Institute of Oceanology, Chinese Academy of Sciences
Article Information
- XU Xiaoming(徐小明), ZHU Qing(祝青), ZHOU Qianzhi(周芊至), LIU Jinzhong(刘金钟), YUAN Jianping(袁建平), WANG Jianghai(王江海)
- An improved method for quantitatively measuring the sequences of total organic carbon and black carbon in marine sediment cores
- Chinese Journal of Oceanology and Limnology, 36(1): 105-113
- http://dx.doi.org/10.1007/s00343-017-6229-8
Article History
- Received Aug. 11, 2016
- accepted in principle Sep. 12, 2016
- accepted for publication Nov. 10, 2016
2 Guangzhou Institute of Geochemistry, Chinese Academy of Sciences, Guangzhou 510640, China
Global climate change is closely associated with global carbon cycling, which involves not only in the carbon allocation among diverse carbon reservoirs but also in the circulation within individual carbon reservoir (Schuur et al., 2015; Talley et al., 2016). It is widely accepted that ocean has been considered as a huge carbon reservoir (Mantoura et al., 1991; Blair and Aller, 2012; Canuel and Hardison, 2016). The organic carbon in marine sediments, especially in shelf seas, is an important part of the global carbon reservoir, and previous researches have shown that a great amount of carbon was sequestrated at high burial rates in continental shelf seas (Hedges et al., 1995). Due to the complexity of carbon sources in shelf seas with high sedimentation rates (Gao et al., 2012), to expound the mechanisms of carbon burial (Lin et al., 2017; Xu et al., 2018; Zhu et al., 2018), and further calculate the carbon storage amounts and their variations (Wang et al., 2018), we should obtain the high-resolution temporal sequences of diflerent carbon forms [including inorganic and organic carbon, as well as black carbon (BC)] in advance on the basis of the studies of marine sediment cores. The total organic carbon (TOC) in marine sediments in the continental shelf seas usually comes from two sources, i.e., terrestrial and marine autogenous sources. The latter is closely related to marine primary productivity, and the former includes inert BC and other labile solid-form organic matters mainly delivered by river to ocean. BC is principally composed of burning remains of fossil fuels and biomass as well as graphite from high-grade metamorphic rocks. It is crucial to identify and measure these diflerent forms of organic carbon in order to understand global carbon cycling and its relationship with global warming.
Previous investigators have already developed diverse methods for quantitatively detecting the contents of TOC or BC in soils/sediments. Recently, there are two commonly adopted methods to pretreat the soil/sediment samples for detecting the TOC contents, i.e., wet oxidation (Snyder and Trofymow, 1984; Leong and Tanner, 1999; Wang et al., 2012; Avramidis et al., 2015) and dry combustion (Dean, 1974; Luzak et al., 1997; Rabenhorst, 1988; Vitti, 2016). Wet oxidation is followed by titration with ferrous ammonium sulfate or photometric determination of Cr3+, or the collection and measurement of evolved CO2 (Avramidis et al., 2015); while dry combustion burns a sample at high temperatures in a furnace with the collection and detection of evolved CO2.
The methods for quantifying BC in soils/sediments are commonly classified into two types, i.e., chemical oxidation (Meredith et al., 2013) and chemothermal oxidation (Gustafsson et al., 2001; Agarwal and Bucheli, 2011; Yang et al., 2012). Chemical oxidation oxidizes labile organic carbon with acidified potassium dichromate for obtaining and quantifying BC in soils/sediments (Meredith et al., 2013); while chemothermal oxidation burns labile organic carbon at 375℃ (CTO-375) for gaining and quantitatively detecting BC (Pohl et al., 2014; Huang et al., 2016). There is a consensus that no single approach may completely measure all forms of BC because BC is a combustion continuum but not any individual compound.
However, if the traditional methods are adopted to quantitatively determine the contents of various carbon forms for establishing the high-resolution sequence of one marine sediment core, then three immediate questions will follow: How to deal with the contradiction between the limited amount of each subsample in one sediment core and diverse analytical items? How to enhance the data reproducibility of carbon contents for sediment cores? How to achieve the rapid TOC or BC measurements of a great number of subsamples for one core?
Therefore, the method for determining the sequences of TOC and BC contents in marine sediment cores should be further improved. In this paper, we will address the above-mentioned issues, and subsequently develop a novel method named as "WXY method" (meaning a method mainly established by Wang J H, Xu X M and Yuan J P) to detect the TOC and BC contents for establishing the high-resolution temporal sequences of marine sediment cores. Simultaneously, the newlyestablished method should also meet the requirements of the high and stable recovery rates of TOC and BC or no involvement of recovery procedures, minimum amount of each subsample, high-efficiency sample pretreatment, and rapid and precise instrumental analysis. Establishment of this novel method will provide a significant technical support for determining the high-resolution temporal sequences of marine sediment cores, and consequently for expounding the mechanism of marine carbon burial.
2 MATERIAL AND METHOD 2.1 MaterialsIn this study, one big fine-grained surface sediment sample (more than 1 000 g) was collected from the South Yellow Sea, and used to develop the novel method for detecting TOC and BC contents. Hydrochloric acid was of analytical grade, and purchased from the Kermel Chemical Co., Ltd. (Tianjin, China). The ultrapure water preparation device (Milli-Q) from the Wenrui Scientific Instrument Co., Ltd. (Guangzhou, China) was used to prepare ultrapure water (resistance at 25℃ higher than 18 MΩ·cm; conductivity at 25℃≤1 μs/cm). The highpurity gases of N2 and O2 (purity 99.995%) were purchased from the Keming Gas Co., Ltd. (Guangzhou, China). The disposable ceramic crucible with the micropore-controlled ability was customized from the Chashan Crucible Factory (Liling, Hunan, China), and its carbon content was less than 3 μg/g.
2.2 InstrumentsThe teflon holder was made with the size of 220 mm×145 mm×18 mm by our group. Other instruments used in this work included the LGJ-50F freeze dryer (Songyuanhuaxing Technology Development Co. Ltd., Beijing, China), CS-230 carbon-sulfur analyzer (Leco Corporation, LECO, Michigan, USA), SG-GL1100 tube furnace with the temperature precision of ±1℃ (customized from the Shanghai Institute of Optics and Fine Mechanics, Chinese Academy of Sciences, Shanghai, China), Sartorius BT 125D high-precision electronic balance (Beijing Sartorius Instrument System Co. Ltd., Beijing, China) and Mettler XP6U Microbalance (Mettler-Toledo Ltd., Zurich, Switzerland).
2.3 Methods 2.3.1 Analytical proceduresGenerally, the analytical procedures of TOC and BC contents includes the following steps, i.e., pretreatment, weighing, acidification, chemothermal oxidation and quantification (Fig. 1).
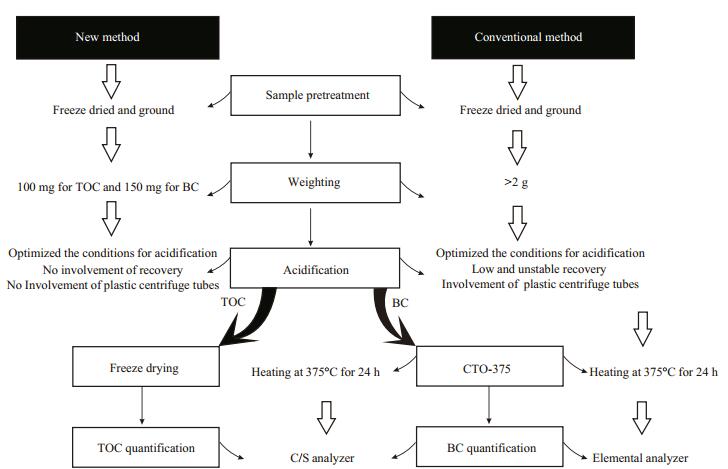
|
| Figure 1 Comparison of the analytical procedures for detecting total organic carbon (TOC) and black carbon (BC) in marine sediment cores between the newly-developed and conventional methods |
Pretreatment: The big marine sediment sample was dried by the LGJ-50F freeze dryer, and then homogenized by ultrasonic vibration. Finally, it was grounded into the powder with the size of about 200 meshes for the following procedures.
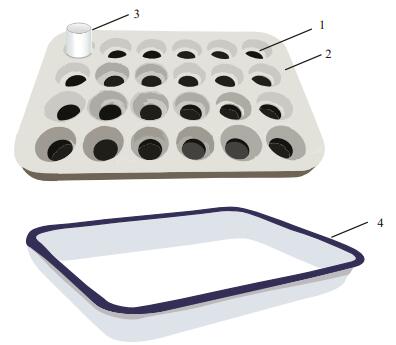
Weighing: About 100 mg or 150 mg of each subsapmle was weighed for the TOC or BC measurement, respectively. Then, they were successively transferred into the disposable ceramic crucibles with the micropore-controlled ability (Fig. 2). Before loading the subsample into each crucible, we burnt the empty crucibles at 1 100℃ for 4 hours in order to remove the absorbed water and impurities.
|
| Figure 2 Device for the acidification procedures 1. hole of the teflon holder; 2. teflon holder; 3. disposable ceramic crucible with the micropore-controlled ability; and 4. white-enameled salver. |
Acidification: The subsample-filled crucibles were labeled and placed accordingly into the holes of a self-made teflon holder, till fully loaded (depend on need). Then, the teflon holder was placed in a whiteenamel salver. Before acidification, the optimization was performed for the concentrations of HCl, duration of HCl soaking, temperatures of HCl soaking, and heating duration before washing. In acidification, the enough amount of 3 mol/L HCl was added into each crucible and the white-enameled salver, and the HCl level inside the crucibles was kept to be consistent with that outside of them. After maintaining 12 hours, the crucibles together with the holder and salver were heated in a thermostatic water bath at 80℃ for 1 hour. After HCl was completely excluded from the crucibles, ultrapure water was added into the crucibles for removing the resultants of inorganic carbon and residual HCl, and the washing procedure needed to repeat at least 6 times until the acidity of the water excluded from the crucibles was neutral. After acidification, the subsample-containing crucibles were dried by a freeze dryer for 24 hours. The dried subsamples were ready to measure the TOC contents according to the following procedure of qualification of TOC. If further analyzing the BC contents, we need to continue the chemothermal oxidation as described below.
Chemothermal oxidation: Closely following the above-mentioned acidification, the crucibles were blow-dried at 60℃. Then, the subsample-filled crucibles were held on the stainless steel plate in a quartz-tube furnace for the chemothermal oxidation at 375℃ for 24 hours under an active airflow. After this step, the labile organic carbon was removed, and BC remained in the crucibles. The subsamples processed by the above-described CTO-375 method were ready to measure the BC contents.
Quantification of TOC or BC: A CS230 carbonsulfur analyzer was employed to measure the TOC or BC contents in the treated subsamples together with the crucibles. The limit of quantification (LOQ) (defined as the mean blank value plus 10 times its standard deviation) in this study was (2.3±0.3) μg/g. The comparison of the analytical procedures for quantifying TOC or BC in marine sediment cores between the newly-developed and conventional methods was illustrated in details in Fig. 1.
2.3.2 Facility for the acidification proceduresThe self-made device for the acidification procedures is illustrated in Fig. 2, which contains three parts, i.e., teflon holder with 24 holes, white-enameled salver with the size of 25 cm×19 cm×3 cm, and disposable ceramic crucible with the microporecontrolled ability. This device may simultaneously process 24 subsamples each batch.
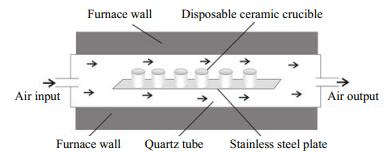
2.3.3 Facility for the CTO-375 methodThe facility for the CTO-375 method is shown in Fig. 3, which contains an SG-GL1100 quartz-tube furnace, a self-made stainless steel plate, disposable ceramic crucibles, and an external air blower. The working temperature of the furnace is at the interval of 0-1 100℃; and its constant temperature zone is 40 cm long. The stainless steel plate is placed in the constant temperature zone; and can hold 96 crucibles each batch.
|
| Figure 3 Cross section diagram of SG-GL1100 tube furnace |
TOC or BC was measured by a LECO CS 230 carbon-sulfur analyzer, which is designed on the basis of the principle of infrared absorption detection. Carbon and sulfur in the samples were burned at high temperatures under the oxygen-rich condition for producing CO2 and SO2 gases, which were then transformed into the measurable signals in their respective infrared absorption cell.
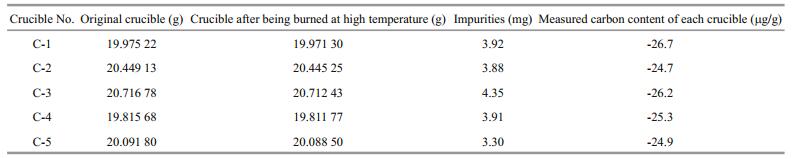
3 RESULT AND DISCUSSION 3.1 Impurities in crucibles and carbon blanksTo enhance the accuracy of TOC or BC data, five new customized crucibles were heated to 1 100℃ for 4 hours for removing the absorbed impurities and water. Their impurities and carbon blanks were accordingly measured, and presented in Table 1, where it can be seen that the diflerences in the masses between original and treated crucibles are at the interval of 3.30-4.35 mg per crucible, implying the existence of absorbed impurities and water. The carbon blanks of the new empty crucibles were measured by a CS-230 carbon-sulfur analyzer, and listed in Table 1, where it is shown that all detected carbon values are negative, indicating that their carbon blanks are lower than the level of LOQ, and there is no carbon in the new crucibles.
To improve accuracy and reliability of TOC and BC data, the following experimental parameters were systematically optimized for acidification: HCl concentration, duration of HCl soaking, HCl cooking temperature, and heating duration before washing.
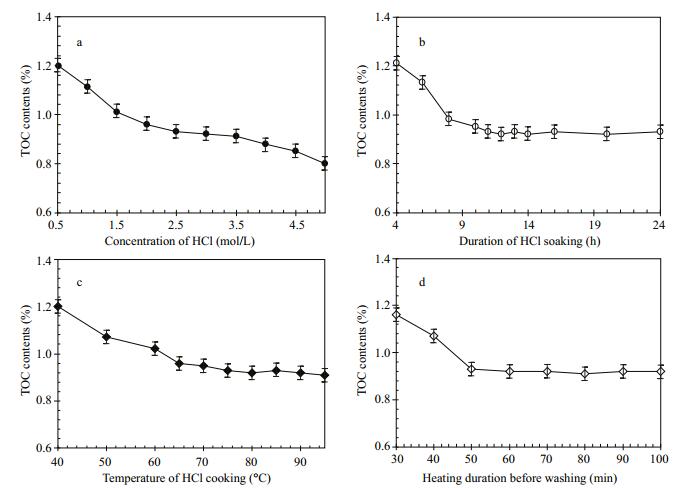
3.2.1 HCl concentrationTen acidification experiments at room temperature were performed for optimizing the concentrations of HCl, and the results are presented in Fig. 4a. It is shown that the TOC contents gradually decrease with increasing HCl concentration at the range of 0.5-4.0 mol/L, and there is no obvious change at the interval of 2.5-3.5 mol/L (Fig. 4a). Clearly, over high (>4.0 mol/L) or low concentrations (< 2.0 mol/L) of HCl will evidently aflect the measured TOC contents, probably due to the formation of volatile halides or remaining carbonates. Thus, we consider that the optimal concentration is 3 mol/L HCl.
|
| Figure 4 Variations of the TOC contents in marine sediment samples with HCl concentrations (a), duration of HCl soaking duration (b), temperatures of HCl cooking (c), and heating duration before washing (d) |
Eleven acidification experiments at room temperature were carried out for gaining the optimal duration of HCl soaking, and the results are illustrated in Fig. 4b. It demonstrates that the TOC contents gradually decline with HCl soaking time in the period of 4-11 hours, and maintain a level of 0.92%-0.93% after 11 hours (Fig. 4b). Therefore, we considered the optimal HCl soaking duration to be 12 hours.
3.2.3 HCl cooking temperatureTen acidification experiments revealed TOC decreased gradually as temperature increased from 40℃ to 75℃, but remained steady at 0.91%-0.93% between 75℃ and 95℃ (Fig. 4c). Therefore, we considered the optimal HCl cooking temperature to be 80℃.
3.2.4 Heating duration before washingTen acidification experiments at 80℃ revealed TOC decreased gradually with increased heating time between 30 min and 50 min, but stayed a level of 0.91%-0.93% from 50 min to 120 min (Fig. 4d). Therefore, we considered the optimal heating duration before washing to be 60 min.
3.3 Limited sample size versus diverse analytical itemsTypically many analyses (e.g., isotope dating, TOC and BC measurement, grain size, and stable carbon/ nitrogen isotope ratios) compete for a subsample from a complete sample of 2-3 g total weight. Therefore, economical analytical procedures for measuring various attributes of a sample are preferred. Our optimization experiments suggest 100 mg or 150 mg of subsample usually suffices for measurement of TOC or BC (Lin et al., 2017; Xu et al., 2018; Zhu et al., 2018). One gram of sample suffices for measurement of detecting the contents of diverse carbon forms and δ13C and δ15N values, in addition to grain-size analysis for marine sediment cores.
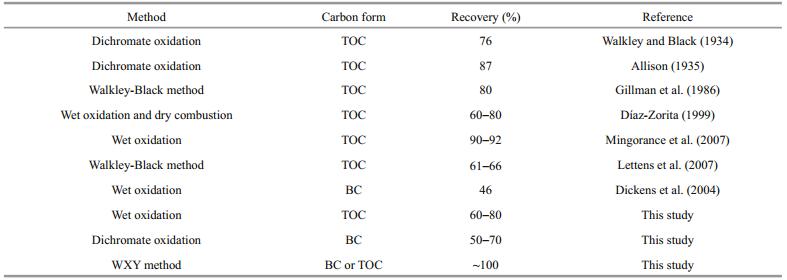
3.4 Low TOC/BC recovery rate versus data reproducibilityBefore measuring TOC or BC using the instruments, samples must be pretreated to remove carbonates, which conventionally involves in the recovery procedure. It is well known that low recovery rates of TOC or BC remarkably influence data reproducibility for sediment cores. In general, TOC or BC recovery from analytical procedures is variable, ranging from 46% to 92% (Table 2). Our own experiments confirmed that the recovery rates of wet oxidation for TOC and dichromate oxidation for BC were 60%-80% and 50%-70%, respectively (Table 2). Century-scale BC sequences recently reported from fine-grained sediment cores from the central South Yellow Sea suggest the relatively low BC recovery rate evidently aflected the pattern of BC sequences, because some BC particles might partially be lost during pretreatment (Xu et al., 2018). Low and/or unstable recovery rates may decrease data reliability and increase fluctuation within temporal TOC or BC sequences, making it more difficult to interpret real variations in temporal sequences of sediment cores. Our use of disposable ceramic crucibles with micropore-controlled ability to remove carbonates, without involvement of the procedures of recovering TOC or BC, eliminated the negative eflects of the low recovery rate, and enhanced the reproducibility of carbon data.
When measuring TOC or BC contents in marine sediment cores, many (usually several hundred) subsamples need to be analyzed. To economize on time and labor, two key strategies are to develop efficient pretreatment facilities using optimal analytical procedures, and analytical instruments capable of rapid detection of TOC or BC contents with the high precision and accuracy. According to our optimal experiments and comprehensive assessment, our pretreatment protocols (Figs. 2 and 3) enable simultaneous treatment of up to 240 TOC subsamples (equivalent to 10 Teflon holders, Fig. 2) and 96 BC subsamples (Fig. 3).
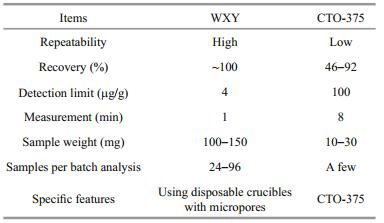
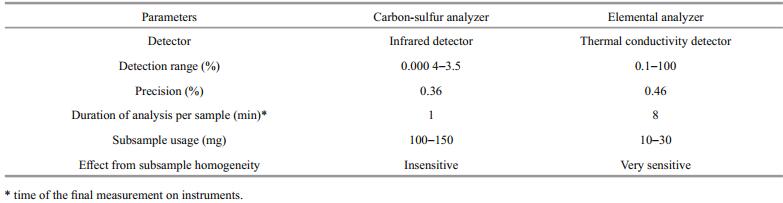
Based on instrument performance, we considered that both carbon/sulfur and elemental analyzers can meet the requirements of quantifying TOC and BC in aspects of accuracy, stability, and efficiency (running cost) (Table 3). However, as the carbon/sulfur analyzer was more efficient (Table 3), and compatible with our disposable ceramic crucibles, it was selected for subsequent TOC/BC measurement.
|
The WXY method uses customized disposable ceramic crucibles with micropore-controlled ability, whose carbon blank is so low that a very low LOQ was reached during analysis. In addition, using disposable ceramic crucibles simplified the experimental procedure, leading to near complete BC recovery compared with conventional methods with complicated operational processes and relatively low BC recovery (Table 4).
The Teflon holder improves the experimental efficiency in that it allows simultaneous processing of 24 subsamples, with the tube furnace capable of accommodating up to four Teflon holders for processing 96 subsamples per batch. The Teflon holders also ensure all subsamples are subject to comparable experimental conditions, and therefore keep the high stability and experimental repeatability of the sample data, which is urgent and important when revealing a high-resolution temporal sequence for one marine sediment core.
The carbon-sulfur analyzer is used in the final measurement, which can easily determine the carbon contents of a relatively large amount of subsample (about 100 mg for TOC, and about 150 mg for BC), more than five times that of the elemental analyzer in conventional methods. Therefore, the homogeneity of large subsamples used for carbon-sulfur analysis is better than that for the elemental analyzer (Table 4).
The WXY method provides a relatively fast tool for obtaining the accurate and reliable data of TOC or BC contents. The novel approach has already contributed to an improved understanding of the mechanisms of carbon burial, accumulation and sequestration in the Yellow Sea and the East China Sea (Lin et al., 2017; Wang et al., 2018; Xu et al., 2018; Zhu et al., 2018).
4 CONCLUSIONThe WXY method for detecting the contents of TOC and BC in marine sediment cores has been successfully developed, which can fully meet the requirements of establishing the high-resolution temporal sequences of TOC or BC. The key techniques of this method use customized disposable ceramic crucible with micropores, several readily made Teflon holding devices for batch processing of subsamples, and optimized analytical procedures. The WXY method will provide a strong technical support for studying on sedimentary organic carbon and its related significant issues such as global climate change, anthropogenic influence on seas, and carbon sequestration into coastal shelf sediments, and further understanding of carbon cycles.
| Agarwal T, Bucheli T D, 2011. Adaptation, validation and application of the chemo-thermal oxidation method to quantify black carbon in soils. Environmental Pollution, 159(2): 532–538. Doi: 10.1016/j.envpol.2010.10.012 |
| Allison L E, 1935. Organic soil carbon by reduction of chromic acid. Soil Science, 40(4): 311–320. Doi: 10.1097/00010694-193510000-00005 |
| Avramidis P, Nikolaou K, Bekiari V, 2015. Total organic carbon and total nitrogen in sediments and soils:a comparison of the wet oxidation-titration method with the combustion-infrared method. Agriculture and Agricultural Science Procedia, 4: 425–430. Doi: 10.1016/j.aaspro.2015.03.048 |
| Blair N E, Aller R C, 2012. The fate of terrestrial organic carbon in the marine environment. Annual Review of Marine Science, 4: 401–423. Doi: 10.1146/annurev-marine-120709-142717 |
| Canuel E A, Hardison A K, 2016. Sources, ages, and alteration of organic matter in estuaries. Annual Review of Marine Science, 8: 409–434. Doi: 10.1146/annurev-marine-122414-034058 |
| Dean Jr W E, 1974. Determination of carbonate and organic matter in calcareous sediments and sedimentary rocks by loss on ignition:comparison with other methods. Journal of Sedimentary Research, 44(1): 242–248. |
| Díaz-Zorita M, 1999. Soil organic carbon recovery by the Walkley-Black method in a typic hapludoll. Communications in Soil Science & Plant Analysis, 30(5-6): 739–745. |
| Dickens A F, Gelinas Y, Masiello C A, Wakeham S, Hedges J I, 2004. Reburial of fossil organic carbon in marine sediments. Nature, 427(6972): 336–339. Doi: 10.1038/nature02299 |
| Gao X L, Yang Y W, Wang C Y, 2012. Geochemistry of organic carbon and nitrogen in surface sediments of coastal Bohai Bay inferred from their ratios and stable isotopic signatures. Marine Pollution Bulletin, 64(6): 1148–1155. Doi: 10.1016/j.marpolbul.2012.03.028 |
| Gillman G P, Sinclair D F, Beech T A, 1986. Recovery of organic carbon by the Walkley and Black procedure in highly weathered soils. Communications in Soil Science & Plant Analysis, 17(8): 885–892. |
| Gustafsson O, Bucheli T D, Kukulska Z, Andersson M, Largeau C, Rouzaud J N, Reddy C M, Eglinton T I, 2001. Evaluation of a protocol for the quantification of black carbon in sediments. Global Biogeochemical Cycles, 15(4): 881–890. Doi: 10.1029/2000GB001380 |
| Hedges J I, Keil R G, 1995. Sedimentary organic matter preservation:an assessment and speculative synthesis. Marine Chemistry, 49(2-3): 81–115. Doi: 10.1016/0304-4203(95)00008-F |
| Huang L, Zhang J, Wu Y, Wang J, 2016. Distribution and preservation of black carbon in the East China Sea sediments:Perspectives on carbon cycling at continental margins. Deep Sea Research Part Ⅱ:Topical Studies in Oceanography, 124: 43–52. Doi: 10.1016/j.dsr2.2015.04.029 |
| Leong L S, Tanner P A, 1999. Comparison of methods for determination of organic carbon in marine sediment. Marine Pollution Bulletin, 38(10): 875–879. Doi: 10.1016/S0025-326X(99)00013-2 |
| Lettens S, De Vos B, Quataert P, Van Wesemael B, Muys B, Van Orshoven J, 2007. Variable carbon recovery of Walkley-Black analysis and implications for national soil organic carbon accounting. European Journal of Soil Science, 58(6): 1244–1253. Doi: 10.1111/ejs.2007.58.issue-6 |
| Lin J, Zhu Q, Hong Y H, Yuan L R, Liu J Z, Xu X M, Wang J H, 2017. Synchronous responses of sedimentary organic carbon accumulation in the inner shelf of the East China Sea to the water impoundment of Three Gorges and Gezhouba Dams. Chinese Journal of Oceanology and Limnology. Doi: 10.1007/s00343-017-6216-0 |
| Luczak C, Janquin M A, Kupka A, 1997. Simple standard procedure for the routine determination of organic matter in marine sediments. Hydrobiologia, 345(1): 87–94. Doi: 10.1023/A:1002902626798 |
| Mantoura R F C, Martin J M, Wollast R, 1991. Ocean margin processes in global change. John Wiley and Sons Ltd. |
| Meredith W, Ascough P L, Bird M I, Large D J, Snape C E, Song J, Sun Y, Tilston E L, 2013. Direct evidence from hydropyrolysis for the retention of long alkyl moieties in black carbon fractions isolated by acidified dichromate oxidation. Journal of Analytical and Applied Pyrolysis, 103: 232–239. Doi: 10.1016/j.jaap.2012.11.001 |
| Mingorance M D, Barahona E, Fernández-Gálvez J, 2007. Guidelines for improving organic carbon recovery by the wet oxidation method. Chemosphere, 68(3): 409–413. Doi: 10.1016/j.chemosphere.2007.01.021 |
| Pohl K, Cantwell M, Herckes P, Lohmann R, 2014. Black carbon concentrations and sources in the marine boundary layer of the tropical Atlantic Ocean using four methodologies. Atmospheric Chemistry and Physics, 14(14): 7413–7443. |
| Rabenhorst M C, 1988. Determination of organic and carbonate carbon in calcareous soils using dry combustion. Soils Science Society of America Journal, 52: 965–969. Doi: 10.2136/sssaj1988.03615995005200040012x |
| Schuur E A G, McGuire A D, Schädel C, Grosse G, Harden J W, Hayes D J, Hugelius G, Koven C D, Kuhry P, Lawrence D M, Natali S M, Olefeldt D, Romanovsky V E, Schaefer K, Turetsky M R, Treat C C, Vonk J E, 2015. Climate change and the permafrost carbon feedback. Nature, 520(7546): 171–179. Doi: 10.1038/nature14338 |
| Snyder J D, Trofymow J A, 1984. A rapid accurate wet oxidation diffusion procedure for determining organic and inorganic carbon in plant and soil samples. Communications in Soil Science & Plant Analysis, 15(5): 587–597. |
| Talley L D, Feely R A, Sloyan B M, Wanninkhof R, Baringer M O, Bullister J L, Carlson C A, Doney S C, Fine R A, Firing E, Gruber N, Hansell D A, Ishii M, Johnson G C, Katsumata K, Key R M, Kramp M, Langdon C, Macdonald A M, Mathis J T, McDonagh E L, Mecking S, Millero F J, Mordy C W, Nakano T, Sabine C L, Smethie W M, Swift J H, Tanhua T, Thurnherr A M, Warner M J, Zhang J Z, 2016. Changes in ocean heat, carbon content, and ventilation:A review of the first decade of GO-SHIP global repeat hydrography. Annual Review of Marine Science, 8: 185–215. Doi: 10.1146/annurev-marine-052915-100829 |
| Vitti C, Stellacci A M, Leogrande R, Mastrangelo M, Cazzato E, Ventrella D, 2016. Assessment of organic carbon in soils:a comparison between the Springer-Klee wet digestion and the dry combustion methods in Mediterranean soils (Southern Italy). Catena, 137: 113–119. Doi: 10.1016/j.catena.2015.09.001 |
| Walkley A, Black I A, 1934. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Science, 37(1): 29–38. Doi: 10.1097/00010694-193401000-00003 |
| Wang J H, Xiao X, Zhou Q Z, Xu X M, Liu J Z, Yuan D, 2018. Rates and fluxes of century-scale carbon storage in the fine-grained sediments from the central South Yellow Sea and Min-Zhe belt, East China Sea. Journal of Oceanology and Limnology, 36(1): 139–152. Doi: 10.1007/s00343-017-6242-y |
| Wang J, Zhu L, Wang Y, Gao S, Daut G, 2012. A comparison of different methods for determining the organic and inorganic carbon content of lake sediment from two lakes on the Tibetan Plateau. Quaternary International, 250: 49–54. Doi: 10.1016/j.quaint.2011.06.030 |
| Xu X M, Hong Y H, Zhou Q Z, Liu J Z, Yuan L R, Wang J H, 2017. Century-scale high-resolution black carbon records in the sediment cores from the South Yellow Sea, China. Journal of Oceanology and Limnology, 36(1): 115–127. Doi: 10.1007/s00343-017-6214-2 |
| Yang W, Lampert D, Zhao N, Reible D, Chen W, 2012. Link between black carbon and resistant desorption of PAHs on soil and sediment. Journal of Soils and Sediments, 12(5): 713–723. Doi: 10.1007/s11368-012-0494-0 |
| Zhu Q, Lin J, Hong Y H, Yuan L R, Liu J Z, Xu X M, Wang J H, 2017. Century-scale records of total organic carbon in the sediment cores from the South Yellow Sea, China. Journal of Oceanology and Limnology, 36(1): 128–138. Doi: 10.1007/s00343-017-6215-1 |
 2018, Vol. 36
2018, Vol. 36