Institute of Oceanology, Chinese Academy of Sciences
Article Information
- HU Shunxin(胡顺鑫), WANG You(王悠), WANG Ying(王影), ZHAO Yan(赵妍), ZHANG Xinxin(张鑫鑫), ZHANG Yongsheng(张永生), JIANG Ming(姜铭), TANG Xuexi(唐学玺)
- Effects of elevated pCO2 on physiological performance of marine microalgae Dunaliella salina (Chlorophyta, Chlorophyceae)
- Chinese Journal of Oceanology and Limnology, 36(2): 317-328
- http://dx.doi.org/10.1007/s00343-018-6278-7
Article History
- Received Oct. 8, 2016
- accepted in principle Dec. 2, 2016
- accepted for publication Feb. 4, 2017
2 Rongcheng Ocean and Fisheries Bureau, Weihai 264300, China
The concentration of atmospheric CO2 has increased from ~280 to 395 μatm since the Industrial Revolution because of human activities such as deforestation, cement manufacture and burning of fossil fuels (Caldeira and Wickett, 2003). As humans continue to burn fossil fuels and biomass, atmospheric pCO2 is predicted to continue to increase by a minimum of 0.5% per year in the next centuries, reaching 1 000 and 2 000 μatm by 2100 and 2300, respectively (Caldeira and Wickett, 2003; IPCC, 2013). Approximately 1/3 of this atmospheric pCO2 will be dissolved in the surface ocean, increasing CO2 (aq) and decreasing the pH, thereby changing the seawater carbonate chemistry in a process called ocean acidification (Guinotte and Fabry, 2008). By 2100 and 2300, oceanic absorption of CO2 will lead to a decrease in pH of 0.4 and 0.7 pH units, respectively (Caldeira and Wickett, 2003).
Marine algae fix inorganic carbon via the enzyme ribulose-1, 5-bisphosphate carboxylase/oxygenase (RubisCO), which utilizes CO2 exclusively as the substrate for the carboxylase reaction. The concentration of CO2(aq) (~10 μmol/L) in oceans at present is far less than the half-saturation constant of Rubisco (~20–200 μmol/L) (Badger et al., 1998). Consequently, most marine algae have developed CO2 concentrating mechanisms (CCMs) to overcome the limitations of Rubisco and compensate for the low CO2(aq) (Rost et al., 2006). Marine algae have evolved diverse types of CCMs, two of which are widely found. In the first CCM, the reversible dehydration of HCO3ˉ to CO2 is catalyzed by extracellular carbonic anhydrase at the cell surface, then taken into the cell by passive diffusion, while in the second, HCO3ˉ is transported across cell membranes via anion exchange (AE), after which CO2 is produced through the dehydration of HCO3ˉ catalyzed by intracellular carbonic anhydrase (Reinfelder, 2011). The types and energy costs of CCMs will largely determine the sensitivity of marine algae to ocean acidification (Reinfelder, 2011).
Functioning of CCMs has been widely investigated in different algal species. In diatoms, the CCMs of Pseudo-nitzschia multiseries, Stellarima stellaris, Phaeodactylum tricornutum and Thalassiosira pseudonana were down-regulated with elevated pCO2, as indicted by reduced photosynthetic affinities for dissolved inorganic carbon (DIC) and CO2 (Trimborn et al., 2008; Wu et al., 2010; Yang and Gao, 2012). Furthermore, these responses were generally accompanied by reduced active transport of HCO3ˉ or lowered extracellular carbonic anhydrase activities. The affinities for CO2 and DIC were also lower under high pCO2 conditions in the coccolithophore Emiliania huxleyi and the cyanobacterium Trichodesmium, while CAext activity appeared to play a minor role in CCMs, and was not affected by elevated pCO2 (Rost et al., 2003, Kranz et al., 2009). Furthermore, the relative expression of genes associated with carbonic anhydrases and aquaporins in the dinoflagellate Thoracosphaera heimii decreased with increasing pCO2 (Van de Waal et al., 2013). Nevertheless, Zou and Gao (2009) and Zou et al. (2011) reported that Hizikia fusiformis and Gracilaria lemaneiformis showed no down-regulation of CCMs with elevated pCO2. These different types of CCMs and their responses to ocean acidification have deepened our knowledge of species-specific responses to ocean acidification in marine algae.
Inevitably, the expression and operation of CCMs involve an energetic investment. Down-regulation under high CO2 conditions could reduce the energetic requirement for photosynthesis. Consequently, the energy saved from down-regulated CCMs could be invested in other physiological processes, such as assimilation of other nutrients, leading to stimulated growth and photosynthesis (Giordano et al., 2005; Riebesell et al., 2007; Wu et al., 2010). Nevertheless, elevated pCO2 is not always beneficial to marine algae. It has been widely reported that calcifying organisms are more sensitive to ocean acidification due to the negative effects they have on the formation of aragonite or calcite armor (Langer et al., 2006; Kurihara et al., 2008; Van de Waal et al., 2013), while negative effects on growth and photosynthesis have been observed in non-calcifying species (Mercado et al., 1999; Iñíguez et al., 2015), likely due to the negative effects on the physiological processes caused by reduced external pH.
Dunaliella salina is a unicellular and halotolerant biflagellate marine microalgal species that has been widely applied as an important model organism to evaluate physiological responses to environmental changes and fundamental molecular mechanisms because of its tolerance to hyper salinity and the simplicity of its cytoarchitecture (Booth and Beardall, 1991; Zhang et al., 2015). However, comprehensive studies of the effects of elevated pCO2 on the physiological performance of this species remain scare. Nevertheless, it is essential to investigate the long-term acclimation of physiological activities such as photosynthesis and respiration under stable carbonate chemistry. Therefore, our study was conducted using semi-continuous cultures to maintain stable carbonate chemistry systems while keeping the microalgae in an exponential stage for a long period of time. The specific goal of this study was to improve our understanding of the physiological responses of the marine microalgae D. salina to elevated pCO2. This was achieved by evaluating the growth, photosynthesis, dark respiration and CCM modes of D. salina under three different pCO2 levels in semicontinuous cultures. The three pCO2 levels included: 390 μatm (pHNBS: 8.10), which is the present pH value, as well as 1 000 μatm (pHNBS: 7.78) and pCO2: 2 000 μatm (pHNBS: 7.49), which are the pH values predicted for 2100 and 2300, respectively. We hypothesized that CO2-induced ocean acidification will change the metabolic energy requirement to balance the reduced external pH, and that elevated CO2 availability and changes in seawater carbonate chemistry may reduce their ability to actively transport CO2 and HCO3ˉ, thereby reducing the energetic costs of CCMs. Consequently, these effects are predicted to lead to different physiological sensitivities to CO2-induced ocean acidification.
2.1 Culture conditions and experimental designD. salina was obtained from the Culture Collection of Algae at the Ocean University of China. The cells were cultured in 0.45 μm-filtered natural seawater collected from Lu Xun seaside Park (Qingdao), which had been autoclaved (30 min, 121℃) and enriched with modified f/2 medium (Guillard, 1975). All cultures were incubated at 20±1℃ and illuminated with 80 μmol photon/(m2∙s) (a sub-saturating light intensity) under a 12 h:12 h light: dark cycle. The salinity of the culture medium was adjusted to 30.
Experiments were conducted in triplicate in 500 mL sterilized and acid-washed Erlenmeyer flasks containing 300 mL medium. Prior to inoculation, the cultures were aerated with three different CO2 levels: 390, 1 000 and 2 000 μatm, corresponding to approximately present-day levels and those predicted for 2100 and 2300, respectively. Different air/CO2 mixtures were generated by plant CO2 chambers (HP400G-D, Ruihua Instrument & Equipment Ltd., Wuhan, China) with a variation of less than 5%. Semi-continuous cultures used to measure the physiological responses of D. salina to elevated pCO2 in the present study have been widely applied in other relevant studies (Fu et al., 2007; Hutchins et al., 2007; Wu et al., 2010). In the present study the culture medium was renewed every 24 h to ensure that the cell concentration remained within a range of 2×104 to 5×104 cells/mL (the dilution rate is about 40%) at their exponential growth phase so that the pH fluctuations during growth were less than 0.06. Cultures were harvested following 4–6 weeks of semi-continuous incubation when their growth rates did not fluctuate significantly for three or more consecutive days, at which time they were considered fully acclimated to their respective experimental treatments.
2.2 Seawater carbonate chemistryThe concentrations of dissolved inorganic carbon (DIC) and pH were measured before and after diluting the culture, as well as during the middle of the light period to ensure stability of the carbonate system in culture. The DIC was determined using a total organic carbon analyzer (TOC-VCPN, Shimadzu) following the method described by Liu et al. (2014). pH values were determined using a pH meter (SevenCompactTM S210k, METTLER TOLEDO) calibrated with the standard National Bureau of Standards (NBS) buffer system in a three-point calibration. The other relevant parameters of the seawater carbonate system were computed according to the known values of pH, DIC, salinity, temperature and pCO2 using the CO2SYS software (Lewis et al., 1998).
2.3 Growth and photosynthetic pigmentThe growth rate of microalgae was monitored daily using a hemocytometer before and after the medium was renewed. The specific growth rate (μ) was calculated from the equation: μ=(lnN1–lnN0)/(t1–t0), where N0 and N1 represent the average cell numbers at times t0 (initial or just after the dilution) and t1 (before the dilution), respectively.
Chlorophyll a (Chl a), chlorophyll b (Chl b) and carotenoid (Car) concentrations were measured according to Wellburn (1994). Briefly, 60 mL of culture were filtered onto glass microfiber filters (GF/F, Whatman), then extracted with 10 mL of methanol overnight at 4℃. Concentrations were calculated according to the following equations:
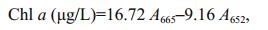
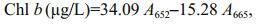
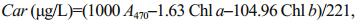
where, A470, A652 and A665 represent absorbance values of the acetone extracts at 470 nm, 652 nm and 665 nm, respectively.
2.4 Chlorophyll fluorescence measurementsFluorescence induction curves and rapid light curves (RLCs) were applied to evaluate the changes in photosynthetic performance of microalgae under different levels of pCO2 using a pulse amplitudemodulated fluorometer (Water-PAM fluorometer, Walz, Effeltrich, Germany).
The RLCs were determined at eight different PAR levels (83, 123, 188, 282, 400, 556, 991 and 1 332 μmol photon/(m2∙s)), each of which lasted 10s. The RLCs were fitted with the empirical equation proposed by Platt et al. (1980) to determine the relevant parameters; namely, rETRmax (maximum relative electron transport rate), α (photosynthetic efficiency), and Ek (light saturation point). The minimum saturating irradiation was derived from rETRmax and α according to the following equation: Ek=rETRmax/α. The following settings ensured convergence of the regression model: iterations=100, step size=100, tolerance=0.000 1, and initial seed value for P=5, α=0.05 and β=0 (Ralph and Gademann, 2005).
For fluorescence induction curves, all of the samples were dark-adapted for 20 min before measurement. The dark-adapted induction curves were then measured with a delay of 40 s between Fv/Fm measurements. The actinic light was set at 188 μmol photon/(m2∙s) to measure the value of effective quantum yield (ΔF/F'm) and nonphotochemical quenching (NPQ).
2.5 Photosynthetic oxygen evolution and respirationPhotosynthetic oxygen evolution and dark respiration were measured using a Clark-type oxygen electrode (Chlorolab 3, Hansatech, UK). Light was supplied by a halogen lamp, and temperature was maintained using a water bath circulator at 20℃. Prior to the determinations, the cells were allowed to acclimate to the light or dark conditions in the reaction chamber for 15 min. The 5 mL reaction medium was continuously magnetically stirred during the measurement.
The evolution of photosynthetic oxygen of D. salina under different pCO2 values with the addition of inhibitors was measured to determine the mechanism of inorganic carbon acquisition. The inhibitors included acetazolamide (AZ), which is an impermeant CA inhibitor and thus inhibits only extracellular CA, ethoxyzolamide (EZ), which is a membrane-permeable carbonic anhydrase inhibitor that can inhibit both extracellular and intracellular CA, and DIDS (4, 4′-diisothiocyanostilbene-2, 2′-disulfonate), which inhibits direct HCO3ˉ uptake by means of the anion-exchange protein. These inhibitors have been widely used to determine the contribution of external CA, internal CA and anion-exchange protein to photosynthetic inorganic carbon uptake (Moroney et al., 1985; Axelsson et al., 1995; Ihnken et al., 2011).
2.6 Determination of ribulose-1, 5-bisphosphate carboxylase/oxygenase (Rubisco) activitiesThe cells were collected by centrifugation at 3 000×g and 4℃ for 15 min. After removing the supernatant, 1 mL buffer solution (40 mmol/L Tris HCl, 5 mmol/L glutathione, 10 mmol/L MgCl2 and 0.25 mmol/L EDTA, pH 7.6) was added, and the cells were ground on ice. The liquid was subsequently concentrated, after which the supernatant was used for further assays. The Rubisco activity in the supernatant was generally determined following the methods described by Gerard and Driscoll (1996). Briefly, the assay mixture contained 5 mmol/L NADH, 50 mmol/L ATP, 50 mmol/L phosphocreatine, 0.2 mmol/L NaHCO3, 160 U/mL creatine phosphokinase, 160 U/mL phosphoglycerate kinase, 160 U/mL glyceraldehyde-3-phosphate dehydrogenase and reaction buffer (0.1 mol/L TrisHCl, 12 mmol/L MgCl2 and 0.4 mmol/L EDTA, pH 7.8). The absorbance values at 340 nm (A340) were measured every 20 s for 3 min to obtain the background NADH oxidation rate. Next, 0.05 mL RuBP (final concentration of 25 mmol/L) was added into the assay mixture, and the A340 was recorded every 20 s for 3 min. The activities of Rubisco were computed by subtracting the background rate of decrease in A340 from the rate determined in the three minutes following RuBP addition and then converting the corrected rate of A340 decrease to a rate of NADH oxidation.
2.7 Measurement of carbonic anhydrase activityCells grown under three different pCO2 levels were collected to determine the internal carbonic anhydrase and external carbonic anhydrase activity according to an electrometric method (Giordano and Maberly, 1989). Briefly, cells were harvested by centrifugation at 4 000×g and 4℃ for 10 min, then re-suspended in Veronal buffer (20 mmol/L, pH 8.2) adjusted to the salinity of the culture medium with NaCl. The cell suspension was initially analyzed for CAext activity, after which it was used for CAint determination. The CA ext was determined based on the time taken for the pH to decrease from 8.2 to 7.2 following the addition of 2 mL CO2-saturated distilled water (also adjusted to the salinity of the culture medium with NaCl) to a 5 mL cell suspension. For measurements of CAint activity, the same method as described above was used; however, the cell suspension was disrupted by a sonicator and the disruption of cells was confirmed by microscopic observation. Enzyme activity was calculated using the following equation:
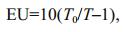
where, T0 is the uncatalyzed reaction and T is the time of the catalyzed reaction.
3 STATISTICAL ANALYSISOne-way ANOVA was conducted to identify significant differences among treatments using the SPSS software (20.0). The LSD (Least Significant Difference) post-hoc comparison test was used if ANOVA indicated a significant difference. Prior to analysis, data were initially examined for normality using the Shapiro-Wilk test and for homogeneity of variances using Levene's test. Dates were presented as the means±SE and the significance was set to P < 0.05. All figures were prepared with Sigmaplot 12.5.
4 RESULT 4.1 Carbonate systemUnder the simulated laboratory conditions of ocean acidification, the seawater carbonate chemistry system under high pCO2 (1 000 μatm and 2 000 μatm) levels showed significant changes compared to under 390 μatm CO2 conditions (Table 1). Throughout the semi-continuous culture systems, the pH varied by less than 0.04 before and after diluting the culture medium.
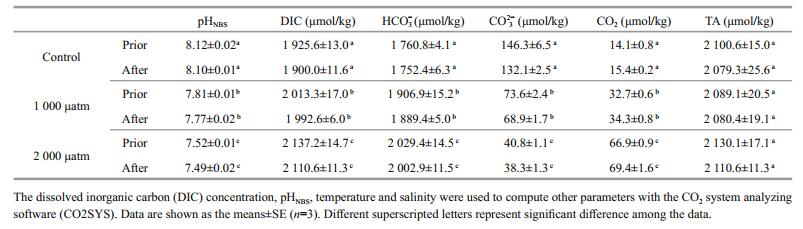
|
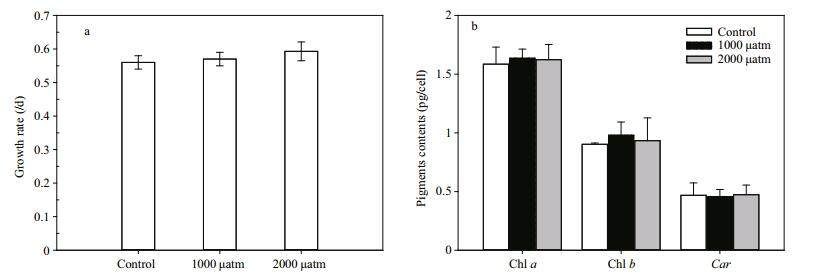
The growth rates of D. salina at three different pCO2 treatments were 0.56±0.02, 0.57±0.02 and 0.59±0.03, respectively (Fig. 1a). However, the growth rates of D. salina did not vary significantly (P > 0.05) among pCO2 treatments. Similar to the growth rates, elevated pCO2 had no significant (P > 0.05) effect on the concentrations of chlorophyll a, chlorophyll b and carotenoids (Fig. 1b).
|
| Figure 1 The growth rate (a) and pigment contents (chlorophyll a, chlorophyll b and carotenoids) (b) of D. salina acclimated to different pCO2 levels Data shown are the means±SE (n=9). |
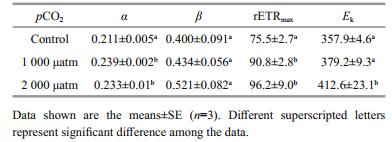
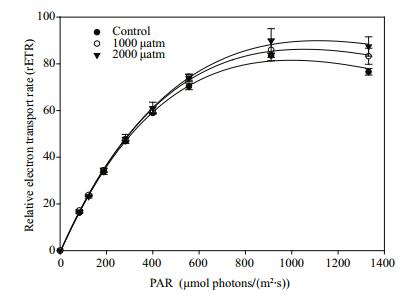
The rapid light curves (RLCs) of D. salina acclimated to different pCO2 levels showed a classical pattern of rETR as a function of PAR (Fig. 2). With regard to the parameters derived from the RLCs (Table 2), the α value increased significantly by 13.4% (P < 0.01) and 10.5% (P < 0.01) when exposed to 1 000 and 2 000 μatm pCO2, respectively. Similar to the trend in α, the rETRmax increased significantly by 20.4% (P < 0.05) and 27.5% (P < 0.01) when exposed to 1 000 and 2 000 μatm CO2, respectively. In addition, the Ek value under 2 000 μatm CO2 was significantly higher than that of the control (P < 0.01), while there was no significant difference between 390 μatm and 1 000 μatm CO2 (P > 0.05). Neither elevated pCO2 conditions significantly influenced the value of β (P > 0.05).
|
|
| Figure 2 The rapid light curves of D. salina acclimated to different pCO2 levels Data shown are the means±SE (n=3). |
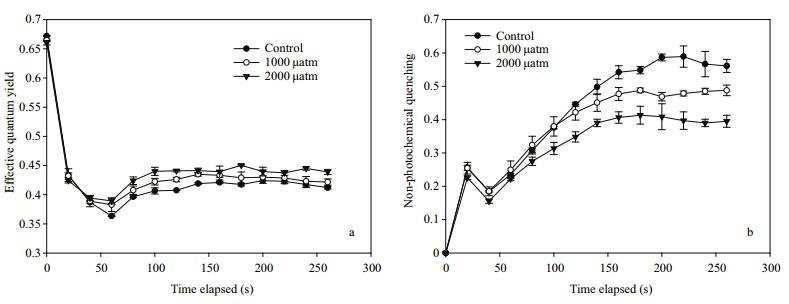
The ΔF/F'm value during the induction curves (Fig. 3a) showed that elevated pCO2 increased the effective quantum yield of D. salina. The ΔF/F'm value was significantly stimulated by 2.2% (P > 0.05) and 6.6% (P < 0.01) under 1 000 and 2 000 μatm CO2 relative to the control. Non-photochemical quenching (NPQ) showed a linear increase, then reached a plateau after 140 s. The cells acclimated to 1 000 and 2 000 μatm CO2 showed a lower NPQ of about 86.6% (P < 0.01) and 70.4% (P < 0.01) of that in 390 μatm CO2 (Fig. 3b).
|
| Figure 3 Effective quantum yield (yield) (a) and non-photochemical quenching (NPQ) (b) of D. salina acclimated to different pCO2 levels Data shown are the means±SE (n=3). |
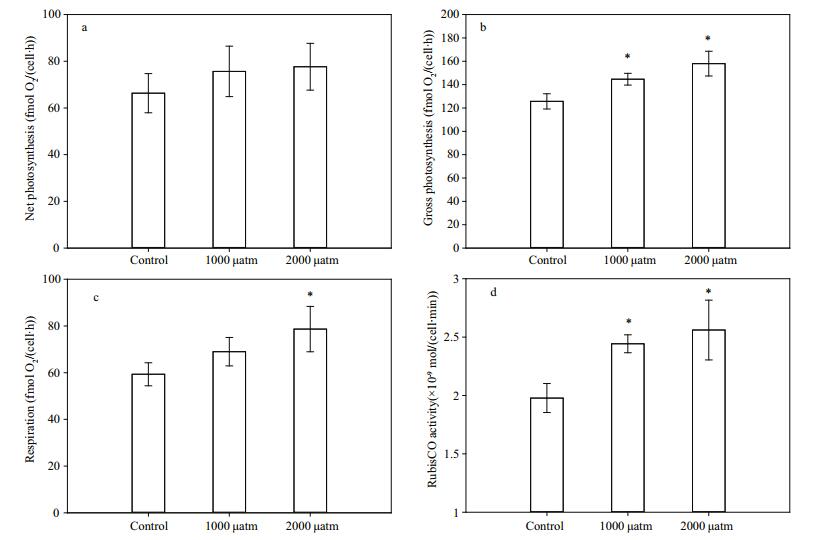
The gross photosynthetic O2 evolution (Fig. 4b) was significantly enhanced by 14.65% (P < 0.05) and 25.73% (P < 0.01) under 1 000 and 2 000 μatm pCO2, while elevated pCO2 levels did not significantly influence the net photosynthetic O2 evolution (Fig. 4a) of D. salina (P > 0.05). Similar to gross photosynthetic O2 evolution, Rubisco activity (Fig. 4d) was significantly stimulated by 23.86% (P < 0.05) and 29.95% (P < 0.01) under 1 000 and 2 000 μatm pCO2. Cells acclimated to 2 000 μatm pCO2 treatments showed a higher dark respiration rate than that of the control group (P < 0.05), while there was no significant difference between the control group and the 1 000 μatm pCO2 group (P > 0.05) (Fig. 4c).
|
| Figure 4 Net photosynthetic oxygen evolution (a), gross photosynthetic oxygen evolution (b), dark respiration (c) and Rubisco activity (d) of D. salina acclimated to different pCO2 levels Data are shown as the means±SE (n=3). The asterisks indicate significant differences with respect to the values of control cultures. |
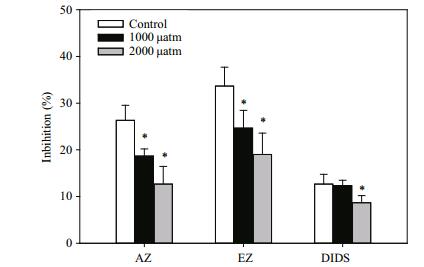
The inhibitors AZ and EZ had significant effects on the photosynthetic O2 evolution (Fig. 5) of D. salina under different pCO2 levels (P < 0.05). The inhibitory effect was more pronounced at 390 μatm pCO2 than at 1 000 and 2 000 μatm pCO2. The inhibitory effect of AZ on the photosynthetic O2 evolution of D. salina was 18.67% and 12.67% under 1 000 and 2 000 μatm pCO2, respectively, which was significantly lower than that of 390 μatm pCO2 (P < 0.05), for which the inhibitory rate was 26.33%. The inhibitory rates of EZ were 24.67% and 19.10% under the two high pCO2 treatments, respectively, while they were significantly lower than those obtained under 390 μatm pCO2 (P < 0.05). The inhibitory effects of DIDS on photosynthetic O2 evolution of D. salina did not differ significantly between the 390 and 1 000 μatm pCO2 treatments (P > 0.05), while the inhibitory effect of DIDS under 2 000 μatm pCO2 was significantly lower than that of 390 μatm pCO2.
|
| Figure 5 Inhibition rate of inhibitors (AZ, EZ and DIDS) on the photosynthetic O2 evolution of D. salina under different pCO2 levels Data are shown as the means±SE (n=3). The asterisks indicate significant differences with respect to the values of control cultures. |
The carbonic anhydrase activities of D. salina under different pCO2 levels are shown in Fig. 6. Relative to the control conditions, both the 1 000 μatm and 2 000 μatm pCO2 groups showed significantly reduced internal carbonic anhydrase activity (CAint), which decreased by 25.7% (P < 0.05) and 34.6% (P < 0.01), respectively. The external carbonic anhydrase activity (CAext) decreased significantly by 27.7% (P < 0.05) when exposed to 2 000 μatm pCO2 levels, while there was no significant difference between the 390 μatm and 1 000 μatm pCO2 groups (P > 0.05).

|
| Figure 6 Carbonic anhydrase activity of D. salina acclimated to different pCO2 levels Data shown are the means±SE (n=3). The asterisks indicate significant differences with respect to the values of control cultures. |
Different species of algae show different types of CCMs. The diatom Thalassiosira pseudonana (Yang and Gao, 2012), the diatom Phaeodactylum tricornutum (Burkhardt et al., 2001) and the chlorophyte Chlorella ellipsoidea (Matsuda and Colman, 1995) can use both CO2 and HCO3ˉ as a source of inorganic carbon, while the chlorophyte Nannochloris atomus (Huertas and Lubián, 1998) and the raphidophyceae Heterosigma akashiwo (Nimer et al., 1997) only use CO2. It is generally believed that CA ext functions to increase the CO2 concentration in the boundary layer by converting HCO3ˉ to CO2, thereby facilitating CO2 uptake. The presence of CCMs in D. salina is well established, and CAext plays an important role in the operation of CCMs (Booth and Beardall, 1991). In the present study, the net photosynthetic oxygen evolution of D. salina was significantly inhibited by acetazolamide (AZ) and 4, 4′-diisothiocyano-stilbene-2, 2′-disulfonate (DIDS), indicating that D. salina can use HCO3ˉ via CAext and anion exchange (AE) protein. Furthermore, the operation of CCMs was down-regulated under high pCO2 conditions, as indicated by the lower CA activity (CAext and CAint) and the lower inhibition of the photosynthetic rate by AZ, EZ and DIDS (Fig. 5) at high pCO2. Such down-regulation of CCMs might be attributed to the elevated availability of CO2 and HCO3ˉ, as well as the increased entry of inorganic carbon into the cell by passive diffusion. Mercado et al. (1997) found that the CAint activity of Porphyra leucosticte was significantly reduced at elevated pCO2 levels, while the CAext activity was unaffected. Conversely, only the CAext activity of Macrocystis pyrifera was reduced when acclimated to 1 200 μatm CO2 for 7 days, while the CAint showed no significant changes (Fernández et al., 2015). These different responses to elevated CO2 might be due to the different types of inorganic carbon uptake mechanisms in different species and the contribution of CCMs to photosynthetic carbon fixation. Trimborn et al. (2009) showed that both CA ext and CAint activities of T. pseudonana are unaffected when acclimated to 800 μatm CO2 for three days, while the CAint activity and the photosynthetic affinity for CO2 were lowered when the same strain was acclimated to 1 000 μatm CO2 for 15 days (more than 20 generations) (Yang and Gao, 2012; Wu et al., 2015). The inconsistent results of the responses to elevated pCO2 in the same strain might be due to the acclimation span or a different seawater carbonate system.
The long term cultivation experiment of D. salina under present and predicted future pCO2 conditions showed that photosynthesis of D. salina was significantly influenced by CO2-induced ocean acidification in terms of rETRmax, ΔF/F'm, α and Rubisco activities. Moreover, the results indicated that the photosynthetic rate in this species is not saturated at present seawater inorganic carbon concentrations. The ΔF'm of D. salina at high pCO2 levels was significantly higher than that observed at the present pCO2 levels. These results indicate that cells grown at high pCO2 can use light more efficiently. The positive response of ΔF/F'm to ocean acidification has also been reported for the rhodophyte Neosiphonia harveyi and the diatom Navicula directa (Torstensson et al., 2012; Olischläger and Wiencke, 2013). Generally, photosynthetic efficiency (α) represents light use efficiency (Fu et al., 2007, 2008). In D. salina, α in the control was significantly lower than in the high pCO2 treatments, suggesting that future high pCO2 levels increased the light use efficiency in D. salina. Moreover, Fu et al. (2007) proposed that an increased α at high pCO2 is probably due to reduced energy allocation to the concentration CO2 by the cell and for more efficient light use. The results of the present study support the conclusions of Fu et al. (2007), who found that elevated pCO2 down-regulated the CCMs in D. salina, as indicated by decreased carbonic anhydrase activity and lowered inhibition of photosynthetic O2 evolution by AZ, EZ and DIDS at high pCO2. Ek (rETRmax /α) represents the optimum light of the photosynthetic apparatus to maintain a balance between photosynthetic energy capture and the capacity to process this energy (Falkowski and Raven, 1997). Liu et al. (2012) reported that CO2-induced seawater acidification down-regulated CCMs in the green alga, Ulva prolifera, and that the subsequent energy savings contributed to a lower Ek. In the present study, although CCMs were also down-regulated at high pCO2 in D. salina, the energy saved did not lead to a lower Ek. On the contrary, increased Ek values at high pCO2 were found in D. salina, probably due to stimulation of the maximal photosynthetic capacity (rETRmax). Notably, the growth illumination of D. salina in our experiment was far below the Ek value, suggesting that D. salina was light-limited. The higher Ek at high pCO2 indicates that the growth of D. salina under future conditions may be closely related to the availability of light, and that light is more likely to be a limiting factor for D. salina in the future. Conversely, the higher Ek at high pCO2 also indicated that elevated pCO2 led to a higher light threshold at which light becomes excessive, so that algae are less likely to experience light stress than under the present conditions.
Non-photochemical quenching (NPQ) is composed of energy-dependent quenching (qE), which is induced by acidification of the thylakoid lumen, state transition quenching (qT), which is concerned with the balance in the distribution of excitation energy between the two photosystems, and photoinhibitory quenching (qI), which is related to photo-inhibition of photosynthesis (Krause and Jahns, 2004). In the present study, CO2-induced ocean acidification significantly decreased the NPQ of D. salina (Fig. 3b). The synthesis of ATP will lead to translocation of the hydrogen ion from the thylakoid lumen to the stroma, thus weakening the acidification of the thylakoid lumen. Because enhanced carboxylation (Fig. 4d) at high pCO2 requires more ATP, more H+ ions are transported out of the thylakoid lumen, leading to decreased qE. However, cyclic electron transports, which play an important role in photo-protection by producing and maintaining the ΔpH, can be accelerated by the operation of CCMs (Heimann and Schreiber 1999). Down-regulated CCMs of D. salina can reduce cyclic electron transport (Moroney and Somanchi, 1999), leading to increased qI. Thus, the response of NPQ to elevated pCO2 appears to reflect the net effects of a decreased qE and an increased qI.
Ribulose-1, 5-bisphosphate carboxylase/oxygenase (Rubisco) catalyzes the first step in photosynthetic carbon fixation and is the rate-limiting reaction of the Calvin cycle (Spreitzer and Salvucci, 2002). In the present study, increased Rubisco activity at high pCO2 indicated that elevated pCO2 enhanced the photosynthetic carbon fixation in D. salina (Fig. 4d). Similar to the trend in rubisco activity, high pCO2 conditions enhanced dark respiration in D. salina (Fig. 4c), indicating a higher energy requirement due to either enhanced biosynthesis in response to increased photosynthetic carbon fixation or increased energy requirements to maintain the intracellular acid-base stability (Geider and Osborne, 1989). The enhanced dark respiration would consume more gross photosynthetic production under future high pCO2 and low pH conditions (del Giorgio and Duarte, 2002). Therefore, the unchanged growth of D. salina could be attributed to the balance of the stimulated carbon assimilation and carbon loss. Similar to D. salina, enhanced photosynthesis and respiration were found in the diatom Thalassiosira pseudonana, while growth was not significantly affected by high pCO2 (Yang and Gao, 2012). Stimulated photosynthesis and growth were found in Phaeodactylum tricornutum, Ulva prolifera and Heterosigma akashiwo (Fu et al., 2008; Wu et al., 2010; Xu and Gao, 2012) under high pCO2 conditions, since energy was saved in downregulated CCMs or when there was enhanced availability of CO2. However, many other studies have shown no significant effects (Fu et al., 2008; Fernández et al., 2015), and even negative effects (Gao et al., 2012; Iñiguez et al., 2016) on the growth and photosynthesis of marine phytoplankton. These studies, together with our results, indicate that the different responses of marine phytoplankton to CO2-induced ocean acidification might be related to the net outcome of the positive and negative (extra carbon loss) effects, although the diverse mechanisms of inorganic carbon acquisition and other environmental factors might affect the responses of marine algae to elevated pCO2.
It has been widely reported that other environmental factors, such as light intensity, temperature and nutrient supply, might modulate the response of algae to increased pCO2. Gao et al. (2012) found that stimulated growth of P. tricornutum, T. pseudonana and Skeletonema costatum under high pCO2 levels was observed under low light levels, whereas a reverse trend was observed at high light levels. Similarly, different light intensity and nitrogen levels modulated the effects of pCO2 on Gracilaria lemaneiformis (Zou and Gao, 2009; Zou et al., 2011). The PSP toxin content of Alexandrium fundyense decreased with elevated pCO2 under N-limited conditions, while it showed an adverse trend under N-replete conditions (Eberlein et al., 2016). For H. akashiwo, the effects of pCO2 on the growth rate were dependent on temperature, with a positive effect observed at 20℃ and no effect observed at 24℃ (Fu et al., 2008). For the red algae Chondrus crispus, a significant effect of elevated pCO2 on photosynthesis and growth was only observed in interactions with either high temperature or reduced light levels (Sarker et al., 2013). Overall, the results of these studies indicate how changeable and complicated the response of D. salina to the predicted future ocean might be, and that this response might be modulated by experimental conditions.
6 CONCLUSIONBased on the results of the present study, CO2-induced ocean acidification might lead D. salina to enhance photosynthesis, down-regulate their CCMs, and stimulate dark respiration, resulting in an unchanged growth rate. The net effect of ocean acidification on microalgae will be determined by the balance of these positive and negative effects. However, further studies are needed to evaluate the interactive effects of pCO2, light, nutrients and temperature on growth, photosynthesis and other physiological processes to determine how this species might respond to future ocean conditions.
7 ACKNOWLEDGMENTWe thank all of the members of the laboratory and Dr. LIANG in the College of Fisheries of Ocean University of China for their help.
| Axelsson L, Ryberg H, Beer S, 1995. Two modes of bicarbonate utilization in the marine green macroalga Ulva lactuca. Plant Cell Environ., 18(4): 439–445. Doi: 10.1111/pce.1995.18.issue-4 |
| Badger M R, Andrews T J, Whitney S M, et al, 1998. The diversity and coevolution of Rubisco, plastids, pyrenoids, and chloroplast-based CO2-concentrating mechanisms in algae. Can J Bot, 76(6): 1 052–1071. |
| Booth W A, Beardall J, 1991. Effects of salinity on inorganic carbon utilization and carbonic anhydrase activity in the halotolerant alga Dunaliella salina (Chlorophyta). Phycologia, 30(2): 220–225. Doi: 10.2216/i0031-8884-30-2-220.1 |
| Burkhardt S, Amoroso G, Riebesell U, Sültemeyer D, 2001. CO2 and HCO3 uptake in marine diatoms acclimated to different CO2 concentrations. Limnol. Oceanogr., 46(6): 1 378–1 391. Doi: 10.4319/lo.2001.46.6.1378 |
| Caldeira K, Wickett M E, 2003. Oceanography:anthropogenic carbon and ocean pH. Nature, 425(6956): 365. Doi: 10.1038/425365a |
| del Giorgio P A, Duarte C M, 2002. Respiration in the open ocean. Nature, 420(6914): 379–384. Doi: 10.1038/nature01165 |
| Eberlein T, van de Waal D B, Brandenburg K M, John U, Voss M, Achterberg E P, Rost B, 2016. Interactive effects of ocean acidification and nitrogen limitation on two bloomforming dinoflagellate species. Mar. Ecol. Prog. Ser., 543: 127–140. Doi: 10.3354/meps11568 |
| Falkowski P G, Raven J A, 2013. Aquatic Photosynthesis. Aquatic Photosynthesis. Princeton University Press, Princeton. |
| Fernández P A, Roleda M Y, Hurd C L, 2015. Effects of ocean acidification on the photosynthetic performance, carbonic anhydrase activity and growth of the giant kelp Macrocystis pyrifera. Photosynth. Res., 124(3): 293–304. Doi: 10.1007/s11120-015-0138-5 |
| Fu F X, Warner M E, Zhang Y H, Feng Y Y, Hutchins D A, 2007. Effects of increased temperature and CO2 on photosynthesis, growth, and elemental ratios in marine Synechococcus and Prochlorococcus (Cyanobacteria). J.Phycol., 43(3): 485–496. Doi: 10.1111/jpy.2007.43.issue-3 |
| Fu F X, Zhang Y H, Warner M E, Feng Y Y, Sun J, Hutchins D A, 2008. A comparison of future increased CO2 and temperature effects on sympatric Heterosigma akashiwo and Prorocentrum minimum. Harmful Algae, 7(1): 76–90. Doi: 10.1016/j.hal.2007.05.006 |
| Gao K S, Xu J T, Gao G, Li Y H, Hutchins D A, Huang B Q, Wang L, Zheng Y, Jin P, Cai X N, Häder D P, Li W, Xu K, Liu N N, Riebesell U, 2012. Rising CO2 and increased light exposure synergistically reduce marine primary productivity. Nat. Climate Change, 2(7): 519–523. |
| Geider R J, Osborne B A, 1989. Respiration and microalgal growth:a review of the quantitative relationship between dark respiration and growth. New Phytol., 112(3): 327–341. Doi: 10.1111/nph.1989.112.issue-3 |
| Gerard V A, Driscoll T, 1996. A spectrophotometric assay for rubisco activity:application to the kelp Laminaria saccharina and implications for radiometric assays1. J.Phycol., 32(5): 880–884. Doi: 10.1111/j.0022-3646.1996.00880.x |
| Giordano M, Beardall J, Raven J A, 2005. CO2 concentrating mechanisms in algae:mechanisms, environmental modulation, and evolution. Annu. Rev. Plant Biol., 56(1): 99–131. Doi: 10.1146/annurev.arplant.56.032604.144052 |
| Giordano M, Maberly S C, 1989. Distribution of carbonic anhydrase in British marine macroalgae. Oecologia., 81(4): 534–539. |
| Guillard R R L. 1975. Culture of phytoplankton for feeding marine invertebrates. In: Smith W L, Chanley M H eds. Culture of Marine Invertebrate Animals. Springer, New York, USA. p. 29-60 |
| Guinotte J M, Fabry V J, 2008. Ocean acidification and its potential effects on marine ecosystems. Ann. N. Y. Acad.Sci., 1134(1): 320–42. Doi: 10.1196/nyas.2008.1134.issue-1 |
| Heimann S, Schreiber U, 1999. Cyt b-559 (Fd) participating in cyclic electron transport in spinach chloroplasts:evidence for kinetic connection with the cyt b6/f complex. Plant Cell Physiol., 40(8): 818–824. Doi: 10.1093/oxfordjournals.pcp.a029610 |
| Huertas I E, Lubián L M, 1998. Comparative study of dissolved inorganic carbon utilization and photosynthetic responses in Nannochloris (chlorophyceae) and Nannochloropsis(eustigmatophyceae) species. Can. J. Bot., 76(6): 1 104–1 108. |
| Hutchins D A, Fu F X, Zhang Y, Warner M E, Feng Y, Portune K, Bernhardt P W, Mulholland M R, 2007. CO2 control of Trichodesmium N2 fixation, photosynthesis, growth rates, and elemental ratios:Implications for past, present, and future ocean biogeochemistry. Limnol. Oceanogr., 52(4): 1 293–1 304. Doi: 10.4319/lo.2007.52.4.1293 |
| Ihnken S, Roberts S, Beardall J, 2011. Differential responses of growth and photosynthesis in the marine diatom Chaetoceros muelleri to CO2 and light availability. Phycologia, 50(2): 182–193. Doi: 10.2216/10-11.1 |
| Iñiguez C, Carmona R, Lorenzo M R, Niell F X, Wiencke C, Gordillo F J L, 2016. Increased CO2 modifies the carbon balance and the photosynthetic yield of two common arctic brown seaweeds:Desmarestia aculeata and Alaria esculenta. Polar Biol., 39(11): 1 979–1 991. Doi: 10.1007/s00300-015-1724-x |
| Iñíguez C, Lorenzo M R, Niell F X, Wiencke C, Gordillo F J, Carmona Fernández R. 2015. Effects of increased CO2 in the carbon budget and the photosynthetic yield of the arctic seaweeds Alaria esculenta and Desmarestia aculeata. http://hdl.handle.net/10630/9265. |
| IPCC. 2013. Climate Change 2013: the physical science basis. In: Stocker TF, Qin D, Plattner GK, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex V, Midgley P M eds. Contribution of working group I to the fifth assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge. p. 1 535. |
| Kranz S A, Dieter S, Richter K U, Rost B, 2009. Carbon acquisition by Trichodesmium:the effect of pCO2 and diurnal changes. Limnol. Oceanogr., 54(2): 548–559. Doi: 10.4319/lo.2009.54.2.0548 |
| Krause G H, Jahns P. 2004. Non-photochemical energy dissipation determined by chlorophyll fluorescence quenching: characterization and function. In: Papageorgiou G C, Govindjee eds. Chlorophyll a Fluorescence. Springer, Netherlands. p. 463-495 |
| Kurihara H, Asai T, Kato S, Ishimatsu A, 2008. Effects of elevated pCO2 on early development in the mussel Mytilus galloprovincialis. Aquat. Biol., 4(3): 225–233. |
| Langer G, Geisen M, Baumann K H, Kläs J, Riebesell U, Thoms S, Young J R, 2006. Species-specific responses of calcifying algae to changing seawater carbonate chemistry. Geochem. Geophys. Geosyst., 7(9): 535–540. |
| Lewis E, Wallace D, Allison L J. 1998. Program developed for CO2 system calculations. Tennessee: Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, US Department of Energy. |
| Liu Y T, Xu J T, Gao K S, 2012. CO2-driven seawater acidification increases photochemical stress in a green alga. Phycologia, 51(5): 562–566. Doi: 10.2216/11-65.1 |
| Liu Z Y, Zhang L J, Cai W J, Wang L, Xue M, Zhang X S, 2014. Removal of dissolved inorganic carbon in the yellow river estuary. Limnol. Oceanogr., 59(2): 413–426. Doi: 10.4319/lo.2014.59.2.0413 |
| Matsuda Y, Colman B, 1995. Induction of CO2 and bicarbonate transport in the green alga chlorella ellipsoidea (Ⅱ.Evidence for induction in response to external CO2 concentration). Plant Physiol., 108(1): 253–260. Doi: 10.1104/pp.108.1.253 |
| Mercado J M, Javier F, Gordillo L, Niell F X, Figueroa F L, 1999. Effects of different levels of CO2 on photosynthesis and cell components of the red alga Porphyra leucosticta. J. Appl. Phycol., 11(5): 455–461. Doi: 10.1023/A:1008194223558 |
| Mercado J M, Niell F X, Figueroa F L, 1997. Regulation of the mechanism for HCO3- use by the inorganic carbon level in 3 Porphyra leucosticta Thur.in Le Jolis (Rhodophyta). Planta, 201(3): 319–325. Doi: 10.1007/s004250050073 |
| Moroney J V, Husic H D, Tolbert N E, 1985. Effect of carbonic anhydrase inhibitors on inorganic carbon accumulation by Chlamydomonas reinhardtii. Plant Physiol., 79(1): 177–183. Doi: 10.1104/pp.79.1.177 |
| Moroney J V, Somanchi A, 1999. How do algae concentrate CO2 to increase the efficiency of photosynthetic carbon fixation?. Plant Physiol., 119(1): 9–16. Doi: 10.1104/pp.119.1.9 |
| Nimer N A, Iglesias-Rodriguez M D, Merrett M J, 1997. Bicarbonate utilization by marine phytoplankton species. J. Phycol., 33(4): 625–631. Doi: 10.1111/j.0022-3646.1997.00625.x |
| Olischläger M, Wiencke C, 2013. Ocean acidification alleviates low-temperature effects on growth and photosynthesis of the red alga Neosiphonia harveyi (rhodophyta). J. Exp.Bot., 64(18): 5 587–5 597. Doi: 10.1093/jxb/ert329 |
| Platt T, Gallegos C L, Harrison W G, 1980. Photoinhibition of photosynthesis in natural assemblages of marine phytoplankton. J. Mar. Res., 38: 687–701. |
| Ralph P J, Gademann R, 2005. Rapid light curves:a powerful tool to assess photosynthetic activity. Aquat. Bot., 82(3): 222–237. Doi: 10.1016/j.aquabot.2005.02.006 |
| Reinfelder J R, 2011. Carbon concentrating mechanisms in eukaryotic marine phytoplankton. Annu. Rev. Mar. Sci., 3(1): 291–315. Doi: 10.1146/annurev-marine-120709-142720 |
| Riebesell U, Schulz K G, Bellerby R G J, Botros M, Fritsche P, Meyerhöfer M, Neill C, Nondal G, Oschlies A, Wohlers J, Zöllner E, 2007. Enhanced biological carbon consumption in a high CO2 ocean. Nature, 450(7169): 545–548. Doi: 10.1038/nature06267 |
| Rost B, Richter K U, Riebesell U, Hansen P J, 2006. Inorganic carbon acquisition in red tide dinoflagellates. Plant Cell Environ., 29(5): 810–822. Doi: 10.1111/pce.2006.29.issue-5 |
| Rost B, Riebesell U, Burkhardt S, Sültemeyer D, 2003. Carbon acquisition of bloom-forming marine phytoplankton. Limnol. Oceanogr, 48(1): 55–67. Doi: 10.4319/lo.2003.48.1.0055 |
| Sarker M Y, Bartsch I, Olischläger M, Gutow L, Wiencke C, 2013. Combined effects of CO2, temperature, irradiance and time on the physiological performance of Chondrus crispus (Rhodophyta). Bot. Mar., 56(1): 63–74. |
| Spreitzer R J, Salvucci M E, 2002. RUBISCO:structure, regulatory interactions, and possibilities for a better enzyme. Annu. Rev. Plant Biol., 53(1): 449–475. Doi: 10.1146/annurev.arplant.53.100301.135233 |
| Torstensson A, Chierici M, Wulff A, 2012. The influence of increased temperature and carbon dioxide levels on the benthic/sea ice diatom navicula directa. Polar Biol., 35(2): 205–214. Doi: 10.1007/s00300-011-1056-4 |
| Trimborn S, Lundholm N, Thoms S, Richter K U, Krock B, Hansen P J, Rost B, 2008. Inorganic carbon acquisition in potentially toxic and non-toxic diatoms:the effect of pHinduced changes in seawater carbonate chemistry. Physiol Plant, 133(1): 92–105. Doi: 10.1111/j.1399-3054.2007.01038.x |
| Trimborn S, Wolf-Gladrow D, Richter K U, Rost B, 2009. The effect of pCO2 on carbon acquisition and intracellular assimilation in four marine diatoms. J. Exp. Mar. Biol. Ecol., 376(1): 26–36. Doi: 10.1016/j.jembe.2009.05.017 |
| Van de Waal D B, John U, Ziveri P, Reichart G J, Hoins M, Sluijs A, Rost B, 2013. Ocean acidification reduces growth and calcification in a marine dinoflagellate. PLoS One, 8(6): e65987. Doi: 10.1371/journal.pone.0065987 |
| Wellburn A R, 1994. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol., 144(3): 307–313. Doi: 10.1016/S0176-1617(11)81192-2 |
| Wu Y P, Beardall J, Gao K S, 2015. Physiological responses of a model marine diatom to fast pH changes:special implications of coastal water acidification. PLoS One, 10(10): e0141163. Doi: 10.1371/journal.pone.0141163 |
| Wu Y, Gao K, Riebesell U, 2010. CO2-induced seawater acidification affects physiological performance of the marine diatom Phaeodactylum tricornutum. Biogeosciences, 7(9): 2 915–2 923. Doi: 10.5194/bg-7-2915-2010 |
| Xu J T, Gao K S, 2012. Future CO2-induced ocean acidification mediates the physiological performance of a green tide alga. Plant Physiol., 160(4): 1 762–1 769. Doi: 10.1104/pp.112.206961 |
| Yang G Y, Gao K S, 2012. Physiological responses of the marine diatom Thalassiosira pseudonana to increased pCO2 and seawater acidity. Mar. Environ. Res., 79: 142–151. Doi: 10.1016/j.marenvres.2012.06.002 |
| Zhang X X, Tang X X, Zhou B, Hu S X, Wang Y, 2015. Effect of enhanced UV-B radiation on photosynthetic characteristics of marine microalgae Dunaliella salina(Chlorophyta, Chlorophyceae). J. Exp. Mar. Biol. Ecol., 469: 27–35. Doi: 10.1016/j.jembe.2015.04.002 |
| Zou D H, Gao K S, Luo H J, 2011. short-and long-term effects of elevated CO2 on photosynthesis and respiration in the marine macroalga Hizikia fusiformis (Sargassaceae, Phaeophyta) grown at low and high N supplies. J. Phycol., 47(1): 87–97. Doi: 10.1111/j.1529-8817.2010.00929.x |
| Zou D H, Gao K S, 2009. Effects of elevated CO2 on the red seaweed Gracilaria lemaneiformis (Gigartinales, Rhodophyta) grown at different irradiance levels. Phycologia, 48(6): 510–517. Doi: 10.2216/08-99.1 |
 2018, Vol. 36
2018, Vol. 36


