Institute of Oceanology, Chinese Academy of Sciences
Article Information
- CAO Xihua(曹西华), YU Zhiming(俞志明), WU Zaixing(吴在兴), CHENG Fangjin(程芳晋), HE Liyan(贺立燕), YUAN Yongquan(袁涌铨), SONG Xiuxian(宋秀贤), ZHANG Jianle(张建乐), ZHANG Yongfeng(张永丰), ZHANG Wanlei(张万磊)
- Environmental characteristics of annual pico/nanophytoplankton blooms along the Qinhuangdao Coast
- Chinese Journal of Oceanology and Limnology, 36(2): 281-292
- http://dx.doi.org/10.1007/s00343-017-5216-4
Article History
- Received Sep. 6, 2015
- accepted in principle Oct. 16, 2015
- accepted for publication Nov. 9, 2016
2 Qinhuangdao Marine Environmental Monitoring Central Station, State Oceanic Administration(SOA), Qinhuangdao 066002, China
Qinhuangdao is a well-known summer resort area in China, which is famous for its beautiful beaches and pleasant climate. However, some marine phytoplankton species have annually bloomed in the coastal waters since 2000, causing poor seawater quality and damaging local tourist industry. According to statistics, only two algal blooms were recorded in the 1990s, but more than 20 occurred from 2000 to 2012 (Zhang et al., 2014). One brown algal bloom has become the most distinctive ecological disaster along the coast since 2009. It severely discolors the coastal seawater every spring and summer, affects thousands of square kilometers along the coast (Zhang et al., 2012), seriously damages local shellfish aquaculture (Zhang et al., 2013), and unbalances local marine ecological systems (Zhang et al., 2012).
It is necessary to ascertain the reasons for the blooms and develop some effective measures to prevent or control these eco-disasters (Qiu, 2012; Zhang et al., 2013; Jin and Yang, 2014). The picosized pelagophyte Aureococcus anophagefferens was once found to be the dominant species in the brown water in 2011, and the blooms were referred to as brown tides (Zhang et al., 2012). However, researchers were unsuccessful in separating the dominant bloom species from the seawater, inhibiting further study which might illuminate the physiological and ecological characteristics of the alga. The year-long survey undertaken in this study has indicated that some pico/nanophytoplankton (cell sizes < 10 μm) were present in abundance, but no species was constantly dominant in the brown seawater in 2013. Little has been known until now about the causes, biological characteristics, and formation and dissipation mechanisms of the blooms in Qinhuangdao.
Some research results suggest that degraded water quality from increased nutrient pollution promotes the development and persistence of many harmful algal blooms (HABs) and is one of the reasons for their expansion worldwide (Anderson et al., 2002; Heisler et al., 2008; O'Neil et al., 2012). However, every HAB species has its special nutrient demands, and so the relationships between nutrient loading and the development of a HAB may be difficult to establish (Anderson et al., 2008; O'Neil et al., 2012). Other environmental factors such as salinity, temperature, or flow velocity can also influence the occurrence of blooms (Zhou et al., 2003; Jiang et al., 2014). It is not easy to identify the main factors affecting the bloom of a single species in the complexity of HAB development. Why have the brown blooms occurred annually along the Qinhuangdao coast since 2009? There were few algal blooms before 2000, although local seawater quality had already been assessed as being slightly eutrophic (Zhang, 1993). How has the coastal seawater changed in recent years, and which alterations contribute to some pico/nanophytoplankton blooms?
A series of studies including field investigation and genomic analysis has now been conducted on the blooms in Qinhuangdao since 2013. In particular, variations in physical and nutrient characteristics following the development of brown blooms in 2013 were investigated and analyzed.
It is well known that the Changjiang River estuary (CRE) and the Long Island estuaries (LIEs) are both thoroughly studied HAB-prone areas. In the past two decades, large-scale blooms of Prorocentrum donghaiense with a cell size of ca 20 μm occurred annually in the CRE (Zhou et al., 2003). In contrast, one picophytoplankton (Aureococcus anophagefferens, 2–5 μm) successively impacted the LIEs (Gobler et al., 2011). Although the dominant species in the blooms differed in the two areas, they shared one similarity: one species bloomed continually in the same area for several years. The blooms along the Qinhuangdao coast had also occurred successively for several years, although identification of the dominant species was still in debate.
In this study, the environmental characteristics in Qinhuangdao were compared with those in the CRE and the LIEs. The recognized findings in the referential areas would, we felt, be helpful in screening out the critical factors in Qinhuangdao.
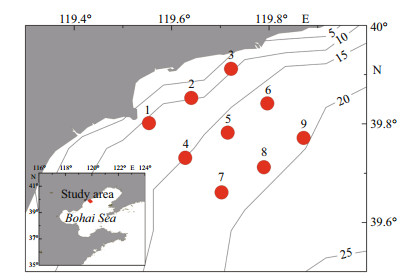
2 SURVEY AND ANALYSIS METHODOur analysis was based on a series of surveys and a review of the literature. Monthly surveys were conducted successively in the algal bloom-prone area of Qinhuangdao from March to December, 2013. Two cruises per month took place in May, June, July, August, and September, and one cruise per month was conducted in the other months. Nine sites were surveyed (Fig. 1), and indicators monitored included water depth and nutrient concentrations (nitrogen, phosphorus, and silicon in various forms). Water depth was measured in situ using an Alec CTD (Alec Electronics Co., Kobe, Japan). Water samples were collected from 0–2 m below the surface at each site with Niskin bottles, taken to the onshore lab within 2 h, and pretreated within 4 h. To measure the concentrations of five nutrients (ammonium nitrogen, nitrite, nitrate, reactive phosphate, and reactive silicate) in the water samples, water was filtered using a glass microfiber filter paper (GF/F), chloroform was added, and filtrates were stored frozen at -20℃ prior to analysis in the lab (GB/T 12763.4-2007). After acidification, water samples for analysis of total nitrogen (TN) and total phosphorous (TP) were also stored frozen at -20℃; later they underwent high pressure oxidation digestion after thawing at room temperature before measurements were made (GB/T 12763.4-2007). Measurements of all nutrient concentrations were performed using a Continuous Flow Analyzer (SKALAR, Breda, the Netherlands) (Grasshoff et al., 1999). The detection limits for ammonium, nitrite, and nitrate were 0.07 μmol/L, 0.03 μmol/L, and 0.03 μmol/L, respectively. The concentration of dissolved inorganic nitrogen (DIN) was the summation of nitrate, nitrite, and ammonium. The average nutrient values of nine sites in the same survey were regarded as the nutrient concentrations of the survey area at that time. Temperature and salinity were measured on every survey at the central site (No. 5) with an Alec CTD (Alec Electronics Co., Kobe, Japan).
|
| Figure 1 Locations of the Qinhuangdao coast in Bohai Sea and the sampling stations |
The preliminary microscopic results from the water samples showed that small-sized phytoplankton were the dominant species in Qinhuangdao coastal water, in agreement with previous reports (Zhang et al., 2012). All pico/nanophytoplankton of < 10 μm in surface seawater were collected at the central site (No. 5) by filtration through 10 μm bolting cloth, and fixed with glutaraldehyde. The collected samples were frozen immediately in liquid nitrogen and stored at -80℃ before flow cytometry analysis. Flow cytometry protocols followed Marie et al. (2000). Fluorescent beads (2 μm, Polysciences, Warrington, PA, USA) were used as the instrument's internal standard (Olson et al., 1993). Cell density of pico/nanophytoplankton was determined by identifying red autofluorescence caused by chlorophyll using a BD FACSVantage SE flow cytometer equipped with an air-cooled 488 nm argon-ion laser (Becton, Dickinson and Company, Franklin Lakes, NJ, USA) (Simon et al., 1994). At least 10 000 cells were sorted for each sample.
According to the variation of local seawater colors in 2013, the survey period was divided into three parts: pre-bloom (pale bluish green from March to mid-June), mid-bloom (brown from late June to August), and post-bloom (pale bluish green from September to December). All parameters surveyed in the different periods were tested to decide the significance of difference by means of Two-Sample t-Testing (Two-Sample t-Testing, Origin 8.5, OriginLab, Northampton, USA). Data relating to local water temperature and salinity during 2004–2013 were considered, and data from the algal bloomprone zones in the CRE and the LIEs were also referred to.
3 RESULT 3.1 Abundance of pico/nanophytoplankton (< 10 μm) during the survey periodBulletin of the marine environmental quality of Hebei Province suggested that the local brown blooms occurred in the latter part of June and disappeared at the end of August in 2013 (Hebei, 2014). Flow cytometry analysis showed that phytoplankton abundance clearly changed with the seasons: there was an obvious increase in the middle of June, a peak of (10.5×108) cells/L around 22 August, and then a sharp decline in September (Fig. 2). Further analysis showed that the local dominant species varied among the survey months (detailed data not shown). Although A. anophagefferens was sporadically dominant in the local pico/nanophytoplankton community, it never reached the bloom density of about 107 cells/L (Adachi, 1973) during the whole survey time. This pattern differed from that of the A. anophagefferens bloom in 2011 (Zhang et al., 2012). However, the high density of pico/nanophytoplankton coincided with the water discoloration that occurred from June to August in 2013, thus the discoloration in summer was attributed to the growth of some pico/nanophytoplankton. In addition, pico/nanophytoplankton density was always higher than 108 cells/L during the entire survey period in 2013. Even in winter when the water temperature dropped to almost 10℃, algae abundance was (1.83×108) cells/L (20 November 2013), the second lowest algal abundance recorded during the surveys.

|
| Figure 2 Abundance of pico/nanophytoplankton (< 10 μm) in the surface seawater of Qinhuangdao during the survey period |
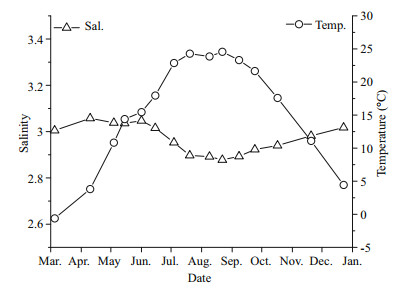
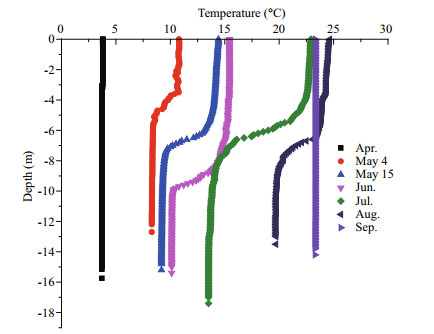
Qinhuangdao is located at 39°–40°N, where the submarine topography presents a very gentle slope. The depth contours are parallel with the shoreline and the location of 20 m isobath is about 30 km from the shoreline (Fig. 1). Qinhuangdao has four distinct seasons and experiences significant seasonal changes in surface seawater temperature. In 2013 the temperature of the surface water dropped below 5℃ in winter and reached ~26℃ in summer (Fig. 3). The temperature rose from March to August and then decreased after September. During late spring and early summer (May–July), the surface water temperature rose rapidly, and an obvious thermocline appeared and gradually intensified until August. The thermocline layer fluctuated within the 5–10 m depth range, and was deepest in June when it occurred at 8–10 m (Fig. 4). The maximum temperature gap of the thermocline layer (7℃ difference between top and bottom) was recorded in July. The surface water temperature peaked in August and then declined rapidly, and the thermocline layer disappeared (Fig. 4).
|
| Figure 3 Annual variations in temperature and salinity in the seawater of Qinhuangdao All data from upper layer water at site No. 5 |
|
| Figure 4 Vertical variations in water temperature from April to September 2013 in site No. 5 in Qinhuangdao |
The annual variations in salinity in the study area ranged from 28.58 to 30.74 (Fig. 3). The salinity changes along the Qinhuangdao coast were generally less than 2.16, and some seasonal fluctuations appeared. Salinity was highest in April and December, and in August it dropped to the lowest value of the year. In the vertical direction, a halocline was not evident in the surveyed areas. A slight vertical leap in salinity was observed only when the thermocline layer was significant, such as in June and July; in these instances the salinity of the bottom layer was slightly higher than in the upper layer (data not shown).
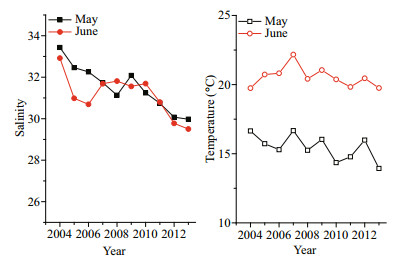
Over the last decade (2004–2013) the surface salinity has shown a decline with some fluctuation in May and June (Fig. 5). Although there were convex plateaus in the salinity curves before and after 2009, the downward slopes were evident (Fig. 5). The salinity in May and June fell by 3.46 and 3.42, respectively, from 2004 to 2013. The contemporaneous surface temperatures also presented a decreasing trend; this was more evident in May than in June. The extreme differences in surface temperature were 2.74℃ in May and 2.43℃ in June over the 10 years.
|
| Figure 5 Changes in salinity and temperature in Qinhuangdao offshore surface seawater in May and June from 2004 to 2013 Data provided by Qinhuangdao Marine Environmental Monitoring Central Station, State Oceanic Administration. |
Ammonium nitrogen (NH4+-N), nitrite nitrogen (NO2--N), and nitrate nitrogen (NO3--N) were found to be the three main forms of DIN in seawater directly used by most phytoplankton species. During the surveys in 2013, the average concentrations of NH4+-N, NO2--N, and NO3--N across all stations were 4.36 μmol/L (1.17–18.10 μmol/L), 1.11 μmol/L (0.11–5.08 μmol/L), and 3.30 μmol/L (0.05–12.35 μmol/L), respectively. Figure 6 shows the annual variations in the three forms of DIN. The error bars indicate spatial variability of the different DIN forms. The average values illustrated that the different forms of nitrogen had different annual change patterns. NH4+-N, which constituted a large proportion of total DIN, showed a sharp decrease in the first half of the survey period and a slight increase after it reached the lowest value in August; NO3--N increased slowly throughout the whole survey period and became the predominant constituent of DIN; nitrite was very stable before August but then increased in September. The total DIN exhibited an oscillatory decrease from March to August and then an abrupt increase.
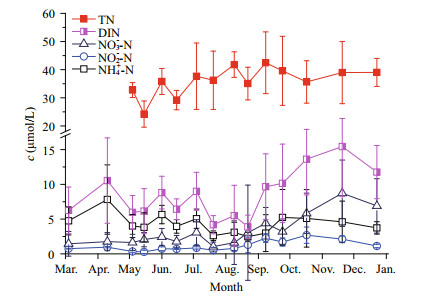
|
| Figure 6 Annual variation in different forms of nitrogen in upper layer seawaters of Qinhuangdao Data are averages of samples from nine sites in one cruise and represent characteristics of the whole area surveyed. |
Table 1 shows the variations in different forms of DIN in the upper layer seawaters in the three different periods. When ammonium and nitrite could be classified as reduced nitrogen (RN), results show that RN constituted a large proportion of DIN (i.e. > 50% in all stages and even > 75% in the pre-bloom phase). Both ammonia and DIN reached the lowest values when the bloom occurred and then increased sharply after the bloom. However, both RN/DIN and NH4+/DIN decreased constantly during the survey.

|
Figure 6 also shows the changes in TN in the seawater over the course of the study. The average concentration of TN during the survey period was about 35.28 μmol/L, with a fluctuation range from 27.34 to 42.45 μmol/L. The average concentration of DIN was only 7.85 μmol/L, fluctuating temporally within a range from 3.91 to 12.62 μmol/L. TN was thus much more abundant than DIN in the seawaters of Qinhuangdao.
The concentration of active phosphate (PO43--P) in Qinhuangdao averaged 0.22 μmol/L, fluctuating temporally within a range from 0.04 μmol/L to 0.94 μmol/L (Fig. 7). The TP values were low before July and gradually increased during the first half of the year, and then plateaued in the second half of the year. The average concentrations of TP and SiO32--Si over the whole time surveyed were 0.86 μmol/L and 7.46 μmol/L, respectively; and they fluctuated temporally within a range from 0.06 μmol/L to 2.86 μmol/L and 0.24 μmol/L to 34.00 μmol/L, respectively. In winter and spring the SiO32--Si was relatively low (< 1 μmol/L), whereas in summer and autumn the concentration increased rapidly, with the highest concentration in some sites reaching 34 μmol/L in August. It decreased rapidly thereafter.

|
| Figure 7 Annual variation in different forms of phosphorus and silicate in upper layer seawaters of Qinhuangdao Data are averages of samples from nine sites in one cruise and represent characteristics of the whole area surveyed. |
We compared the dissolved nutrient concentrations in the other two typical HAB areas with these in Qinhuangdao (Table 2). When there were no blooms (the non-bloom period), the CRE had the highest DIN concentration, followed by Qinhuangdao and the LIEs. DIN in Qinhuangdao was 10.20 μmol/L, which was two times higher than that in the LIEs. When algal blooms occurred (bloom period), concentrations of DIN in all three areas decreased, with decreasing amplitudes of 43%, 40%, and 64%, respectively. The PO43--P concentrations in the three bloom areas were all lower than 0.5 μmol/L, and the highest occurred in the CRE when there was no bloom. Qinhuangdao presented the lowest PO43--P concentrations and showed no obvious change between the different periods. Arranged in descending order, the SiO32--Si concentrations of the three areas when blooms occurred were the LIEs, the CRE and the Qinhuangdao coast in turn. The SiO32--Si concentration in the Qinhuangdao coast was only one-third of that in the LIEs, and showed an obvious increase than its nonbloom period.

|
The water temperature in the study area may favor development of the local pico/nanophytoplankton blooms. According to the literature, water temperature defines the geographic distribution of pico/nanophytoplankton (Kulk et al., 2012; Chen et al., 2014). Although the most appropriate temperature for growth may vary among species, the biomass and production of many species were positively related to temperature (Agawin et al., 2000; Kulk et al., 2012). For example, A. anophagefferens, which was one of the dominant species in 2013 and the bloomed algae in 2011 along the Qinhuangdao coast (Zhang et al., 2012) and had frequently bloomed in the LIEs since 1985 (Bricelj and Lonsdale, 1997), had a probability of explosive proliferation within the range of 15℃ to 20℃ (Gobler and Sunda, 2012). Andersson et al. (1994) reported that picoplankton showed a stronger temperature response and higher photosynthetic efficiency than nano-and microplankton at temperatures above 10℃.
Our unpublished chlorophyll data of sizefractionated phytoplankton showed that the ratio of the pico-sized chlorophyll a (Chl a) increased as the water temperature rose. There was no significant correlation between pico/nanophytoplankton abundance and the upper layer seawater temperature at site No. 5. However, the ratio of pico-sized Chl a to total Chl a in seawater was found to be significantly positively correlated with temperature (data not shown), which indicated that an increase in temperature would promote the contribution of picoplankton to the whole biomass.
In recent years, the surface water temperature in Qinhuangdao has ranged from -1℃ to 26℃ annually, and has reached 15℃ around May, thereby becoming warm enough for some pico/nanophytoplankton to bloom. The surface water temperature rose rapidly after May, usually increasing from below 15℃ in May to almost 20℃ in June. Our survey results showed that the temperature was about 18℃ in midJune 2013, when pico/nanophytoplankton abundance began to increase abruptly and the water in Qinhuangdao first turned brown (Figs. 2 and 3). The surface temperature rose until August, when it reached the yearly maximum (Fig. 3), and pico/nanophytoplankton abundance (> 109 cells/L) rose correspondingly (Fig. 2). The survey data thus suggested that the varying patterns of water temperature in 2013 were consistent with regular trends and allowed some pico/nanophytoplankton to flourish.
In the last 10 years the surface temperature showed a decreased trend in May and June. There were no significant differences in temperature between the corresponding month before 2008 and after 2009 when brown blooms first occurred (Two-Sample t-Testing, P=0.05). This indicated that the decrease in water temperature in May or June of different years might not significantly stimulate blooming of local pico/nanophytoplankton, as the interannual variations were far less than the seasonal ones.
The decline of surface salinity in May and June has been more obvious in the last 10 years. The salinity in May showed a significant variation between the presence and absence of brown blooms (Two-Sample t-Testing, P=0.05) in May, but not in June. The seasonal changes in salinity were small in the Qinhuangdao coastal areas because there were no large freshwater inputs (e.g., surface runoff or underground freshwater systems) (Ren, 2014). The difference in salinity in the presence or absence of a bloom in 2013 was also tested, and there was no significance (Two-Sample t-Testing, P=0.05). The contribution of salinity variation to local blooms remained confused, and the relationship needed to be studied intensively once the causative species had been identified.
4.2 Influence of nutrients on the occurrence of pico/nanophytoplankton bloomsAlthough nitrogen is usually a key nutrient in eutrophication (Vitousek et al., 1997), the nitrogen utilization strategies of pico/nanophytoplankton are still far from clear (Bricelj and Lonsdale, 1997; Pustizzi et al., 2004; Smayda, 2008). Some studies have suggested that there are significant differences in the growth rates of the tested picophytoplankton species under different inorganic nitrogen conditions (Muhlstein and Villareal, 2007). The appropriate amount of DIN could help picophytoplankton use organic nitrogen and multiply rapidly (Gobler and Sañudo-Wilhemly, 2001a; Pustizzi et al., 2004). Increasing numbers of studies found that the ability to use organic nutrients ensures picophytoplankton a competitive advantage in the field (Zubkov et al., 2003; Sieracki et al., 2004).
The composition of different forms of nitrogen in Qinhuangdao waters showed some special features. First, the RNs were the dominant forms in DIN. The concentration of ammonium clearly decreased after a brown bloom (Table 1), which indicates the pico/nanophytoplankton bloom might thus consume a great deal of ammonium. This is consistent with recent findings that the reduced nitrogen (such as NH4+-N and urea) were more important than NO3--N for A. anophagefferens in South Africa (Probyn et al., 2010). Based on these phenomena, we concluded that the high ratio of ammonium to DIN in Qinhuangdao would be beneficial for the explosive proliferation of pico/nanophytoplankton. Second, the ratios of DIN/TN were small (< 30% most of the time), which meant that other forms of nitrogen were richer than DIN. Many previous studies reported that organic nutrients in seawater, especially organic nitrogen, played an important role in the explosive proliferation of some picophytoplankton in other estuarine systems (Gobler et al., 2004; Kana et al., 2004; Bronk et al., 2007; Dong et al., 2014). Data relating to organic nutrients such as dissolved organic nitrogen (DON) were, however, lacking. We remain unable to determine the contribution of organic nutrients to the local pico/nanophytoplankton bloom. Further studies are needed to better understand the extent to which organic nutrients influence the occurrence of brown blooms in Qinhuangdao.
The seawater phosphate concentration of Qinhuangdao is relatively low according to the empirical nutrients ratio. The Redfield ratio indicates that Si/P > 22 and DIN/P > 22 are indicative of phosphorus limitation, whereas DIN/P < 10 and Si/DIN > 1 are indicative of nitrogen limitation (Zhang et al., 2014). Previous data indicated that the offshore waters of Qinhuangdao were under conditions of nitrogen limitation in the 1990s (Zhang, 1993; Cui et al., 1996), but these changed to phosphorus limitation in recent years (Zhang et al., 2014). Our survey results showed that although Si/N was > 1 during the pico/nanophytoplankton bloom (June–August), DIN/P < 10 did not co-occur. This indicates that the local nutrient state was not nitrogen limited. At the same time, Si/P > 22 and DIN/P > 22 occurred in many months, and Si/P in June was nearly 22. These data suggest that the coastal water of Qinhuangdao was, theoretically, lacking phosphorus in most months. There was, however, no evidence of phosphorus limitation in the development of blooms continuing until September. The local active silicate concentrations in seawater were high and there was no evident silicate limitation in any month, although levels were very low in March and November.
4.3 Comparison of environmental characteristics of Qinhuangdao waters with those of other typical algal bloom areas worldwideThe blooms in the CRE were caused by microalgae with cell sizes about 20 μm. In contrast, there were pico/nanophytoplankton blooms with algae sizes below 10 μm in both Qinhuangdao and the LIEs. Blooms in these two regions share some similarities in biology. Do these similarities imply that Qinhuangdao and the LIEs have many similarities in environmental characteristics, and that these characteristics differ substantially from the CRE, which has microplankton blooms?
4.3.1 Physical characteristicsOf these three estuarine regions, the CRE shows distinct hydrological characteristics. The seawater in the CRE is affected by the various water masses, including Changjiang Diluated Water, Taiwan Warm Current Water, Shelf Mixed Water and Kuroshio Water (Zhou et al., 2003). Although the local water temperature and salinity have wide variation ranges, major P. donghaiense blooms usually develop in May. There is a consensus that acute, complex hydrological characteristics are among the most important contributors to the frequent, large-scale P. donghaiense blooms (Zhou et al., 2003). In contrast, both the Qinhuangdao coast and the LIEs had very mild hydrological characteristics when blooms occurred.
The Qinhuangdao coast and the LIEs share strong similarities in geographical location, seasonal variation characteristics, water temperature, and hydrodynamic conditions. Long Island is located at 40.5°–41.5°N, which is very close to the geographical latitude of Qinhuangdao. The water flow in the LIEs is weak for they are closed or semi-closed shallow water bays (Bricelj and Lonsdale, 1997). Previous studies have concluded that the long residence time of the LIEs could lead to local nutrient accumulation, which was thought to be an important hydrological characteristic that could induce A. anophagefferens to bloom (Gobler et al., 2005). Similarly, the Qinhuangdao coastal circulation is so weak that the long water exchange time is detrimental to the marine self-purification capacity (Wei et al., 2002). Tidal movement is the main contributor of seawater transport in the whole Bohai Sea, but the subtidal currents along the Qinhuangdao coast are weaker than those in the neighboring areas (Bi, 2013). Qinhuangdao is one of the only two amphidromic points in the coastal areas of China. While there is limited tidal movement, a weaker flux occurs in spring in the studied area (Bi, 2013).
The mean monthly temperatures in the LIEs fluctuate between -1.7℃ and 24.5℃ (Karentz and Smayda, 1984), which are nearly the same as those of Qinhuangdao (Fig. 3). A. anophagefferens blooms occurred in the LIEs even when the temperature was close to 0℃, as well as during the main season for blooms (Gobler et al., 2002; Nuzzi and Waters, 2004). The occurrence of brown tides in the LIEs is more uncertain compared with the CRE. To date the pico/nanophytoplankton blooms only emerge in spring and summer in the Qinhuangdao coastal areas. Considering A. anophagefferens is also one dominant algal species and the biomasses in winter were still abundant in Qinhuangdao in our survey (Fig. 2), it should take precaution against the occurrence of the pico/nanophytoplankton blooms in winter in Qinhuangdao.
The LIEs bloom species grow optimally at salinities > 24 (Gobler et al., 2011), which agrees with salinity characteristics in Qinhuangdao. In fact, the seawater salinity in Qinhuangdao is relatively more stable as there is less freshwater input (Fig. 3). Based on the salinity variations in different areas of blooms, we speculated that the picophytoplankton could adapt to a certain salinity range, and that salinity might not be a critical factor in determining the occurrence of pico/nanophytoplankton blooms; this was also previously suggested by Laroche et al. (1997).
There are also some different physical characteristics between Qinhuangdao and Long Island, such as temperature differences between surface and bottom seawater. The temperature difference was never more than 2℃ in the LIEs (Gobler et al., 2005). There was an obvious thermocline in the Qinhuangdao coast during the bloom period (Fig. 4).
4.3.2 Eutrophication characteristicsSome previous studies have suggested that water quality along the Qinhuangdao coast was better than neighboring seawaters, and that its eutrophication was slight (Wang et al., 2009; Liu et al., 2011). In contrast to the CRE and the LIEs, comprehensive studies of the relationship between regional eutrophication and brown blooms in Qinhuangdao were lacking prior to this study.
Rich inorganic nutrients and an annual change in the nutrient structure are characteristic of the CRE (Zhou et al., 2003; Li et al., 2014). The effects of inorganic nutrients on the annual large-scale algal blooms in the CRE have been widely discussed (Chai et al., 2006; Zhang et al., 2007; Li et al., 2013), and there is common agreement that phosphorus limitation arising from consumption of the pre-bloomed diatom benefits P. donghaiense because it can adapt well to low phosphorus conditions (Zhu et al., 2009).
Unlike in the CRE, there is low DIN, but high DON and DOC in the LIEs (Gobler et al., 2002; Gobler et al., 2004; Nuzzi and Waters, 2004). The abundant organic nutrients in the LIEs contribute to A. anophagefferens blooming into brown tides (Gobler and Sañudo-Wilhemly, 2001a; Berg et al., 2008; Gobler et al., 2011; Gobler and Sunda, 2012). Qinhuangdao shares some similarities in nitrogen and phosphorus nutrients with the LIEs. Although there is still a lack of some directly observed data for the local dissolved organic nutrient concentrations in Qinhuangdao, there was a large gap between TN and DIN in 2013, which may include large amounts of dissolved nitrogen.
The obvious reduction in DIN in both the LIEs and Qinhuangdao indicates that the bloomed pico/nanophytoplankton can consume considerable amounts of DIN. This is consistent with the previous conclusion that the picophytoplankton can use different states of nitrogen (Gobler and SañudoWilhelmy, 2001a; Gobler et al., 2004; Bronk et al., 2007; Probyn et al., 2010). In addition, DIN in Qinhuangdao is two times higher than that in the LIEs, which suggests that the picophytoplankton may acclimate to a very wide range of DIN contents.
Although the PO43--P concentrations in the three bloom areas were all low, there were no evident P nutrient-limited phenomena in the annual large-scale algal blooms. There was no drawdown in PO43--P concentration in Qinhuangdao and the LIEs, which indicates that when in bloom the phytoplankton can tolerate low phosphorus; or they may store phosphorus within their cells, as reported for some other algae in bloom (Cui et al., 1996; Zhu et al., 2009). Some organic phosphorus chemicals can be used as nutrients by many phytoplankton species (Fisher et al., 1992; Huang et al., 1999; Muhlstein and Villareal, 2007). For example, brown tide species may be able to use forms of phosphorus other than inorganic phosphate, such as beta glycerophosphate (Muhlstein and Villareal, 2007). Multiple phosphorus utilization modes may provide algal blooms with the ability to flourish even if the absolute concentration of reactive phosphate in the waters is below the theoretic phosphorus limitation value. However, to date there are no data for the phosphorus dynamics of pico/nanophytoplankton blooms in Qinhuangdao, so the direct effects of phosphorus on the algal blooms in this area remain unknown.
4.3.3 Other relevant characteristicsIn addition to the characteristics described above, Qinhuangdao and the LIEs are similar in another way: both are important shellfish culture areas. Bay scallop (Argopecten irradians) farming has been taking place in Qinhuangdao for more than 30 years, and after 2000 the scale of aquaculture expanded quickly, accounting for more than 70% of production in China (Cao et al., 2010). The LIEs are an important shellfish culture area in the USA, where the main species are blue mussels (Mytilus edulis) and bay scallops (Hoagland et al., 2002). Many studies have focused on how algal blooms affect shellfish farming, but few have considered the influence of long-term, largescale shellfish culture on the formation of pico/nanophytoplankton blooms.
Another characteristic shared by these three algal bloom-prone areas is that other types of phytoplankton bloomed sporadically before the occurrence of the local notorious and large-scale blooms. For example, many studies reported that diatom blooms appeared in the CRE; Noctiluca scintillans blooms occurred in Qinhuangdao; and other types of microalgal blooms occurred in the LIEs before the main blooms occurred (Gobler and Sañudo-Wilhelmy, 2001b; Gobler et al., 2005; Heisler et al., 2008; Zhu et al., 2009; Gobler and Sunda, 2012). This pre-bloom phenomenon was a factor that induced the formation of picoplankton blooms (Gobler and Sunda, 2012), because the early algal bloom species consumed many of the nutrients in the seawater. This changed the trophic structure, limited the growth of most microalgae, and provided the conditions necessary for the occurrence of picoplankton blooms. In addition, decomposition of the pre-bloom algae generated large quantities of organic nutrients that fueled dinoflagellates and pico/nanophytoplankton species (Berg et al., 2008; Gobler et al., 2011).
5 CONCLUSIONOur study found the physical conditions along the Qinhuangdao coast to be very suitable for the explosive proliferation of some pico/nanophytoplankton species, such as A. anophagefferens. The water temperature rose rapidly into the suitable range of 15–25℃ in the late spring and early summer, at which time the local pico/nanophytoplankton rapidly proliferated and bloomed. At the same time, salinity remained nearly stable, with a variation of < 3. The water exchange was so limited that the diffusion ability of terrestrial pollution was comparatively poor. Eutrophication was slight in the surveyed water, but there were relatively high concentrations of reduced nitrogen (especially ammonium); this constituted a large proportion of DIN and acted as an important nutrient to support the local pico/nanophytoplankton to bloom. Before the pico/nanophytoplankton blooms occurred, other species of algae bloomed, and their biological processes might have promoted the formation of a reduced environment and provided many organic nutrients. All of these factors may together have caused favorable conditions for the pico/nanophytoplankton to bloom into an ecological disaster.
6 ACKNOWLEDGMENTThe authors gratefully acknowledge the support to this research of Cruise QYY66666 for data sampling, and would like to thank the anonymous reviewers for their helpful and constructive comments; these greatly contributed to improving the final version of the paper.
| Adachi R, 1973. Red tide organisms and red tide. Fisheries Engineering, 9: 31–36. |
| Agawin N S R, Duarte C M, Agustí S, 2000. Nutrient and temperature control of the contribution of picoplankton to phytoplankton biomass and production. Limnology and Oceanography, 45(3): 591–600. Doi: 10.4319/lo.2000.45.3.0591 |
| Anderson D M, Burkholder J M, Cochlan W P, Glibert P M, Gobler C J, Heil C A, Kudela R M, Parsons M L, Rensel J E J, Townsend D W, Trainer V L, Vargo G A, 2008. Harmful algal blooms and eutrophication:Examining linkages from selected coastal regions of the United States. Harmful Algae, 8(1): 39–53. Doi: 10.1016/j.hal.2008.08.017 |
| Anderson D M, Glibert P M, Burkholder J M, 2002. Harmful algal blooms and eutrophication:nutrient sources, composition, and consequences. Estuaries, 25(4B): 704–726. |
| Andersson A, Haecky P, Hagström A, 1994. Effect of temperature and light on the growth of micro-nano-and pico-plankton:impact on algal succession. Marine Biology, 120(4): 511–520. Doi: 10.1007/BF00350071 |
| Berg G M, Shrager J, Glöckner G, Arrigo K R, Grossman A R, 2008. Understanding nitrogen limitation in Aureococcus anophagefferens (Pelagophyceae) through cDNA and qRT-PCR analysis. Journal of Phycology, 44(5): 1 235–1 249. Doi: 10.1111/jpy.2008.44.issue-5 |
| Bi C C. 2013. A Numerical Study on the Seasonal Variability and Dynamic Mechanism of Circulation in the Bohai Sea. Ocean University of China, Qingdao. (in Chinese) |
| Bricelj V M, Lonsdale D J, 1997. Aureococcus anophagefferens:Causes and ecological consequences of brown tides in U.S. mid-Atlantic coastal waters. Limnology and Oceanography, 42(5): 1 023–1 038. |
| Bronk D A, See J H, Bradley P, Killberg L, 2007. DON as a source of bioavailable nitrogen for phytoplankton. Biogeosciences, 4(3): 283–296. Doi: 10.5194/bg-4-283-2007 |
| Cao X F, Xia X L, He J P, 2010. Status of Scallop aquaculture in Qinhuangdao and some strategies for its sustainable development. Hebei Fisheries(4): 45–47. |
| Chai C, Yu Z M, Song X X, Cao X H, 2006. The status and characteristics of eutrophication in the Yangtze River(Changjiang) estuary and the adjacent East China Sea, China. Hydrobiologia, 563(1): 313–328. Doi: 10.1007/s10750-006-0021-7 |
| Chen B Z, Liu H B, Huang B Q, Wang J, 2014. Temperature effects on the growth rate of marine picoplankton. Marine Ecology Progress Series, 505: 37–47. Doi: 10.3354/meps10773 |
| Cui Y, Chen B J, Ren S M, Chen J F, Song Y L, 1996. Study on status of bio-physic-chemical environment in the Bohai Sea. Journal of Fishery Sciences of China, 3(2): 1–12. |
| Dong H P, Huang K X, Wang H L, Lu S H, Cen J Y, Dong Y L, 2014. Understanding strategy of nitrate and urea assimilation in a Chinese strain of Aureococcus anophagefferens through RNA-Seq analysis. PLoS One, 9(10): e111069. Doi: 10.1371/journal.pone.0111069 |
| Fisher T R, Peele E R, Ammerman J W, Harding L W, 1992. Nutrient limitation of phytoplankton in chesapeake bay. Marine Ecology Progress Series, 82: 51–63. Doi: 10.3354/meps082051 |
| GB/T 12763.4-2007. 2007. Specifications for oceanographic survey-Part 4: survey of chemical parameters in sea water. China Standard Press, Beijing. p. 3-7, p. 13-29. (in Chinese) |
| Gobler C J, Berry D L, Dyhrman S T, Wilhelm S W, Salamov A, Lobanov A V, Zhang Y, Collier J L, Wurch L L, Kustka A B, Dill B D, Shah M, VerBerkmoes N C, Kuo A, Terry A, Pangilinan J, Lindquist E A, Lucas S, Paulsen I T, Hattenrath-Lehmann T K, Talmage S C, Walker E A, Koch F, Burson A M, Marcoval M A, Tang Y Z, LeCleir G R, Coyne K J, Berg G M, Bertrand E M, Saito M A, Gladyshev V N, Grigoriev I V, 2011. Niche of harmful alga Aureococcus anophagefferens revealed through ecogenomics. Proceedings of the National Academy of Sciences of the United States of America, 108(11): 4 352–4 357. Doi: 10.1073/pnas.1016106108 |
| Gobler C J, Boneillo G E, Debenham C J, Caron D A, 2004. Nutrient limitation, organic matter cycling, and plankton dynamics during an Aureococcus anophagefferens bloom. Aquatic Microbial Ecology, 35(1): 31–43. |
| Gobler C J, Cullison L A, Koch F, Harder T M, Krause J W, 2005. Influence of freshwater flow, ocean exchange, and seasonal cycles on phytoplankton-nutrient dynamics in a temporarily open estuary. Estuarine, Coastal and Shelf Science, 65(1-2): 275–288. Doi: 10.1016/j.ecss.2005.05.016 |
| Gobler C J, Renaghan M J, Buck N J, 2002. Impacts of nutrients and grazing mortality on the abundance of Aureococcus anophagefferens during a New York brown tide bloom. Limnology and Oceanography, 47(1): 129–141. Doi: 10.4319/lo.2002.47.1.0129 |
| Gobler C J, Sañudo-Wilhelmy S A, 2001a. Effects of organic carbon, organic nitrogen, inorganic nutrients, and iron additions on the growth of phytoplankton and bacteria during a brown tide bloom. Marine Ecology Progress Series, 209: 19–34. Doi: 10.3354/meps209019 |
| Gobler C J, Sañudo-Wilhelmy S A, 2001b. Temporal variability of groundwater seepage and brown tide blooms in a Long Island embayment. Marine Ecology Progress Series, 217: 299–309. Doi: 10.3354/meps217299 |
| Gobler C J, Sunda W G, 2012. Ecosystem disruptive algal blooms of the brown tide species, Aureococcus anophagefferens and Aureoumbra lagunensis. Harmful Algae, 14: 36–45. Doi: 10.1016/j.hal.2011.10.013 |
| Grasshoff K, Kremling K, Ehrhardt M. 1999. Methods of Seawater Analysis. 3rd edn. John Wiley & Sons, Chichester. p. 253-364. |
| Hebei P O A. 2014. Bulletin of marine environmental quality of Hebei Province in 2013. http://www.hebgt.gov.cn/apps/cms/docforward.do?id=16404 (in Chinese) |
| Heisler J, Glibert P M, Burkholder J M, Anderson D M, Cochlan W, Dennison W C, Dortch Q, Gobler C J, Heil C A, Humphries E, Lewitus A, Magnien R, Marshall H G, Sellner K, Stockwell D A, Stoecker D K, Suddleson M, 2008. Eutrophication and harmful algal blooms:a scientific consensus. Harmful Algae, 8(1): 3–13. Doi: 10.1016/j.hal.2008.08.006 |
| Hoagland P, Anderson D M, Kaoru Y, White A W, 2002. The economic effects of harmful algal blooms in the United States:estimates, assessment issues, and information needs. Estuaries, 25(4B): 819–837. |
| Huang B Q, Huang S Y, Weng Y, Hong H S, 1999. Effect of dissolved phosphorus on alkaline phosphatase activity in marine microalgae. Acta Oceanologica Sinica, 21(1): 55–60. |
| Jiang Z B, Liu J J, Chen J F, Chen Q Z, Yan X J, Xuan J L, Zeng J N, 2014. Responses of summer phytoplankton community to drastic environmental changes in the Changjiang (Yangtze River) estuary during the past 50 years. Water Research, 54: 1–11. Doi: 10.1016/j.watres.2014.01.032 |
| Jin L, Yang Z X, 2014. Harmful algal blooms occurrence characteristics and control countermeasures of the coastal waters in Qinhuangdao. Journal of EMCC, 24(1): 40–42. |
| Kana T M, Lomas M W, MacIntyre H L, Cornwell J C, Gobler C J, 2004. Stimulation of the brown tide organism, Aureococcus anophagefferens, by selective nutrient additions to in situ mesocosms. Harmful Algae, 3(4): 377–388. Doi: 10.1016/j.hal.2004.06.008 |
| Karentz D, Smayda T J, 1984. Temperature and seasonal occurrence patterns of 30 dominant phytoplankton species in narragansett bay over a 22-year period (1959-1980). Marine Ecology Progress Series, 18(3): 277–293. |
| Kulk G, de Vries P, van de Poll W H, Visser R J W, Buma A G J, 2012. Temperature-dependent growth and photophysiology of prokaryotic and eukaryotic oceanic picophytoplankton. Marine Ecology Progress Series, 466: 43–55. Doi: 10.3354/meps09898 |
| Laroche J, Nuzzi R, Waters R, Wyman K, Falkowski P, Wallace D, 1997. Brown Tide blooms in Long Island's coastal waters linked to interannual variability in groundwater flow. Global Change Biology, 3(5): 397–410. Doi: 10.1046/j.1365-2486.1997.00117.x |
| Li H M, Shi X Y, Chen P, Zhang C S, 2013. Distribution of dissolved inorganic nutrients and dissolved oxygen in the high frequency area of harmful algal blooms in the East China Sea in spring. Environmental Science, 34(6): 2 159–5 165. |
| Li H M, Tang H J, Shi X Y, Zhang C S, Wang X L, 2014. Increased nutrient loads from the Changjiang (Yangtze)River have led to increased harmful algal blooms. Harmful Algae, 39: 92–101. Doi: 10.1016/j.hal.2014.07.002 |
| Liu S G, Lou S, Kuang C P, Huang W R, Chen W J, Zhang J L, Zhong G H, 2011. Water quality assessment by pollutionindex method in the coastal waters of Hebei Province in western Bohai Sea, China. Marine Pollution Bulletin, 62(10): 2 220–2 229. Doi: 10.1016/j.marpolbul.2011.06.021 |
| Marie D, Simon N, Guillou L, Partensky F, Vaulot D. 2000. Flow cytometry analysis of marine picoplankton. In: Diamond R A, Demaggio S eds. In Living Color. Springer, Berlin Heidelberg. p. 421-454. |
| Muhlstein H I, Villareal T A, 2007. Organic and inorganic nutrient effects on growth rate-irradiance relationships in the Texas brown-tide alga Aureoumbra lagunensis(Pelagophyceae). Journal of Phycology, 43(6): 1 223–1 226. Doi: 10.1111/jpy.2007.43.issue-6 |
| Nuzzi R, Waters R M, 2004. Long-term perspective on the dynamics of brown tide blooms in Long Island coastal bays. Harmful Algae, 3(4): 279–293. Doi: 10.1016/j.hal.2004.04.001 |
| Olson R J, Zettler E R, DuRand M D. 1993. Phytoplankton analysis using flow cytometry. In: Kemp P F, Sherr B F, Sherr E B, Cole J J eds. Handbook of Methods in Aquatic Microbial Ecology. Lewis Publishers, Boca Raton. p. 175-186. |
| O'Neil J M, Davis T W, Burford M A, Gobler C J, 2012. The rise of harmful cyanobacteria blooms:the potential roles of eutrophication and climate change. Harmful Algae, 14: 313–334. Doi: 10.1016/j.hal.2011.10.027 |
| Probyn T A, Bernard S, Pitcher G C, Pienaar R N, 2010. Ecophysiological studies on Aureococcus anophagefferens blooms in Saldanha Bay, South Africa. Harmful Algae, 9(2): 123–133. Doi: 10.1016/j.hal.2009.08.008 |
| Pustizzi F, MacIntyre H, Warner M E, Hutchins D A, 2004. Interaction of nitrogen source and light intensity on the growth and photosynthesis of the brown tide alga Aureococcus anophagefferens. Harmful Algae, 3(4): 343–360. Doi: 10.1016/j.hal.2004.06.006 |
| Qiu J. 2012. China third country to be hit by 'brown tide'. Nature News, https://doi.org/10.1038/nature.2012.11015. |
| Ren X L, 2014. Analysis of water resources in Qinhuangdao city water supply. Water Sciences and Engineering Technology, 2: 1–4. |
| Sieracki M E, Gobler C J, Cucci T L, Thier E C, Gilg I C, Keller M D, 2004. Pico-and nanoplankton dynamics during bloom initiation of Aureococcus in a Long Island, NY bay. Harmful Algae, 3(4): 459–470. Doi: 10.1016/j.hal.2004.06.012 |
| Simon N, Barlow R G, Marie D, Partensky F, Vaulot D, 1994. Characterization of oceanic photosynthetic picoeukaryotes by flow cytometry. Journal of Phycology, 30(6): 922–935. Doi: 10.1111/j.0022-3646.1994.00922.x |
| Smayda T J, 2008. Complexity in the eutrophication-harmful algal bloom relationship, with comment on the importance of grazing. Harmful Algae, 8(1): 140–151. Doi: 10.1016/j.hal.2008.08.018 |
| Vitousek P M, Aber J D, Howarth R W, Likens G E, Matson P A, Schindler D W, Schlesinger W H, Tilman D G, 1997. Human alteration of the global nitrogen cycle:sources and consequences. Ecological Applications, 7(3): 737–750. |
| Wang X L, Cui Z G, Guo Q, Han X R, Wang J T, 2009. Distribution of nutrients and eutrophication assessment in the Bohai Sea of China. Chinese Journal of Oceanology and Limnology, 27(1): 177–183. Doi: 10.1007/s00343-009-0177-x |
| Wei H, Tian T, Zhou F, Zhao L, 2002. Numerical study on the water exchange of the Bohai Sea:simulation of the halflife time by dispersion model. Journal of Ocean University of Qingdao, 32(4): 519–525. |
| Zhang C S, Wang X L, Shi X Y, Tang H J, Han X R, Xin Y, 2007. Seasonal variation and spatial distribution of nutrients and their relationships with harmful algal blooms in coastal area of the East China Sea. Environmental Science, 28(11): 2 416–2 424. |
| Zhang J L, 1993. Eutrophication status of the Qinghuangdao coastal areas. Marine Environmental Science, 12(1): 59–63. |
| Zhang Q C, Qiu L M, Yu R C, Kong F Z, Wang Y F, Yan T, Gobler C J, Zhou M J, 2012. Emergence of brown tides caused by Aureococcus anophagefferens Hargraves et Sieburth in China. Harmful Algae, 19: 117–124. Doi: 10.1016/j.hal.2012.06.007 |
| Zhang W L, Zhang Y F, Zhang J L, Zhao S L, 2014. The changes of nutrient and eutrophication of Beidaihe red tide monitoring area. Transactions of Oceanology and Limnology(1): 143–147. |
| Zhang Y F, Li X Y, Zhang W L, Zhang J L, 2013. Spatial and temporal distribution of silicate and chlorophyll a in the coastal waters with picophytoplankton algal bloom. Ecological Science, 32(4): 509–513. |
| Zhou M J, Yan T, Zou J Z, 2003. Preliminary analysis of the characteristics of red tide areas in Changjiang River estuary and its adjacent sea. Chinese Journal of Applied Ecology, 14(7): 1 031–1 038. |
| Zhu M Y, Xu Z J, Li R X, Wang Z L, Shi X Y, 2009. Interspecies competition for nutrients between Prorocentrum donghaiense Lu and Skeletonema costatum (Grev.) Cleve in mesocosm experiments. Acta Oceanologica Sinica, 28(1): 72–82. |
| Zubkov M V, Fuchs B M, Tarran G A, Burkill P H, Amann R, 2003. High rate of uptake of organic nitrogen compounds by Prochlorococcus cyanobacteria as a key to their dominance in oligotrophic oceanic waters. Applied and Environmental Microbiology, 69(2): 1 299–1 304. Doi: 10.1128/AEM.69.2.1299-1304.2003 |
 2018, Vol. 36
2018, Vol. 36


