nucifera (of which T. bispinosa Roxb. was dominant), and submerged macrophytes including Ceratophyllum demersum L., P. crispus L., Myriophyllum aquaticum (Vell.) Verdc., Vallisneria natans (Lour.) Hara and Najas marina L. (among which C. demersum and P. crispus L. were dominant) (Table 1). In 2013, the total aquatic plant area and coverage in Zone A were 1.41 km2 and 9.24%, respectively. The total aquatic plant area in Zone A in 2014 was 1.61 km2, which increased by 0.20 km2 compared with that in 2013. Although the aquatic plants were restored to some extent, submerged macrophyte coverage remained low, and it was mainly dominated by pollution-resistant species and located in shallow depths no more than 1.2 m.
Large mechanical equipment for cyanobacteria removal reduces phytoplankton biomass, nitrogen, and phosphorus, as well as organic matter in the water. According to field monitoring and simulation experiments, when TN and TP concentrations were greater than 3.56 and 0.40 mg/L, respectively, the removal rates were greater than 80% and 60%, respectively (Fig. 4a, b). When the range of chl a concentrations in inflowing water was between 23.12 and 7 336.01 μg/L and 6.71 and 43.76 μg/L in outflowing water, the removal rate was high (Fig. 4c). When the range of chl a concentrations in inflowing water was less than 30 μg/L, the range of the average chl a concentrations in outflowing water was only 10.87 μg/L, with a removal rate of approximately 58.1%. By nonlinear fitting the CODMn in inflowing water and the removal rate, the CODMn removal rate increased with increasing CODMn in water inflow. When the CODMnin inflowing water was > 19.00 mg/L, the removal rate was > 68% (Fig. 4d).
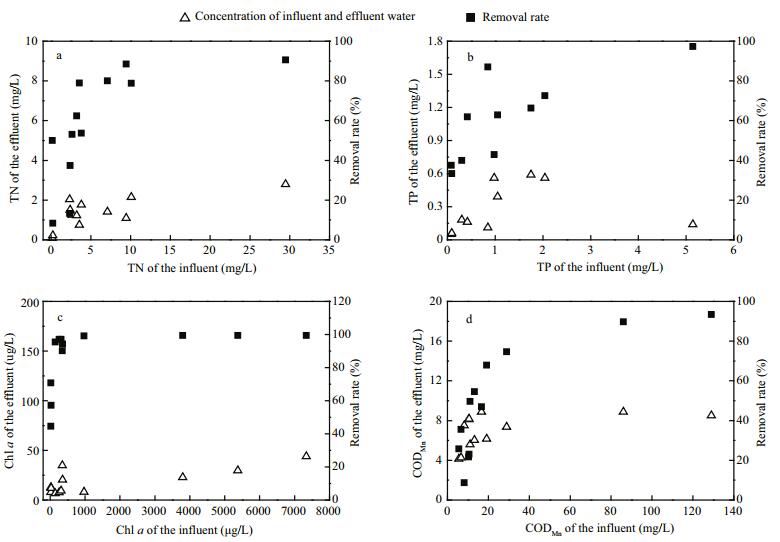
|
| Figure 4 The removal rate of TN, TP, chl a and CODMn by the large mechanical equipment for cyanobacteria removal |
After the restoration, pollutants flowing into Zone A area are greatly reduced. Take the data in 2012 for example, TN input was reduced to about 1 211.2 t from 3 460.7 t, TP input was reduced to about 128.8 t from 357.9 t, and CODMn input to about 1 837.7 t from 5 250.4 t with their outputs in Zone A being 580.3 t, 65.5 t and 745.2 t respectively.
Before restoration in 2008, mean TP concentration in Zones A and B reached (0.182±0.054) and (0.160±0.040) mg/L, respectively, that is, the mean TP concentration in Zone A was 1.14 times that in Zone B (Fig. 5). After dredging was finished in 2011, mean TP concentration in Zone A decreased to (0.109±0.017) mg/L. By 2014, the mean TP concentration in Zone A had decreased to (0.091±0.025) mg/L, which represents a highly significant (P < 0.01) decrease of 50% compared with that in 2008. In 2014, the mean TP concentration in Zone B was (0.146±0.031) mg/L, and in Zone A it was 62.3% of that in Zone B, this difference was highly significant (P < 0.01).
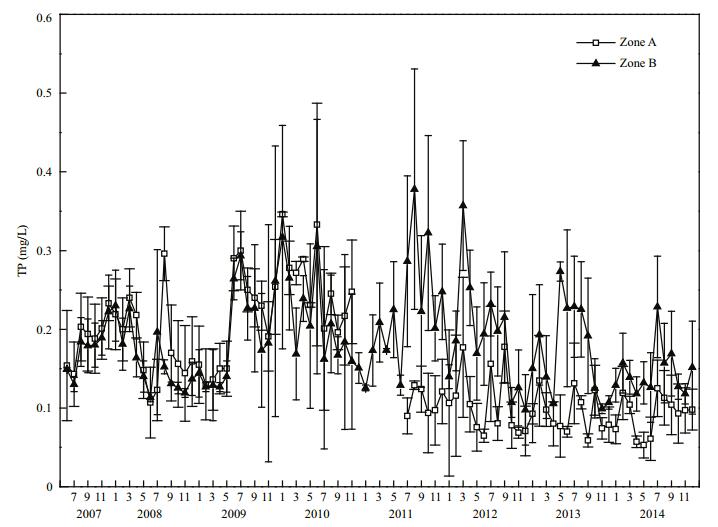
|
| Figure 5 TP variation in Zone A and Zone B |
Before restoration, the mean TN concentration in Zone A was (5.17±1.34) mg/L, and in Zone B it was (4.39±1.48) mg/L, thus, the mean TN concentration in Zone A was 1.18 times that in Zone B (Fig. 6). After dredging was finished in 2011, the mean TN concentration in Zone A decreased to (1.52±0.30) mg/L. By 2014, the mean TN concentration in Zone A had decreased significantly (P < 0.05) to (2.53±0.65) mg/L, representing a decrease of 42.4% compared with that in 2008. In 2014, the mean TN concentration in Zone B was (3.07±0.65) mg/L, and in Zone A it was 82.4% of that in Zone B, this difference was not significant (P > 0.05).
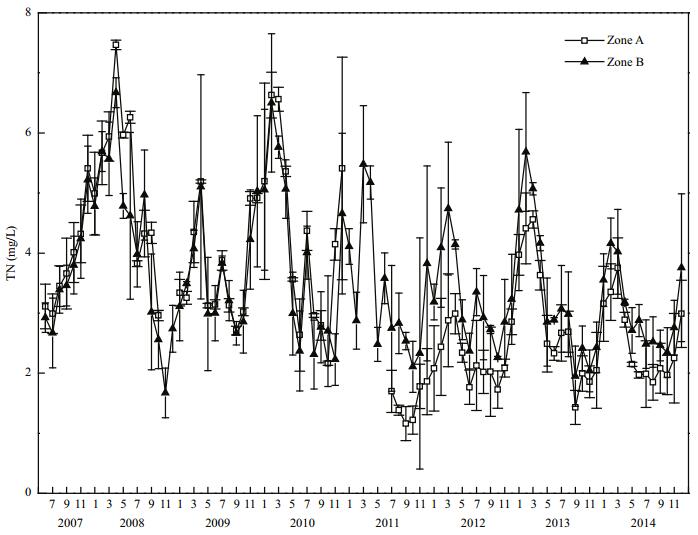
|
| Figure 6 TN variation in Zone A and Zone B |
The mean chemical oxygen demand (CODMn) in Zone A was (6.49±0.94) mg/L before restoration. By 2014, CODMn in Zone A had decreased to (3.84± 0.74) mg/L, which represents a decrease of 40.8% compared with that in 2008, this difference was highly significant (P < 0.01). In 2014, CODMn in Zone B was (4.37±0.54) mg/L, and in Zone A it was 87.9% of that in Zone B (Fig. 7).
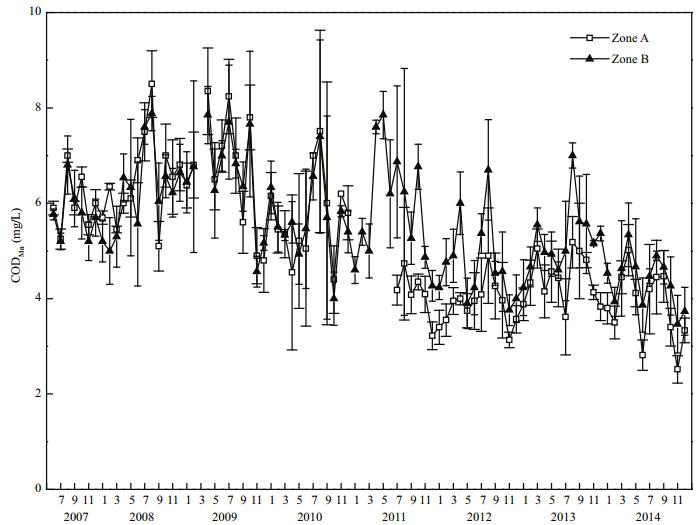
|
| Figure 7 CODMn variation in Zone A and Zone B |
In 2008, the mean SD in Zone A was (30.0± 10.1) cm, and that in Zone B was (32.3±6.4) cm. Thus, the mean SD in Zone B was very slightly higher than that of Zone A (Fig. 8). After the dredging was completed in 2011, the mean SD in Zone A increased to (40.7±5.0) cm. By 2014, the mean SD in Zone A had increased to (42.5±9.8) cm, which was 1.42 times that prior to restoration, and the difference between them was significant (P < 0.05). In 2014, the mean SD in Zone B was (30.4±6.1) cm, and the difference between Zones A and B was significant (P < 0.05).
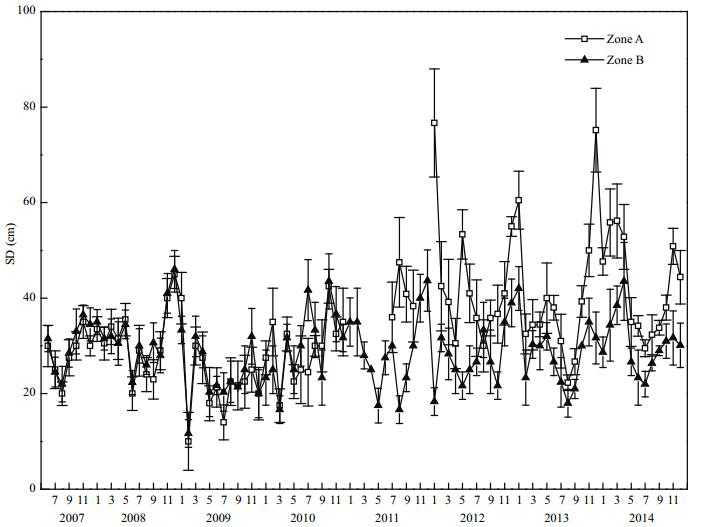
|
| Figure 8 Secchi depth variation in Zone A and Zone B |
Underwater light intensity in Zone A was appreciably improved by restoration (Fig. 9). In the summers of 2009 and 2010, the Zeu depth in Zone A tended to decrease from 110 to 91 m, but increased to 130 m in the summer of 2013, such that the underwater light intensity had improved significantly (P < 0.01). The Zeu depth in Zone B in the summer of 2013 decreased to 113 cm. In winter 2010, the Zeu depth in Zone A was higher than that of Zone B. By the winter of 2014, the Zeu depth in Zones A (223 cm) and B (131 cm) differed significantly (P < 0.05).
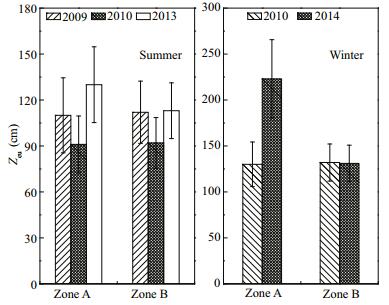
|
| Figure 9 Zeu depth variation in Zone A and Zone B in summer and winter |
The mean chl a concentration in Zone A decreased significantly (P < 0.01) from 34.8 to 20.2 μg/L from 2008 to 2014. Compared with that of Zone B, the mean chl a concentration in Zone A decreased by 22.1%. According to the algae concentration monitoring from 2008 to 2014, the variation in algae concentration was identical to that of chl a. In July 2009, the phytoplankton in Zone A was composed of Cyanophyta, Chlorophyta, and Bacillariophyta, among which Cyanophyta accounted for 98.8% of the total. Until July 2014, the phytoplankton in Zone A was composed of Cyanophyta, Chlorophyta, Bacillariophyta, Cryptophyta, Pyrrophyta, Euglenophyta, and Xanthophyta, among which Cyanophyta and Chlorophyta were overwhelmingly dominant. The number of phytoplankton phyla increased from three to six (Fig. 10). The average Shannon-Wiener index in Zone A increased from 1.7 to 2.2 from 2010 to 2014, representing an increase of 28.7%.
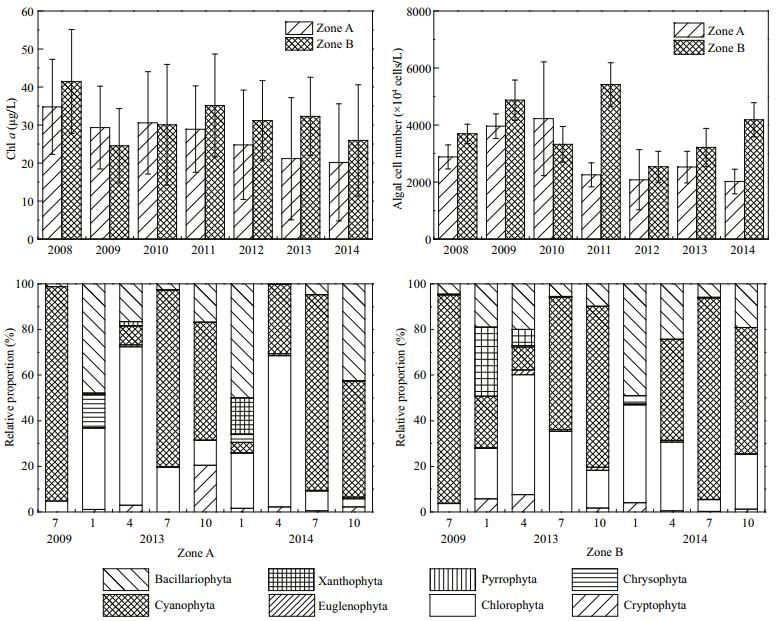
|
| Figure 10 Chl a, algal density, and relative proportion of phytoplankton in Zone A and Zone B |
Before dredging was carried out, TP concentrations in Zones A and B were 0.149 and 0.125 mg/g, respectively (Fig. 11). By 2014, after dredging had been carried out for 2 years, the TP concentration in Zone A decreased to 0.054 mg/g, representing a decrease of 0.095 mg/g. The TP concentration in the Zone A sediment before and after dredging differed significantly (P < 0.01). Before dredging, TN concentration in Zone A sediment was 2.69 mg/g, which was similar to that in Zone B sediment. After dredging was carried out, TN concentration in Zone A sediment decreased to 1.41 mg/g, which was very different from that before dredging (P < 0.01).
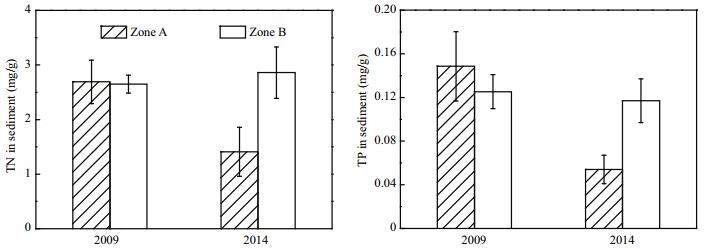
|
| Figure 11 TP and TN in surface sediments in Zone A and Zone B |
Wave action can be an important factor for plants in shallow, wind-exposed lakes even if the sediment is solid enough to prevent uprooting (Scheffer, 1998). Lake Gehu is a typical shallow lake in the subtropical zone. It is long and narrow, hence it has a long fetch. Under the effect of the southeast and northwest monsoons (typhoon and cold wave), wind waves can be generated downwind, which in turn decreases lake transparency. Phytoplankton concentrations can be reduced by controlling the level of nutrients in shallow lakes, but these lakes still have high turbidity. One important reason is that the sediment is resuspended by wind waves, and the concentration of suspended particles in the outlet increases as a result. Many other researchers have studied this phenomenon (Jackson and Starrett, 1959; Andersen and Lastein, 1981; Kristensen et al., 1992; James and Barko, 1994; Ekholm et al., 1997; Van Zuidam and Peeters, 2015). In Lake Apopka in Florida (USA), only 10% of the suspended particles live in frustules, and changes in chl a concentration do not tend to be identical to that of TSS. The phosphorus concentration decreased by 80%, and lake transparency increased from 0.23 to 0.34 m. However, the increase in light intensity was not sufficient for submerged macrophytes (Bachmann et al., 1999). But Lowe et al. (2001) think that the turbid state has been maintained by excessive P loading.during a period with no change in mean wind speeds measured at Lake Apopka.
Loss of aquatic plants can be caused by mechanical disturbance from wind waves, which influences plant reproduction by preventing them from being reestablished (Van Zuidam and Peeters, 2015). When the ecosystem of a grass-type lake is in an unstable state, a heavy stormy wave period is enough to transform the ecosystem from a grass-to an algae type. Lake Apopka had an abundance of submerged macrophytes. However, after a hurricane or storms, the aquatic plant community in that lake was damaged and the water gradually changed to a turbid algae state (Havens et al., 2001; James and Havens 2005; James et al., 2008; Jin et al., 2011; Beave et al., 2013). Hence, wave dissipation was the precondition and foundation for restoring aquatic plants. Many experiments have shown that aquatic plants can be restored by building enclosures to block out wave action, such as in Lake Breukeleveen (Netherlands) (Scheffer, 1998).
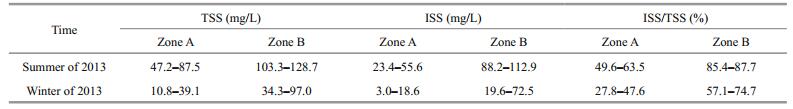
By separating, Zone A has formed a relatively independent lake region within Lake Gehu, and its widest part from north to south is only 2.25 km, whereas the widest part from east to west is 7.82 km. Because of the terrain modification in Zone A, several shallows formed there in the meantime, which has increased the lake's spatial heterogeneity. Because of this, the region's fetch has been shortened from 30 km to less than 1 km with stormy waves being significantly dampened. Bachmann et al. (1999) found that the non-algal source of TSS may come from resuspended sediment particles. In summer 2013, the mean ISS concentration differed significantly between Zones A ((35.0±9.2) mg/L) and B ((101.3±12.4) mg/L) (P < 0.05) (Table 2). In winter 2013, mean ISS concentration differed significantly between Zones A ((8.0±1.9) mg/L) and B ((38.4±6.3) mg/L) (P < 0.05). The ratio of ISS/TSS also differed significantly between Zones A and B both in summer and winter (P < 0.05). Overall, the wind waves in Zone A were attenuated, and the suspended solids also decreased appreciably, providing a relatively stable environment for restoring submerged macrophytes.
After Lake Gehu had been separated, a relatively enclosed region was formed in Zone A. Moreover, the water quality in Zone A had improved appreciably by isolating the rivers emptying into the lake, dredging, restoration of emergent aquatic plants, blocking algae by building enclosures, and removing algae by machines and biomanipulation. In 2014, the TP, TN, CODMn, and chl a concentrations in Zone A were significantly lower than those of 2008, when the restoration measures were carried out.
Rivers flowing into Lake Gehu are the main exogenous pollution sources. The amount of pollutants contributed by the Biandan and Xiaxi Rivers accounts for approximately 49.5% of the total input into Zone A (the data for 2010 had not been published at the time of writing). By diverting rivers flowing into the lake, the pollution load in Zone A decreased by approximately 64.2%. At the same time, the prereservoir was built in 2013 on the mouth of the river emptying into the lake, with a total area of 1.67 km2. The TP, TN, and CODMn removal rates in the river flowing into the lake decreased by 21.1%, 6.7%, and 17.9%, respectively, because of the pre-reservoir, which effectively reduced the pollution load in Zone A.
Dredging has been used to restore many eutrophic lakes (Moss et al., 1986). For example, approximately 70% percent of the mud sediment sand associated P were removed in Lake Okeechobee, but areas where the sediment thickness was < 10 cm dredging was considered too inefficient (James and Pollman, 2011). From 2009 to 2011, the river flowing into the lake was dredged for approximate 13.6 km, with an earth volume from desilting and excavation of approximate 2.267 million m3. As a result, the mean TP and TN concentrations in Zone A sediment decreased by 63.8% and 45.8%, respectively, indicating that the removal of sediment played an important role in decreasing nutrient concentrations in surface sediment.
Algal control measures in Zone A have included blocking phytoplankton by enclosures and refloating blue-green algae by applying large mechanical equipment for cyanobacteria removal and biomanipulation. In 2012, enclosures were set on three exits between Zones A and B to block phytoplankton drifting along with the southeast monsoon. Monitoring has shown that the enclosure effectively blocked phytoplankton in Zone B from entering Zone A, with an interception rate of approximately 58.4 %.
Biomanipulation has been applied to improve water quality in lakes and reservoirs (Hansson et al., 1998; Drenner and Hambright, 1999). Stocking of silver carp (H. molitrix) and bighead carp (H. nobilis) is an optional measure for algal control, which has been applied in different climate systems (Starling, 1993; Radke and Kahl, 2002; Xie, 2003) and in Lake Donghu and Lake Dianchi, China (Liu and Xie, 1999; Xie, 2003). From 2012 to 2014, approximately 10 million silver and bighead carp were stocked in Zone A. Because the water is open, the contribution to water quality by silver and bighead carp cannot be confirmed, but the improvement in water quality has been confirmed by many experiments (Chen et al., 1991; Xie and Liu, 2001; Liu et al., 2004; Ke et al., 2007).
Several restoration techniques have been adopted to promote regime shifts in lakes, but restoring lakes to their previous stable state has been practiced for far longer (Dokulil et al., 2011). After the restoration measures were carried out, the water quality in Zone A improved appreciably, but blue-green algae biomass levels are still high in summer, which suggests that the restoration of eutrophic lakes is characterized by hysteresis; other eutrophic lakes (such as Lake Taihu and Lake Wuli), along with the middle and lower reaches of the Changjiang River, exhibit the same characteristics. In the temperate zone, low nutrient levels in shallow lakes reached a new balance, where N and P phases were < 5 and < 10–15 years, respectively (Jeppesen et al., 2005). The restoration phases are longer for some lakes than others. Although TP decreased, the original restoration of the lake was delayed because of a high SRP concentration in the lake. Algal biomass only decreases when SRP concentration is reduced and finally phytoplankton community composition changes (Phillips, 2005). Scheffer et al. (1993) have reported that although nutrient levels decrease, high fish predation results in insufficient zooplankton grazing, which in turn leads to high phytoplankton production.
4.3 Probability of achieving a switch in stable statesLake ecosystems tend to have the capacity for spontaneous recovery against changes in the external environment. Only when the threshold needed to change the ecosystem becomes overwhelmed by external forces exerted, an appropriate system state can be formed to adapt to the new environmental conditions. (Scheffer et al., 2001). If the TP concentration decreases to 0.050–0.100 mg/L, submerged macrophytes in shallow lakes can switch from the turbid to the clear water state (Scheffer et al., 1993, 2001; Scheffer and Jeppesen, 1998). Long-term surveying and monitoring has shown that Lake Gehu is in a macrophyte-stable state, with a TP concentration of 0.030–0.094 mg/L. By 2014, the mean TP concentration in Zone A decreased to 0.091 mg/L. In terms of TP concentration, Zone A has met the requirements that confirm the switch, but there are some uncertainties. Although exogenous pollution has been controlled to a certain degree, the exogenous pollution load entering Zone A is still heavy, which is not good for further reduction of nutrient levels and restoration of the ecosystem. After dredging was carried out, the abundance of aquatic plant propagules in the sediment decreased, which is not good for the spontaneous restoration of aquatic vegetation. Moreover, the water depth increased after subaqueous relief was reconstructed. Under the existing conditions of transparency, the water depth in 70% of the total region has exceeded the euphotic depth, which does not meet the light intensity requirements for restoring submerged macrophytes.
In the process of eutrophic lake restoration, the precondition for rehabilitating submerged macrophytes is to improve their habitats. The choice of appropriate tolerant plant species is also important (Dong et al., 2014). To restore submerged macrophytes and reconstruct a clear-water stable state, relevant restoration measures have to be continuously carried out. The input of pollutants into the lake has to be reduced further, and the purifying capacity of the prereservoir in Zone A has to be improved. The area of pioneer species, including C. demersum and P. crispus L. must be enlarged to induce ecesis and submerged macrophyte reproduction. To improve the submerged macrophyte restoration rate, propagules have to be planted in the dredging region. In the early spring, underwater light intensity can be improved by decreasing the water level to provide favorable conditions for the submerged macrophytes propagules to sprout and the seedlings to grow.
5 CONCLUSIONAfter Lake Gehu was dissipated, a relatively enclosed region was formed in Zone A. Moreover, the water quality in Zone A improved considerably by isolating the rivers flowing into the lake, dredging, restoration of aquatic plants, blocking algae by building enclosures, and mechanical algae removal and biomanipulation. Until 2014, the TP, TN, CODMn, and chl a concentrations in Zone A significantly decreased compared with those in 2008, which was when restoration measurements were carried out. Transparency also improved significantly, which indicates that controlling eutrophication in Zone A by restoration achieved desirable results. However, some problems remain. To achieve the switch from an algae-to a macrophyte-stable state, further measures must be continuously carried out, such as controlling the pollutant load flowing into the lake, introducing submerged macrophytes, and water level regulation.
6 ACKNOWLEDGEMENTWe thank FAN Fan, GAO Ya and WANG Qing for their assistance in sampling.
| American Public Health Association. 1980. Standard Methods for the Examination of Water and Wastewater. 15th edn. APHA, Washington, DC. 642p. |
| Andersen F Ø, Lastein E, 1981. Sedimentation of resuspension in shallow eutrophic Lake Arreskov, Denmark. Verh. Int.Ver. Limnol., 21: 425–430. |
| Bachmann R W, Hoyer M V, Canfield Jr D E, 1999. The restoration of Lake Apopka in relation to alternative stable states. Hydrobiologia, 394: 219–232. Doi: 10.1023/A:1003638329772 |
| Beaver J R, Casamatta D A, East T L, Havens K E, Rodusky A J, James R T, Tausz C E, Buccier K M, 2013. Extreme weather events influence the phytoplankton community structure in a large lowland subtropical lake (Lake Okeechobee, Florida, USA). Hydrobiologia, 709(1): 213–226. Doi: 10.1007/s10750-013-1451-7 |
| Bornette G, Puijalon S, 2011. Response of aquatic plants to abiotic factors:a review. Aquat. Sci., 73(1): 1–14. Doi: 10.1007/s00027-010-0162-7 |
| Chen F Z, Shu T T, Jeppesen E, Liu Z W, Chen Y W, 2013. Restoration of a subtropical eutrophic shallow lake in China:effects on nutrient concentrations and biological communities. Hydrobiologia, 718(1): 59–71. Doi: 10.1007/s10750-013-1603-9 |
| Chen K N, Bao C H, Zhou W P, 2009. Ecological restoration in eutrophic Lake Wuli:a large enclosure experiment. Ecol.Eng., 35(11): 1646–1655. Doi: 10.1016/j.ecoleng.2008.10.009 |
| Chen S L, Liu X F, Hua L, 1991. The role of silver carp and bighead in the cycling of nitrogen and phosphorus in the East Lake ecosystem. Acta Hydrobiol. Sin., 15(1): 8–26. |
| Cooke G D, Welch E B, Peterson S A, Newroth P R. 1993. Restoration and Management of Lakes and Reservoirs. 2nd edn. Lewis Publishers, Boca Raton, FL. |
| Cooke G D, Welch E B, Peterson S, Nichols S A, 2005. Restoration and Management of Lakes and Reservoirs. CRC Press, Boca Raton529p. |
| Dennison W C, Orth R J, Moore K A, Stevenson J C, Carter V, Kollar S, Bergstrom P W, Batiuk R A, 1993. Assessing water quality with submersed aquatic vegetation. BioScience, 43(2): 86–94. Doi: 10.2307/1311969 |
| Dokulil M T, Donabaum K, Pall K. 2011. Successful restoration of a shallow lake: a case study based on bistable theory. In: Ansari A A, Gill S S, Lanza G R, Rast W eds. Eutrophication: Causes, Consequences and Control. Springer, Netherland. p. 285-294. |
| Dong B L, Qin B Q, Gao G, Cai X L, 2014. Submerged macrophyte communities and the controlling factors in large, shallow Lake Taihu (China):sediment distribution and water depth. J. Great Lakes Res., 40(3): 646–655. Doi: 10.1016/j.jglr.2014.04.007 |
| Drenner R W, Hambright K D, 1999. Biomanipulation of fish assemblages as a lake restoration technique. Archiv für Hydrobiologie, 146(2): 129–165. Doi: 10.1127/archiv-hydrobiol/146/1999/129 |
| Ekholm P, Malve O, Kirkkala T, 1997. Internal and external loading as regulators of nutrient concentrations in the agriculturally loaded Lake Pyhäjärvi (southwest Finland). Hydrobiologia, 345(1): 3–14. Doi: 10.1023/A:1002958727707 |
| Gulati R D, Pires L M D, Van Donk E, 2008. Lake restoration studies:failures, bottlenecks and prospects of new ecotechnological measures. Limnologica, 38(3-4): 233–247. Doi: 10.1016/j.limno.2008.05.008 |
| Hansson L A, Annadotter H, Bergman E, Hamrin S F, Jeppesen E, Kairesalo T, Luokkanen E, Nilsson P Å, Søndergaard M, Strand J, 1998. Biomanipulation as an application of food-chain theory:constraints, synthesis, and recommendations for temperate lakes. Ecosystems, 1(6): 558–574. Doi: 10.1007/s100219900051 |
| Havens K E, Jin K R, Rodusky A J, Sharfstein B, Brady M A, East T L, Iricanin N, James R T, Harwell M C, Steinman A D, 2001. Hurricane effects on a shallow lake ecosystem and its response to a controlled manipulation of water level. Sci. World J., 1: 44–70. |
| Hu H J, Wei Y X, 2006. The Freshwater Algae of China:Systematics, Taxonomy and Ecology. Science Press, Beijing, Chinap.23-903. |
| Jackson H O, Starrett W C, 1959. Turbidity and sedimentation at Lake Chautauqua, Illinois. The Journal of Wildlife Management, 23(2): 157–168. Doi: 10.2307/3797636 |
| James R T, Chimney M J, Sharfstein B, Engstrom D R, Schottler S P, East T, Jin K R, 2008. Hurricane effects on a shallow lake ecosystem, Lake Okeechobee, Florida(USA). Fund. Appl. Limnol., 172(4): 273–287. Doi: 10.1127/1863-9135/2008/0172-0273 |
| James R T, Havens K E, 2005. Outcomes of extreme water levels on water quality of offshore and nearshore regions in a large shallow subtropical lake. Arch. Hydrobiol., 163(2): 225–239. Doi: 10.1127/0003-9136/2005/0163-0225 |
| James R T, Pollman C D, 2011. Sediment and nutrient management solutions to improve the water quality of Lake Okeechobee. Lake and Reservoir Management, 27(1): 28–40. Doi: 10.1080/07438141.2010.536618 |
| James W F, Barko J W, 1994. Macrophyte influences on sediment resuspension and export in a shallow impoundment. Lake and Reservoir Management, 10(2): 95–102. Doi: 10.1080/07438149409354180 |
| Jeffrey S W, Humphrey G F, 1975. New spectrophotometric equations for determining chlorophyll a, b, c1 and c2 in higher plants, algae and natural phytoplankton. Biochem.Physiol. Pflanz., 167(2): 191–194. Doi: 10.1016/S0015-3796(17)30778-3 |
| Jeppesen E, Søndergaard M, Jensen J P, Havens K E, Anneville O, Carvalho L, Coveney M F, Deneke R, Dokulil M T, Foy B, Gerdeaux D, Hampton S E, Hilt S, Kangur K, Köhler J, Lammens E H H R, Lauridsen T L, Manca M, Miracle R, Moss B, Nõges P, Persson G, Phillips G, Portielje R, Romo S, Schelske C L, Straile D, Tatrai I, Willén E, Winder M, 2005. Lake responses to reduced nutrient loading-an analysis of contemporary long-term data from 35 case studies. Freshwater Biol., 50(10): 1747–1771. Doi: 10.1111/fwb.2005.50.issue-10 |
| Jeppesen E, Søndergaard M, Lauridsen T L, Davidson T A, Liu Z W, Mazzeo N, Trochine C, Özkan K, Jensen H S, Trolle D, Starling F, Lazzaro X, Johansson L S, Bjerring R, Liboriussen L, Larsen S E, Landkildehus F, Egemose S, Meerhoff M, 2012. Biomanipulation as a restoration tool to combat eutrophication:recent advances and future challenges. Adv. Ecol. Res., 47: 411–487. Doi: 10.1016/B978-0-12-398315-2.00006-5 |
| Jeppesen E, Søndergaard M, Søndergaard M, Christoffersen K, 1998. The Structuring Role of Submerged Macrophytes in Lakes. Springer-Verlag, New York425p. |
| Jin K R, Chang N B, Ji Z G, James R T, 2011. Hurricanes affect the sediment and environment in lake Okeechobee. Crit.Rev. Environ. Sci. Technol., 41(S1): 382–394. |
| Ke Z X, Xie P, Guo L G, Liu Y Q, Yang H, 2007. In situ study on the control of toxic Microcystis blooms using phytoplanktivorous fish in the subtropical Lake Taihu of China:a large fish pen experiment. Aquaculture, 265(1-4): 127–138. Doi: 10.1016/j.aquaculture.2007.01.049 |
| Keith D J, Yoder J A, Freeman S A, 2002. Spatial and temporal distribution of coloured dissolved organic matter (CDOM) in Narragansett Bay, Rhode Island:implications for phytoplankton in coastal waters. Estuarine, Coastal and Shelf Science, 55(5): 705–717. Doi: 10.1006/ecss.2001.0922 |
| Kristensen P, Søndergaard M, Jeppesen E, 1992. Resuspension in a shallow eutrophic lake. Hydrobiologia, 228(1): 101–109. Doi: 10.1007/BF00006481 |
| Lin S Y, Zou T, Gao H W, Guo X Y, 2009. The vertical attenuation of irradiance as a function of turbidity:a case of the Huanghai (Yellow) Sea in spring. Acta Oceanol.Sin., 28(5): 66–75. |
| Liu J K, Xie P, 1999. Unraveling the enigma of the disappearance of water bloom from the East lake (Lake Donghu) of Wuhan. Resour. Environ. Yangtze Basin, 8(3): 312–319. |
| Liu Q Z, Chen M K, Tong H Y, He G X, Hong R H, Chen L S, Chen L Q, 2004. Study on the possible cause of water blooming and the bloom-prevention technology in Lake Qiandaohu. Agric. Sci. China, 3(8): 627–633. |
| Lowe E F, Battoe L E, Coveney M F, Schelske C L, Havens K E, Marzolf E R, Reddy K R, 2001. The restoration of Lake Apopka in relation to alternative stable states:an alternative view to that of Bachmann et al. (1999). Hydrobiologia, 448(1-3): 11–18. |
| Moss B, Balls H, Irvine K, Stansfied J, 1986. Restoration of two lowland lakes by isolation from nutrient-rich water sources with and without removal of sediment. J. Appl.Ecol., 23(2): 391–414. Doi: 10.2307/2404025 |
| Phillips G, Jackson R., Bennet C, Chilvers A, 1994. The importance of sediment phosphorus release in the restoration of very shallow lakes (The Norfolk Broads, England) and implications for biomanipulation. Hydrobiologia, 275/276: 445–456. Doi: 10.1007/BF00026733 |
| Phillips G, Kelly A, Pitt J A, Sanderson R, Taylor E, 2005. The recovery of a very shallow eutrophic lake, 20 years after the control of effluent derived phosphorus. Freshwater Biol., 50(10): 1628–1638. Doi: 10.1111/fwb.2005.50.issue-10 |
| Pu P M, 1993. An experimental study on the physio-ecological engineering for improving Taihu Lake water quality in water source area of Mashan Drinking Waterplant. J. Lake Sci., 5(2): 171–180. Doi: 10.18307/1993.0210 |
| Qin B Q, Gao G, Zhu G W, Zhang Y L, Song Y Z, Tang X M, Xu H, Deng J M, 2013. Lake eutrophication and its ecosystem response. Chin. Sci. Bull., 58(9): 961–970. Doi: 10.1007/s11434-012-5560-x |
| Qin B Q, Xu H, Dong B L, 2011. The Principle and Practice of Eutrophic Lake Restoration and Management. Higher Education Press, Beijing, Chinap.281-282. |
| Qin B Q, Zhang Y L, Gao G, Zhu G W, Gong Z J, Dong B L, 2014. Key factors affecting lake ecological restoration. Progress in Geography, 33(7): 918–924. |
| Qin B Q, 2002. Approaches to mechanisms and control of eutrophication of shallow lakes in the middle and lower reaches of the Yangze River. J. Lake Sci., 14(3): 193–202. Doi: 10.18307/2002.0301 |
| Radke R J, Kahl U, 2002. Effects of a filter-feeding fish[silver carp, Hypophthalmichthys molitrix (Val.)] on phyto-and zooplankton in a mesotrophic reservoir:results from an enclosure experiment. Freshwater Biol., 47(12): 2337–2344. Doi: 10.1046/j.1365-2427.2002.00993.x |
| Sas H. 1989. Lake Restoration by Reduction of Nutrient Loading: Expectations, Experiences, Extrapolations. Academia Verlag Richarz, Sankt Augustin. 497p. http://onlinelibrary.wiley.com/doi/10.4319/lo.1990.35.6.1412/full |
| Scheffer M, Carpenter S, Foley J A, Folke C, Walker B, 2001. Catastrophic shifts in ecosystems. Nature, 413(6856): 591–596. Doi: 10.1038/35098000 |
| Scheffer M, Hosper S H, Meijer M L, Moss B, Jeppesen E, 1993. Alternative equilibria in shallow lakes. Trends Ecol.Evol., 8(8): 275–279. Doi: 10.1016/0169-5347(93)90254-M |
| Scheffer M, Jeppesen E. 1998. Alternative stable states. In: Jeppesen E, Søndergaard M, Søndergaard, M, Christoffersen K eds. The Structuring Role of Submerged Macrophytes in Lakes. Springer Verlag, New York. p. 397-407. |
| Scheffer M, 1998. Ecology of Shallow Lakes. Chapman & Hall, London375p. |
| Søndergaard M, Liboriussen L, Pedersen A R, Jeppesen E, 2008. Lake restoration by fish removal:short-and longterm effects in 36 Danish lakes. Ecosystems, 11(8): 1291–1305. Doi: 10.1007/s10021-008-9193-5 |
| Starling F L D R M, 1993. Control of eutrophication by silver carp(Hypophthalmichthys molitrix) in the tropical Paranoá Reservoir (Brasília, Brazil):a mesocosm experiment. Hydrobiologia, 257(3): 143–152. Doi: 10.1007/BF00765007 |
| State Environmental Protection Administration, 2002. Water and Exhausted Water Monitoring Analysis Method. China Environmental Science Press, Beijing, Chinap.216-219. |
| Van Zuidam B G, Peeters E T H M, 2015. Wave forces limit the establishment of submerged macrophytes in large shallow lakes. Limnol. Oceanogr., 60(5): 1536–1549. Doi: 10.1002/lno.10115 |
| Xie P, Liu J K, 2001. Practical success of biomanipulation using filter-feeding fish to control cyanobacteria blooms:a synthesis of decades of research and application in a subtropical hypereutrophic lake. Sci. World J., 1: 337–356. Doi: 10.1100/tsw.2001.67 |
| Xie P, 2003. Silver Carp and Bighead, and Their Use in the Control of Algal Blooms. Science Press, Beijing, China134p. |
| Xu L G, Pan J Z, Jiang J H, Zhao H B, Liu C H, 2013. A history evaluation modelling and forecastation of water quality in shallow lake. Water Environ. J., 27(4): 514–523. Doi: 10.1111/wej.2013.27.issue-4 |
| Yang J K, Xiao B, Liu N F, Li J J, He G L, 2005. Forecasting the influence of south-to-north project on water quality in Xiangfan section by using WASP6. China Water & Wastewater, 21(9): 103–104. |
| Yu X F, Hu J Q, Mao C Z, Liao D, Jia H B, Shao X Y, 2008. Comparison of optical characters of Hangzhou west lake in winter and spring. Res. Environ. Sci., 21(4): 119–125. |
| Zhang Y L, Qin B Q, Chen W M, Hu W P, Gao G, Zhu G W, Luo L C, 2005. Attenuation of photosynthetically available radiation (PAR) in Meiliang Bay under different winds and waves. Chin. J. Appl. Ecol., 16(6): 1133–1137. |
 2017, Vol. 35
2017, Vol. 35