Institute of Oceanology, Chinese Academy of Sciences
Article Information
- HE Yunhong(何云红), SUN Chengjun(孙承君), LI Wenjuan(李文娟), YANG Gui-Peng(杨桂朋), DING Haibing(丁海兵)
- Degradation of lipids in seasonal hypoxic seawater under different oxygen saturation
- Chinese Journal of Oceanology and Limnology, 36(5): 1570-1585
- http://dx.doi.org/10.1007/s00343-018-7110-0
Article History
- Received Apr. 7, 2017
- accepted in principle Jun. 19, 2017
- accepted for publication Oct. 9, 2017
2 Marine Ecology Center, the First Institute of Oceanography, State Oceanic Administration(SOA), Qingdao 266061, China;
3 Laboratory for Marine Ecology and Environmental Science, Qingdao National Laboratory for Marine Science and Technology, Qingdao 266071, China;
4 College of Chemistry and Chemical Engineering, Ocean University of China, Qingdao 266100, China;
5 Qingdao Collaborative Innovation Center of Marine Science and Technology, Ocean University of China, Qingdao 266100, China
Organic matter degradation in seawater is an important process in marine carbon and nutrient cycle. Generally, organic matter degradation in marine environments is affected by physical, chemical and biological processes, which can be considered as internal and external factors. The internal factors include the composition and structure of organic matter (Sun et al., 2000; Ding and Sun, 2005; Vieler et al., 2007) and the external factors includes redox condition, light intensity, wind speed, temperature, salinity, bioturbation, microorganism consumption and etc. (Aller, 1984; Canfield, 1994; Harvey et al., 1995; Sun and Wakeham, 1998; Sun et al., 2002; Sun and Dai, 2005; Dai and Sun, 2007, Pruski et al., 2017). Redox condition, determined by oxygen saturation, is among fundamental factors. However, the role of oxygen on organic matter degradation is still a controversial issue. In earlier studies, the aerobic and anaerobic degradation was similar (Foree and McCarty, 1970; Jewell and McCarty, 1971; Otsuki and Hanya, 1972a, b; Sun et al., 2004). For example, Lee (1992) reported that the degradation rates of organic matter had no clear distinction in aerobic and anaerobic sediments in subsequent researches. On the other side, series of later studies indicated that existence of oxygen may promote organic matter degradation (Emerson and Hedges, 1988; Sun et al., 1997; Hartnett et al., 1998; Caradec et al., 2004; Lv et al., 2010). Harvey et al. (1995) analyzed the alga carbon under oxic and anoxic experimental incubations and estimated the degradation rates according to the multi-G model reported by Westrich and Berner (1984). They found that oxic decomposition of organic matter had much higher degradation rates than those of anoxic decomposition. More recent studies consider that existence of oxygen can promote organic matter degradation, and anoxic condition is favorable for organic matter preservation in sediment (Ding and Sun, 2005a; Pan et al., 2014, 2017). However, degradation and preservation of organic matter in marine environment is complex, and data accumulation about organic matter degradation is very limited, especially in water column. The mechanism, pathway and control factors about organic matter degradation are still far away from completely understanding.
In some coastal areas and estuaries, redox condition keeps changing because of seasonal hypoxia phenomena. In the Changjiang estuary, a developing anoxic zone (around 122°35′E, 31°45′N) has been observed in summer from the 1950s. The annual cycle of hypoxia off the Changjiang estuary has been reported by Wang et al. (2012). As the time going on, the area is becoming wider and the hypoxia situation deteriorates more seriously. The oxygen saturation (a ratio of the concentration of dissolved oxygen (O2) in the seawater to the maximum amount of oxygen that will dissolve in the seawater at that temperature and pressure under stable equilibrium) has a large span ranging from over 100% on the surface to about 25% at the 20–50 m of the water column in August sectional survey (Liu et al., 2012). Just like that, the Zhujiang (Pearl) River estuary is also experiencing hypoxia in summer time since 1985. In these areas, coexistence of seasonal hypoxia and red tide were occurred frequently and caused serious ecological problems (Sun et al., 2008; Sun and Song, 2009). However, little research focuses on the degradation of organic matter in the seasonal hypoxic condition under various oxygen saturations. Therefore, further research is necessary to investigate organic matter degradation in the marine environments with continuously changing oxygen saturation.
To study organic matter degradation in marine environment, tracking variations of lipids is a frequently used method. In fact, organic compounds in seawater include lipids, carbohydrates, amino acids or proteins, humic substance (HS) and etc. They undergo different decomposition processes under complex redox condition (Westrich and Berner, 1984). Compared with proteins and carbohydrates, lipids are less reactive and more easily to be preserved in various natural environment (Volkman et al., 1987; Wakeham and Lee, 1993; Sun et al., 2004). Therefore, lipids are considered as typical biomarkers in studying organic matter degradation (Gagosian et al., 1983; Derieux et al., 1998). In marine microalgae, lipids have important physiological and metabolic functions. For example, in algal cells, phospholipids are fundamental component in cell membrane; and triacylglycerols are used for metabolism as energy storage substances. Because of structural difference, lipids have various degradation activities even under the same redox conditions. For example, Sun et al. (2002) indicated that the oxic conditions accelerated cell-associated lipids degradation. Ding et al. (2005) confirmed that fatty acids on membrane degraded more easily than those on intracellular compounds under anaerobic conditions. Although series previous studies investigated degradation of lipids under various redox conditions, their degradation in marine environment with different oxygen saturation is still undocumented.
In this study, series of laboratory incubation experiments with algae were conducted to simulate organic matter degradation in seasonal hypoxic area along China coast and estuaries with various oxygen saturations. Skeletonema costatum, a typical red-tide diatom in the hypoxic area, was selected for the incubation experiments. Variations of lipids in the experimental systems were tracked to evaluate the effects of oxygen saturation on organic matter degradation in seasonal hypoxic seawater. This is the first study about lipid degradation in seawater under various oxygen saturations, providing fundamental results to understand organic carbon cycle in marine environment more deeply.
2 MATERIAL AND METHOD 2.1 Sampling and materialsThe natural sea waters for laboratory incubation were collected in October 2011 from P01 station (122.72°E, 31.04°N) (Fig. 1) nearby the estuary of the Changjiang River during fall cruise of a 973 Project (China) in the East China Sea. The seawater was collected from the sea surface and bottom (50 m). The surface water was oxic and the bottom water was hypoxic (oxygen saturation was about 40%). The mixture of the surface and bottom seawater (v/v 1:1) was used for the experiment. The marine alga Skeletonema costatum used for incubation experiments is widely distributed along coastal China as a typical red-tide algae (Sun et al., 2008; Sun and Song, 2009). Skeletonema costatum was obtained from the Marine Interface Chemistry Lab in the Ocean University of China and continuously cultured in 1-L conical flask with f/2 medium at 20±0.5℃ (Yang et al., 2002; Urbani et al., 2005). The light-dark cycle and luminous intensity were 12-h/12-h and 4 000 lx, respectively. After about two weeks, the alga was collected at the end of exponential phase by centrifugation and stored at -18℃ for later incubation experiment.
|
| Figure 1 Sampling station closed to the Changjiang River estuary in the East China Sea |
Before the experiment, all glassware, such as 500 mL flask, 250 mL separating funnel, 50 mL centrifuge tube, 20 mL glass tube, glass pipette, 100 mL and 500 mL beaker, 50 mL and 100 mL pearshaped bottle, 4 mL vial et al., was soaked by FL-70 detergent at least for 48 h, then orderly washed three times by tap water, high purity water and Milli-Q H2O. After drying in the oven at 100℃, the glassware was put into the muffle furnace to remove residual organic matter at 450℃ for 6 h. Besides, the washed plastics including plug and lid with Teflon material was saved by burned aluminum-foil paper, and rinsed three times by methanol, dichloromethane and n-hexane before use.
2.2 Microcosm setupIncubation experiments were conducted based on series previous designed degradation experiments (Lee, 1992; Sun et al., 1997; Sun and Wakeham, 1998; Sun et al., 2000, 2002; Ding and Sun, 2004, 2005a, b; Sun and Dai, 2005). The incubation microcosm contained a series of 500 mL flasks filled with mixed seawater under different oxygen saturation. Every flask had 200 mL seawater and about 300 mg thawed algal cells (~90% water) (if carbon account for 40% of algal dry weitht, the concentration of fresh carbon in the seawater should be 60 mg C/L). This biomass is constant with the most serious situation of the red tide in the East China Sea (The maximum concentration of POC in the seawater was up to about 60 mg C/L (Lin, 2007) after red tide season around the Changjiang estuary). All flasks were sealed with rubber plugs with inserted glass pipes for gas exchange. Four series incubation experiments were set up as anoxic, hypoxic and oxic seawater with oxygen saturations of 0%, 25%, 50% and 100% to simulate marine environment changing from anoxic to oxic condition, respectively. The oxic and anoxic sea water with 100% and 0% oxygen saturation was obtained by bubbling pure air and N2 into it until liquid-gas balance, respectively. Mixture gases with N2/air (v/v) ratios 1:1 and 3:1 were blown into the incubation system to form hypoxic sea water with oxygen saturations 25% and 50%, respectively. A little CO2 was also purged into the sea water to maintain pH value. During the incubation, the N2 and mixture gases with a little CO2 were purged into the seawater samples at least twice a day to stabilize the oxygen saturations for anoxic and hypoxic sea water. Pure air was continuously bubbled into oxic seawater to keep 100% oxygen saturation. Preliminary experiments confirmed that oxygen saturations in the incubation were stable by treating the sea water using the described method. All flasks were placed in an incubator (GPX-250, Hunan, China) in dark at 20±0.5℃, similar as the water temperature in summer around the P01 station. The whole experimental period was 90 days. At t=0, 5, 10, 17, 24, 35, 50, 70, 90 d, one flask from each series were taken out from the incubator, and the alga in the sea water was separated by centrifugation (10 min, 2 500 r/min, Era Beili GTR-16B centrifuge, Ding and Sun, 2005b) and stored at -20℃ for future treatment.
2.3 Lipid extractionThe method for lipid extraction was based on published procedure (Sun et al., 1997). Briefly, a small part of alga (about 1/10) was heated at 100℃ for 24 h to measure algal water content. The residue was firstly soaked with 10 mL methanol and vortexed for 1 min. After that, the mixture was sonicated for 1–2 min, followed by centrifuging at 2 500 r/min for 10 min at 4℃. The supernatant was decanted from centrifuge tube and transferred to separating funnel. Then the residual was extracted with 3×10 mL dichloromethane-methanol (2:1 v/v). During each extraction, samples were vortexed for 1 min, sonicated for 1–2 min and centrifuged for 10 min. Combined extracts were partitioned into a methylene phase formed by adding 5% NaCl solution in the separating funnel. The organic phase was released to pyriform flask. In order to increase the efficiency of extraction, the aqueous phase was re-extracted by 3×10 mL dichloromethane and all organic phases were transferred to the flask. Recovery test indicated that relative standard deviation of the extraction was ±2.6% (Sun et al., 1997).
After evaporating the organic phase with a rotary evaporator, the lipids in the flask were rinsed by 3×1 mL 0.5 mol/L potassium hydroxide-methanol solution and saponified at 100℃ for 2 h. The neutral lipids were extracted from the basic solution and the fatty acids were extracted after addition of HCl (pH < 2) by 3×5 mL hexane, respectively. After rotary evaporation, the neutral lipids were washed with 3×1 mL n-hexane and then transferred to 4 mL vial. The hexane was blew-dried by pure nitrogen gas and the neutral lipids were reacted with 100 μL BSTFA (Bis(trimethylsilyl)trifluoroacetamide) in 200 μL acetonitrile at 100℃ for 2 h. The acetonitrile was also blew-dried by pure N2 and the silylated neutral lipids were stored at 4℃ for future neutral lipid analysis. The fatty acids solution was rotary evaporated and the flask was washed by 3×1 mL 10% BF3-methanol (RS-Supelco cat 33021) and 3× 1 mL methanol. After esterification at 100℃ for 2 h, the fatty acids were converted into fatty acid methyl esters (FAMEs). The FAMEs was extracted by 3×5 mL n-hexane and dried with a rotary evaporator, followed by 3×1 mL n-hexane rinsing and transferring to 4 mL vial. The hexane in the vial was blow-dried by pure N2, and the fatty acids were also stored at 4℃ for future analysis.
2.4 Lipid analysisGas chromatography-mass spectrometry (GC-MS) and gas chromatography (GC) were used for qualitative and quantitative analysis of lipids. Prior to analysis, 50 μL 5α-cholestane (1 mg/mL in hexane) and nonadcanoic acid methyl ester (2 mg/mL in hexane) as internal standards for silylated neutral lipids and FAMEs were added into the vials, respectively. An Agilent 7890N GC-MS system equipped with a 30 m×0.25 mm×0.25 μm column (DB-5MS, Agilent) coated with phenyl arylene polymer virtually equivalent to a (5%-phenyl)- methylpolysiloxane was applied for lipid identification. The GC-MS operating conditions were: injection volume 1 μL, mass range 50–550 amu, ionizing energy 70 eV and ion source temperature 240℃. The carrier gas was helium. The oven temperature gradient for neutral lipids was: 50–200℃ by 30℃/min and stayed at 200℃ for 10 min, then increased to 200–215℃ by 5℃/min, continued to 215–220℃ by 1℃/min and held at 220℃ for 2 min, then increased to 220–280℃ by 10℃/min and stayed at 280℃ for 30 min. The oven temperature gradient for fatty acids was: 30–200℃ by 30℃/min, increased to 200–215℃ by 5℃/min, continued to 215–220℃ by 1℃/min, held at 220℃ for 2 min, then increased to 220–280℃ by 10℃/min and stayed at 280℃ for 4 min, and finally increased to 280–310℃ by 20℃/ min and stopped at 310℃ for 5 min. The neutral lipids and fatty acids were identified based on their MS profile and retention time.
For quantitative analysis of lipids, an Agilent 6890N GC system with an automatic injector and a flame ionization detector (FID) was used. The neutral lipids and fatty acids were separated by a 30.0 m× 250 μm×0.25 μm column (HP-5MS, Agilent) coated with (5%-phenyl)-methylpolysiloxane copolymer. The temperature gradient of the oven was the same as the GC-MS temperature gradient. A Chemstation Software was applied to quantify the identified lipids by comparing their peak areas with the peak area of the internal standards. The errors of peaks area and retention time by the GC analysis was less than 3%.
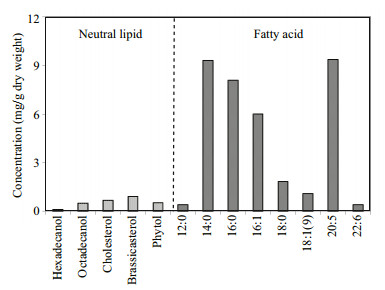
3 RESULT 3.1 Lipids in S. costatum and in microcosmIn cultured S.costatum cells, five neutral lipids and eight fatty acids were identified based on GC and GCMS profiles and their concentrations (based on dry weight) in the algae were shown in Fig. 2. As a whole, the total concentration of the thirteen lipids was 39.17 mg/g dw (dry weight), account for approximately 4% of algal cell dry weight. Among five neutral lipids (Fig. 3a), concentrations of cholesterol and brassicasterol were up to 0.50 mg/g dw and 0.68 mg/g dw, respectively, higher than two normal fatty alcohols (referred as alcohol below) and phytol. Concentration of hexadecanol was the lowest (0.11 mg/g dw). The total concentration of the five neutral lipids was only 2.74 mg/g, a factor lower than that of fatty acids and account for less than 7% of total lipids. The fatty acids were the major components in the algal lipids, including four saturated (12:0, 14:0, 16:0 and 18:0), two monounsaturated (16:1 and 18:1(ω9)) and two polyunsaturated (20:5 and 22:6) ones. Among them, concentrations of 14:0, 16:0, 16:1 and 20:5 were all higher than 5 mg/g dw and their relative percentage to total fatty acids was about 90% (about 84% of total lipids). The two most abundant fatty acids were 14:0 and 20:5, their concentrations were up to 9.30 mg/g dw and 9.35 mg/g dw, respectively. As a comparison, concentrations of 12:0 and 22:6 were as low as 0.40 mg/g dw and 0.41 mg/g dw, respectively. In the cultured cells, bacterial specific fatty acids, such as branched iso- and anteiso-15:0, were not detected, indicating the culture was anxenic.
|
| Figure 2 Concentrations (based on dry weight) of five neutral lipids and eight fatty acids in S. costatum |
|
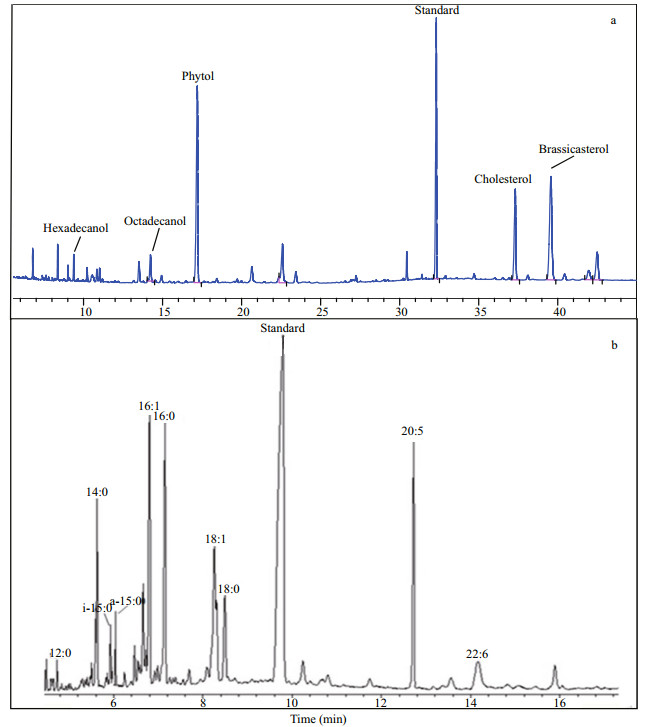
| Figure 3 Gas chromatography profile of lipids in S. costatum a. netural lipids; b. fatty acids. |
After mixing with collected hypoxic seawater, two more fatty acids iso- and anteiso- 15:0 were detected in the microcosm at the beginning of incubation (Fig. 3b). Concentrations of these two bacterial specific fatty acids were 108.78 μg/L and 152.08 μg/L, respectively. The occurrence of iso- and anteiso- 15:0 indicated existence of active bacteria in the incubated microcosm.
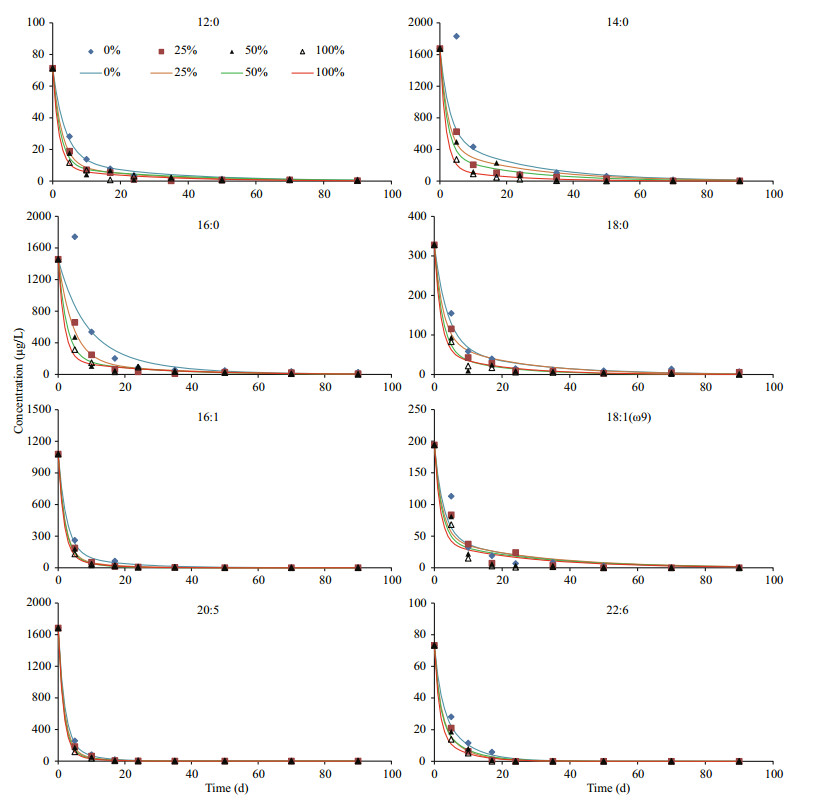
3.2 Time dependent variations of neutral lipidsIn general, variation patterns of all five algal neutral lipids were similar under four different oxygen saturations during incubations (Fig. 4). Their concentrations declined significantly after 2–3 weeks and then decreased slowly until the end of incubation. However, there were obvious differences among the degradations of various lipids under four oxygen saturations. By comparing concentrations of lipids after 10-d and 90-d incubation with their initial concentration, we calculated percentage of each compound (including both neutral lipids and fatty acids) remained in the algal residue at the two time points (Table 1). After 10-d of incubation, except octadecanol, more than 60% of the neutral lipids remained in the seawater under anoxic condition. With the existence of oxygen, most of alcohols degraded but a large fraction of cholesterol, brassicaterol and phytol were preserved in the microcosms. After 90-d, hexadecanol, octadecanol and phytol completely or almost completely disappeared and more than 90% of the two sterols degraded under 100% oxygen saturation, most of alcohols degraded under all four oxygen saturations. In hypoxic seawater (25% and 50% oxygen saturation) and anoxic seawater, two sterols and phytol still presented in significant amounts (12.4% to 36.8%). Expectedly, after 90-d of incubation, remained fraction of each lipid in the seawater had negative relationship with oxygen saturation.
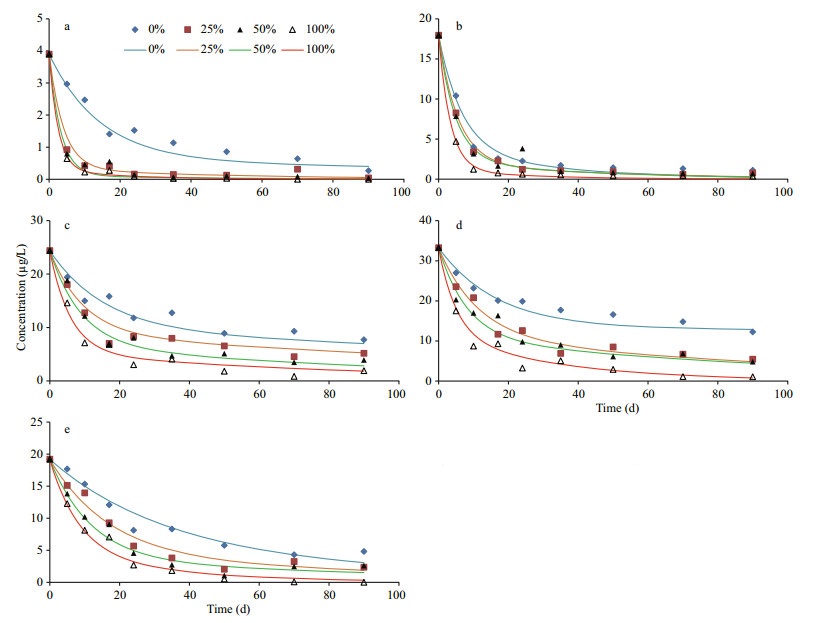
|
| Figure 4 Variations of concentrations of neutral lipids during incubations under four different oxygen saturations The points are experimental data and the curves are simulated results based on multi-G model. a. hexadecanol; b. octadecanol; c. phytol; d. cholesterol; e. brassicaterol. |
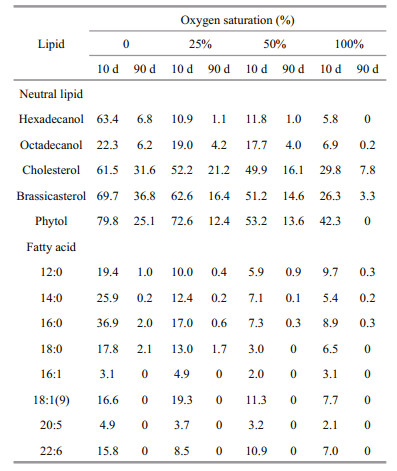
|
Similar to neutral lipids, the concentrations of all eight identified algal fatty acids, dropped dramatically in the first two weeks of incubation and then decreased slowly, although there were some differences among various oxygen saturations (Fig. 5). Generally, with increasing oxygen saturation, the algal fatty acids degraded faster. After 10 days, less than 10% of all the fatty acids remained in oxic seawater. More than 95% of 16:1 and 20:5 degraded in the seawater under all four oxygen saturations, but a large fraction (8.5%–36.9%) of other six fatty acids remained in anoxic and severely hypoxic seawater. As a whole, difference of remaining fractions of fatty acids in 100% and 50% oxygen saturation seawater were insignificant. After three month of incubation, four unsaturated fatty acids degraded completely and only a small amount of saturated fatty acids remained in the seawater under all four oxygen saturations. Unlike neutral lipids, there was no obvious difference of preservations of fatty acids under various oxygen saturations.
|
| Figure 5 Variations of concentrations of algal fatty acids during incubations under four different oxygen saturations The points are experimental data and the curves are simulated results based on multi-G model. |
Unlike the neutral lipids and algal fatty acids, two bacterial specific fatty acids iso and anteiso-15:0 showed different variation patterns under various oxygen saturations during incubation in residue (Fig. 6). Under anoxic condition, concentration of iso and anteiso-15:0 increased 40% and 32%, respectively, to the maximum of their concentrations after 5-d of incubation, and then declined very quickly in the next 10 days. After the dramatic declining, concentrations of the two fatty acids varied with a fluctuation pattern at low level until the end of incubation. In hypoxic seawater with 25% and 50% oxygen saturation, concentration of iso-15:0 varied similarly as under anoxic condition. However, concentration of anteiso-15:0 declined continuously from the beginning to the end of the incubation under hypoxic conditions. Under oxic condition, concentrations of both iso- and anteiso- 15:0 kept decreasing to very low levels until the end of incubation. Comparing to the peaks of their concentrations, only about 2%–3% of iso-15:0 and less than 0.6% of ante-iso 15:0 still remained in residue after 90-d of incubation. Iso- and anteiso- 15:0 were the only two bacterial fatty acids identified in our incubation system. Time-dependent variations of the bacterial fatty acids might reflect the variation of bacterial biomass that influence degradation of algal material during incubation.
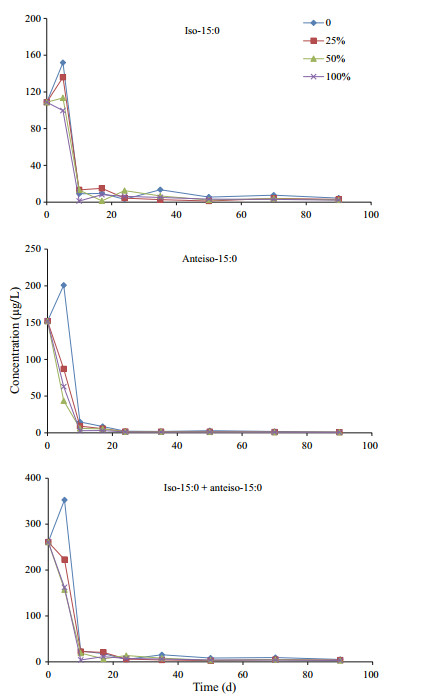
|
| Figure 6 Variations of bacteria-specific fatty acid concentrations during incubations under four different oxygen saturations |
Our data showed that the major fatty acids in S. costatum were 14:0, 16:0, 16:1 and 20:5. This result was constant with Suzuki and Matsuyama's (1995) record. They also detected small fractions of fatty acid 18:0, 18:1 and 22:6 in the algae. In their culture, the algae grew at 22℃ under 100 μE m2/s (8 000 lux) of cool-white fluorescent illumination on a 12-h/12-h light-dark cycle, similar as our culture. However, they did not identify 12:0 in their culture. In fact, many previous studies indicated that the existence of fatty acid 12:0 in the algal cells might be related to artificial sea water media. For instance, in Ukeles culture medium (Sánchez et al., 1993) and Conway medium (Servel et al., 1994), 12:0 was non-detectable. When the algae grew in f/2 medium as we applied, 12:0 was detected in triacylglycerol but not in phospholipids (Popovich et al., 2012).
In our culture, total amount of neutral lipids in S. costatum was much less than that of fatty acids. Lv et al. (2010) also described similar results as ours. All the five identified neutral lipids were typical lipid compounds in the algae (Lv et al., 2010). In fact, the two most abundant neutral lipids, brassicasterol and cholesterol, were considered as the major fraction of neutral lipids and account for over 50% of neutral lipids in cultured S. costatum (Mohammady, 2004; Lv et al., 2010). However, relative lipid compositions of marine microalgae are dependent on their growth condition, especially the culture medium and growth phase (Hallegraeff et al., 1991; Fidalgo et al., 1998; Mohammady, 2004; Lv et al., 2010). Therefore, proportions of various lipids in our culture diatom were different with previous studies. For example, in Mohammady's culture, the ratio between brassicasterol and cholesterol in S. costatum was about 4:1, but in our culture, this ratio was only about 1.4:1.
4.2 Preservation of lipids originated from S. costatumThe preservation percentages of lipids after short term (10-d) and long term (90-d) incubation in seawater are listed in Table 1. From the results, several major factors should be considered controlling preservation of lipids in seawater. The first one is degradation time, reflecting residence time of lipids in seawater. Under the same oxygen saturation, much more lipids were preserved in seawater after short time incubation than after long time incubation. The second one is oxygen saturation. Our data showed that with decreasing oxygen saturations, for all algal lipids, their preservation percentages increased in the seawater after 10-d and 90-d incubation in general. The third one is chemical property of organic compound. After 10-d incubation, more than 80% fatty acids and alcohols disappeared under oxic and hypoxic condition. However, much less sterols and phytol degraded in the seawater at that period. At the end of incubation, all fatty acids degraded almost completely under oxic, hypoxic and anoxic conditions, but a fraction of neutral lipids were still exist in the microcosms, especially for sterols, indicating the important role of chemical properties of lipids for their preservation. This result is consistent with Pan et al.'s (2017) study. In fact, which factors control organic matter preservation in marine environments is one of the most complex and controversial issues in contemporary biogeochemistry (Hedges and Keil, 1999). Previous studies showed that after 2–3 months of incubation, under oxic condition, algal lipids degraded completely in seawater, but under anoxic condition, 10%–40% of algal lipids still remained in seawater (Sun et al., 2004). Our results are consistent with their results regarding lipid degradation under oxic and anoxic conditions. Many previous studies showed that most of biosynthesised organic material was mineralized to CO2 in seawater, while a small fraction of the carbon fixed by photosyntheis formed recalcitrant dissolved organic matter and eventually generated a large carbon reservoir in seawater (Jiao et al., 2010). Our results indicate that due to the chemical property difference of various algal organic compounds, microorganism in seawater might have preference to utilize different compounds. Generally, fatty acids widely distributed in both eukaryotic and prokaryotic cells and similar enzymes are shared by all the cells to synthesize and degrade fatty acids. Therefore, the microorganism could utilize algal fatty acids directly and easily as carbon and energy sources. As a comparison, sterols naturally occur in eukaryotic cells and their chemical feature are stable. In fact, abiotic processes such as photooxidation and autoxidation affect sterol degradation and preservation remarkably (Rontani and Marchand, 2000). With stable chemical structure and dark incubation, both biotic and abiotic degradattion for sterols were more diffiuclt in our microcosm. Then more sterols were preserved in seawater and eventually transformed into recalcitrant materials.
4.3 Degradation rate constants of algal lipidsVariations of concentrations of all identified lipids, including both neutral lipids and fatty acids, showed similar degradation pattern: initial fast decrease in the first 2–3 weeks followed by slower or little change till the end of incubation. This variations pattern was widely observed in many previous studies for organic matter degradation and has been quantitatively described by well-known multi-G model. The multi-G model was modified from the G-model which was firstly established by Berner (1964):
dGt/dt= kGt.
In the G-model, degradation of organic matter was considered as whole. Gt is the concentration of total organic matter. Organic matter degradation rate was proportional to its concentration and degradation rate constant k, which keeps constant in the whole degradation process. However, the G-model did not match the reality well due to complex organic matter degradation in marine environment. In fact, in sediment and seawater, various organic matter has different degradation rate due to the difference of the biochemical activity, and varying environmental factors, especially oxygen concentration. To overcome the defect of the G-model, Westrich and Berner (1984) developed multi-G model. In this model, different fractions of organic compounds are assumed to have various reactivities and degrade independently at distinct rates. The sum of individual degradation rates of organic matter equal to the overall degradation rate of the organic compounds, as shown by the following equations:
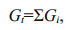 (1)
(1)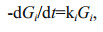 (2)
(2)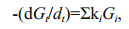 (3)
(3)where ki is the first-order degradation rate constant of fraction i; Gi is the concentration of fraction i, which is considered as organic matter with similar activities; -(dGt/dt) is the degradation rates of all fractions. From Eq.2, ki=1/Δt×(ln(Gi)0/(Gi) then Gi=(Gi)0exp(-kit). Therefore,
Gt=ΣGi=(G1)0exp(- k1t)+(G2)0exp(- k2t)+…,
and (G1)0 and (G2)0 are initial concentrations of fraction 1 and 2. Each fraction is considered as a pool in multi-G model.
The validity of the multi-G model has been confirmed by many field observation and laboratory studies (Westrich and Berner, 1984; Sun and Wakeham, 1998; Ding and Sun, 2005). However, even for one compound, ki keep decreasing with time due to variations of controlling factors, such as decreasing concentration of oxygen. Considering variation of ki, Meddelburg (1989) developed power model to describe organic matter degradation. In the power model, ki is a function with time. Since at least two points are necessary to determine the initial ki, which may not reflect the reality and calculation errors still occurs. To reduce the errors, initial ki need to be adjusted frequently according to real conditions. The adjustment causes complex calculation. In our study, based on the preservation results, individual lipids showed variable kinetic features during their degradation course, indicating that even the same type of molecules can degrade at different rates. Previous study also showed that lipid locating in different cellular regions and associating with different components, might have various degradation rate constants (Ding and Sun, 2005a, b). It is reasonable to assume that pool size of different cellular components varied under various oxygen saturations for different compounds. ki can be considered as a constant in short time. Based on these assumptions and the degradation pattern of the algal lipids in our study, to avoid complex power model and simplify calculation lipid degradation by using Eq.3, we test two-pool G model to determine their degradation rate constants. The Eq.3 was modified as:
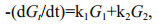 (4)
(4)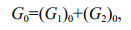 (5)
(5) (6)
(6)To obtained k1 and k2 from the equations above, more assumptions are necessary. If degradation of both (G1)0 and (G2)0 match G-model, Eq.6 can be expressed as:
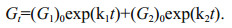 (7)
(7)Based on Eq.7, the assumption k1>> k2 was applied. Under this assumption, the degradation rate was determined by G1 pool in early degradation, and by G2 pool in late degradation. Therefore, the modeling processes involved two steps to obtain initial k1 and k2 according to our data set: 1. plotting ln(Gt) vs. incubation time (t), then the first four point and the last four point constructed two straight lines. The slopes of the two straight lines were considered as the initial k1 and k2. The intersection point of the two straight lines was used to estimate relative pool size based on Eqs.5 and 7; and 2. fitting the original (Gt) data to the two-pool G model by modifying k1 and k2. Based on the two-pool G model, the average degradation rate constant of a lipid compound from two different pools is defined as:
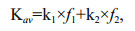 (3)
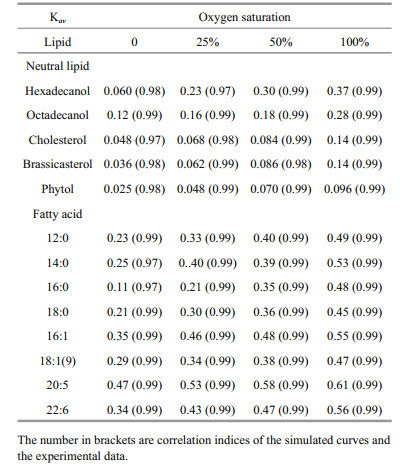
(3)where f1 and f2 are relative proportions of pool 1 and pool 2 (f1=(G1)0/(Gt)0 and f2=(G2)0/(Gt)0. The Kav reflected how fast a lipid compound degraded in the microcosm as a whole during incubation period.
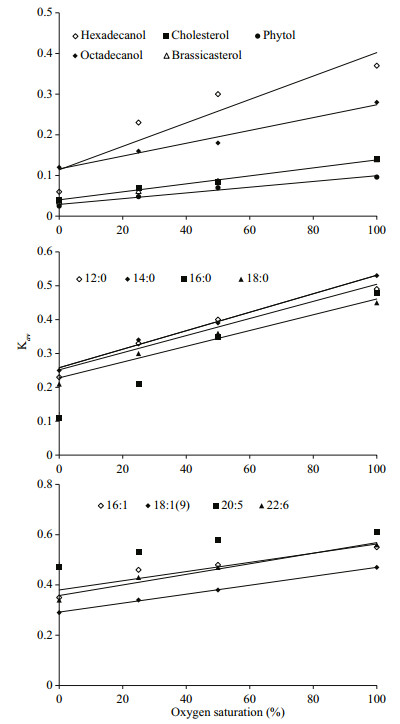
The simulated degradation curves based on the two pool G-model that are plotted in Fig. 4 (neutral lipids) and 5 (fatty acids) matched the experimental data very well (all correlation indices were higher than 0.97). All calculated Kav of various lipids are listed in Table 2. Comparison of Kav showed several interesting implications for degradations of the algal lipids under various oxygen saturations. First, for each lipid, degradation rate constants increased with increasing oxygen saturations. Second, under the same oxygen saturation, fatty acid degraded faster than neutral lipids. Third, unsaturated fatty acid degraded faster than saturated fatty acid.
Our results indicate that under the same oxygen saturation in seawater, the degradation of lipids was strongly dependent on their structures. For the eight algal fatty acids, unsaturated fatty acids degraded faster than saturated fatty acids, in general, under the same redox condition. Fatty acids in S. costatum originate from two positions, namely from the membrane and from the intracellular part of the cell (Berge et al., 1995). In the intracellular fraction, the main modality of fatty acids are triacylglycerol and esters. In the membrane, fatty acids are bound with glycerol, phosphoric acid or glucose to from complex lipids with specific function, including phospholipids and glycolipid. Thus, these lipids have different degradation behavior in various surroundings with various structures (Ding and Sun, 2005). In a word, based on common biochemical principles, cellassociated fatty acids decomposition had two major steps: the first is that the enzymes acted on ester bond between fatty acids and other organic compound (e.g. glycerol) to release fatty acids from structural matrices, such as triacylglycerol, phospholipids and etc., called enzymatic hydrolysis; the next is to further degrade the released free fatty acids into some smaller molecules by β-oxidation or other lipid oxidation pathways (Sun et al., 2000; Ding and Sun, 2004). The end product of lipid degradation is CO2 (Nealson and Cox, 2012). The first degradation step is complicated because lipids and other organic macromolecules can form different compounds. The structure of these componds are complexes and various kinds of enzymes, such as lipase and phospholipase, are required to degrade them. Thus, their degradate rates are limited. The second step is relatively simple for the plain and doubtless structure of fatty acids. Unsaturation and chain lengths of free fatty acids strongly controll the degradation rates. From Fig. 7, it proved that unsaturated fatty acids degraded faster than the saturated ones at same redox conditions, like 16:0-16:1 and 18:0-18:1(9), which was identical with other studies (Harvey and Macko, 1997; Sun et al., 1997, 2000). The result of the incubation experiments also showed that the short-chain saturated fatty acids (12:0 and 14:0) were more labile than long-chain ones (16:0 and 18:0) in seawater.
|
| Figure 7 Variations of Kav of algal lipids in the incubation systems |
For the five neutral lipids, under the same redox conditions, two alcohols had the highest degradation rate constants, and sterols degraded faster than phytol (Fig. 7), which indicated the structures and chemical properties of the five organic compounds had remarkable influence on their degradation. The alcohols convert into fatty acids through a series of oxidation processes, which could be directly used by various kind of microorganism or subject to the next degradation procedure. As a result, alcohols experience fast degradation rates. Sterols are characteristic constituents of eukaryotic organisms and absent from prokaryotes (Galea and Brown, 2009). Hopanoids are considered as sterol surrogate in prokaryotes. Prokaryotes lack of enzymes for sterol degradation and then should not utilize sterol as their preferential carbon source with existence of other more labile carbon sources. So their degradation rates were slower. In addition, the cholesterol and brassicaterol degradation rates were mostly the same under the same oxygen saturation due to the same heterocyclic structure (Rantani and Aubert, 1997). Phytol is the most slowly degraded that could be related to the position in the intracellular part. Phytol is the product of side chain ester bond rupture on chlorophyll that can be combined with protein and polypeptide into macromolecular compound (Schoch and Brown, 1987; Ding and Sun, 2005b) in plant or algae cytoplasm. Therefore, the degradation process of this compound was very complicated involving many processes and enzymes (Louda et al., 1998). The degradation of chlorophyll constantly produced new phytol as the released phytol degraded, so phytol decomposed slower as a whole. On the other hand, based on our results, phytol and sterols were partially preserved in the seawater under different oxygen saturation, which showed their degradation became abnormally gentle trending into inert at the end of incubation. The results of degradation constants support Jiao et al.'s (2010) report that the preserved organic matter after the bacteria utilization could be stored at long term in seawater as inert dissolved organic carbon, forming biological pump. The undecomposed neutral lipids from S. costatum could become the origin of the recalcitrant dissolved organic matter (RDOM) in seawater.
4.5 The role of marine microbe on organic matter degradationMicrobes have double effects in organic matter degradation. Their growth can increase the amount and types of organic matter, but they can also degrade most macromolecular compounds as their carbon and energy sources (Azam et al., 1983; Deming and Baross, 1993). In this study, we detected two bacteriaspecific fatty acids iso and anteiso-15:0, which indirectly indicate the bacteria abundance (Cranwell et al., 1987; Wakeham and Beier, 1991). In different oxygen saturation incubation systems, the bacterial community varies according to their respiration types, such as aerobic, facultative anaerobic and strictly anaerobic respiration, which result in development of different kinds of bacterial fatty acids (Parkes and Taylor, 1983). Variations of bacterial fatty acids such as iso and anteiso-15:0 reflect changes of bacteria abundance and biomass. Ding and Sun's results (2005) showed the two fatty acids had similar variation pattern in the same period under oxic and anoxic conditions. In our study, the results indicate that the sum of concentrations of iso and anteiso-15:0 increased significantly in the first week under anaerobic conditions, and then dramatically decreased during the second week (Fig. 6), while it kept declining under hypoxic and aerobic conditions with a rapid decrease in the first week. This result indicate that high abundance of microbe contributed to faster degradation of lipids at the beginning of incubation under various redox conditions. However, although bacteria were considered to play critical roles on organic matter degradation (Harvey and Macko, 1997; Teece et al., 1998; Ding and Sun, 2005), no simple relationship between bacterial biomass and lipid degradation rate constant was detected duo to the complexity of the degradation processes in this study.
4.6 Abiotic degradation of lipidsPhotooxidation and autoxidation are two typical abiotic processes to degrade organic matter in marine environment (Rontani et al., 2013). Since our incubation was conducted in dark, there was no light effect on lipid degradation in the microcosm. Previous study suggested that autoxidation, defined as O2 spontaneous reacting with organic compounds, proceeds by a three step radical chain: initiation, propagation and temination, and mainly acts on compounds with double bonds (Fossey et al., 1995; Rontani et al., 2013). However, autoxidation seems to be induced by homolytic cleavage (catalyzed by redox-active metal ions) of photochemically produced hydorperoxides. Therefore, it was difficult to initiate autoxidation in our incubation microcosm without photooxidation (Rontani et al., 2014). Despite the difficulty, several evidences might indicate autoxidation during the incubation period. First, multi-double bond compounds (cholesterol and brassicasterol) degraded much slower than alcohol and fatty acids. Generally, the cyclic skeleton of this kind of compounds is considered to relatively resistant to bacterial mineralization (Gagosian et al., 1982; Rontani et al, 2014) and then autoxidation should play key role to degrade them. Second, under anoxic condition, preservations of cholesterol and brassicasterol were much higher than under oxic or hypoxic condition, indicating the importance of oxygen on autoxidation. The initiation of autoxidation might depend on existed hydroperoxide in collected seawater. However, we did not directly detect autoxidative products of various lipids in our experiment, more future work is necessary to evaluate the effect of abitic degradation on algal lipids.
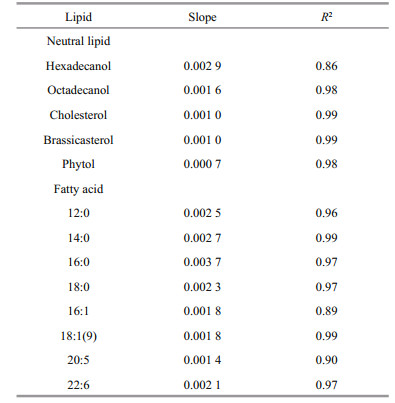
4.7 Oxygen saturation as key factor controlling lipids degradationFigure 7 shows that there is a positive and highly significant linear relationship between oxygen saturation and the average degradation rate constant (Kav) for all lipids. Therefore, the average degradation rate constants of all lipids could be expressed as Kav=k×SO2+ Kan (k is the slope of the each line, SO2 is the saturation of oxygen, and Kan is the degradation rate constant of lipids under anoxic condition). The slope of each line present the effect of oxygen saturation on the degradation of various lipids. High slope value reflects that oxygen saturation affects lipid degradation more remarkably. The linear relationship between oxygen saturation and degradation rate constants of lipids suggested that oxygen saturation was the critical factor that determined redox condition of seawater and played key role on controlling organic matter degradation.
All the slopes for various lipids were listed in Table 3. The data indicate that oxygen saturation affect degradation of saturated fatty acids more significantly than that of unsaturated fatty acids. For sterols, oxygen saturation had much lower impact on their degradation as compared to fatty acids. Sterols and unsaturated fatty acids are important constituents of algal cell membrane (Ding and Sun, 2005a). The difference of effects of oxygen saturation on degradation of lipids might reflect the location of various lipids in algal cells. Previous study reported that oxygen contributed to the decomposition of cellassociated lipids in the sediments, and their degradation rates had linear or nonlinear relationship with oxygen exposure time (Sun et al., 2002). Although series of studies confirmed that complex organic matter degraded faster under oxic condition than under anoxic condition (Canfield, 1994; Sun et al., 2004; Ding and Sun, 2005), our results are the first report about the linear relationship between oxygen saturation and degradation rate constants of various lipids in seawater. These results suggest that although microbial community were very complex and might vary dramatically in oxic, hypoxic and anoxic seawater, oxygen saturation could be considered as the sole factor controlling degradation of various organic compounds. In fact, biotic degradation of lipids in seawater was conducted by various microbes. This kind of biochemical process is similar for different microorganism. For example, β-oxidation of fatty acids is a normal degradation process in all kinds of organisms to generate acetyl-CoA and then entering citric acid cycle to generate redox hydrogen. Oxygen as most common electron acceptor in aerobic respiration reacts with the redox hydrogen to form water and produce ATP (Voet and Voet, 2011). From this perspective, oxygen saturation in seawater might determine aerobic respiration rate of whole microbial community. On the other side, higher oxygen saturation might generate more active oxygen radials (Canfield, 1994). Formation of oxygen radials might promote abiotic degradation of organic double-bond compounds and complex organic matter (Canfield, 1994; Rontani et al., 2013, 2014). However, more future work is necessary to investigate the mechanism of oxygen saturation controlling organic matter degradation in seawater.
|
This is the first study on degradation of algal lipids in seasonal hypoxic seawater. The results indicated that residence time, oxygen saturation, and selective utilization of lipids by microorganisms were key factors controlling preservation of different lipids in seawater. The degradation of all algal lipids in seawater could be well described by multi-G model. Under same oxygen saturation, structural differences of various lipids determine their degradation rate constants in seawater. The positive linear relationship of degradation rate constants and oxygen saturation suggests the critical role of oxygen on organic matter degradation in seawater. This study provided new fundamental results for understanding organic carbon cycling in seawater in varying redox condition.
6 ACKNOWLEDGEMENTThe authors are grateful to all of members help in the Marine Interface Chemistry Lab in the Ocean University of China. The authors also appreciate Ms. Rose Jaffe and Mr. Dani Cox from the University of California at Santa Barbara to help us polish this paper.
Aller R C. 1984. The importance of relict burrow structures and burrow irrigation in controlling sedimentary solute distributions. Geochimica et Cosmochimica Acta, 48(10): 1 929-1 934.
DOI:10.1016/0016-7037(84)90375-2 |
Azam F, Fenchel T, Field J G, Gray J S, Meyer-Reil L A, Thingstad F. 1983. The ecological role of water column microbes in the sea. Marine Ecology Progress Series, 10(3): 257-263.
|
Berge J P, Gouygou J P, Dubacq J P, Durand P. 1995. Reassessment of lipid composition of the diatom, Skeletonema costatum. Phytochemistry, 39(5): 1 017-1 021.
DOI:10.1016/0031-9422(94)00156-N |
Berner R A. 1964. An idealized model of dissolved sulfate distribution in recent sediments. Geochimica et Cosmochimica Acta, 28(9): 1 497-1 503.
DOI:10.1016/0016-7037(64)90164-4 |
Canfield D E. 1994. Factors influencing organic carbon preservation in marine sediments. Chemical Geology, 114(3-4): 315-329.
DOI:10.1016/0009-2541(94)90061-2 |
Caradec S, Grossi V, Gilbert F, Guigue C, Goutx M. 2004. Influence of various redox conditions on the degradation of microalgal triacylglycerols and fatty acids in marine sediments. Organic Geochemistry, 35(3): 277-287.
DOI:10.1016/j.orggeochem.2003.11.006 |
Cranwell P A, Eglinton G, Robinson N. 1987. Lipids of aquatic organisms as potential contributors to lacustrine sediments-Ⅱ. Organic Geochemistry, 11(6): 513-527.
DOI:10.1016/0146-6380(87)90007-6 |
Dai J H, Sun M Y. 2007. Organic matter sources and their use by bacteria in the sediments of the Altamaha estuary during high and low discharge periods. Organic Geochemistry, 38(1): 1-15.
DOI:10.1016/j.orggeochem.2006.10.002 |
Deming J W, Baross J A. 1993. The early diagenesis of organic matter: bacterial activity. In: Engel M H, Macko S A eds.Organic Geochemistry: Principles and Applications.Springer, Boston, MA. p.119-144.
|
Derieux S, Fillaux J, Saliot A. 1998. Lipid class and fatty acid distributions in particulate and dissolved fractions in the north Adriatic sea. Organic Geochemistry, 29(5-7): 1 609-1 621.
DOI:10.1016/S0146-6380(98)00089-8 |
Ding H, Sun M Y. 2005a. Biochemical degradation of algal fatty acids in oxic and anoxic sediment-seawater interface systems:effects of structural association and relative roles of aerobic and anaerobic bacteria. Marine Chemistry, 93(1): 1-19.
DOI:10.1016/j.marchem.2004.04.004 |
Ding H, Sun M Y. 2005b. Effects of intracellular structural associations on degradation of algal chloropigments in natural oxic and anoxic seawaters. Geochimica et Cosmochimica Acta, 69(17): 4 237-4 252.
DOI:10.1016/j.gca.2005.04.005 |
Emerson S, Hedges J I. 1988. Processes controlling the organic carbon content of open ocean sediments. Paleoceanography, 3(5): 621-634.
DOI:10.1029/PA003i005p00621 |
Fidalgo J P, Cid A, Torres E, Sukenik A, Herrero C. 1998. Effects of nitrogen source and growth phase on proximate biochemical composition, lipid classes and fatty acid profile of the marine microalga Isochrysis galbana. Aquaculture, 166(1-2): 105-116.
DOI:10.1016/S0044-8486(98)00278-6 |
Foree E G, McCarty P L. 1970. Anaerobic decomposition of algae. Environmental Science & Technology, 4(10): 842-849.
|
Fossey J, Lefort D, Sorba J. 1995. Free Radicals in Organic Chemistry. Masson, Paris.
|
Gagosian R B, Smith S O, Nigrelli G E. 1982. Vertical transport of steroid alcohols and ketones measured in a sediment trap experiment in the equatorial Atlantic Ocean. Geochimica et Cosmochimica Acta, 46(7): 1 163-1 172.
DOI:10.1016/0016-7037(82)90002-3 |
Gagosian R B, Volkman J K, Nigrelli G E. 1983. The use of sediment traps to determine sterol sources in coastal sediments off Peru. In: Advances in Organic Geochemistry, 1981. Wiley, Chichester. p.369-379.
|
Galea A M, Brown A J. 2009. Special relationship between sterols and oxygen:were sterols an adaptation to aerobic life?. Free Rradical Biology and Medicine, 47(6): 880-889.
DOI:10.1016/j.freeradbiomed.2009.06.027 |
Hallegraeff G M, Nichols P D, Volkman J K, Blackburn S I, Everitt D A. 1991. Pigments, fatty acids, and sterols of the toxic dinoflagellate Gymnodinium catenatum. Journal of Phycology, 27(5): 591-599.
DOI:10.1111/j.0022-3646.1991.00591.x |
Hartnett H E, Keil R G, Hedges J I, Devol A H. 1998. Influence of oxygen exposure time on organic carbon preservation in continental margin sediments. Nature, 391(6667): 572-575.
DOI:10.1038/35351 |
Harvey H R, Macko S A. 1997. Kinetics of phytoplankton decay during simulated sedimentation:changes in lipids under oxic and anoxic conditions. Organic Geochemistry, 27(3-4): 129-140.
DOI:10.1016/S0146-6380(97)00077-6 |
Harvey H R, Tuttle J H, Bell J T. 1995. Kinetics of phytoplankton decay during simulated sedimentation:changes in biochemical composition and microbial activity under oxic and anoxic conditions. Geochimica et Cosmochimica Acta, 59(16): 3367-3377.
DOI:10.1016/0016-7037(95)00217-N |
Hedges J I, Keil R G. 1999. Organic geochemical perspectives on estuarine processes:sorption reactions and consequences. Marine Chemistry, 65(1-2): 55-65.
DOI:10.1016/S0304-4203(99)00010-9 |
Jewell W J, McCarty P L. 1971. Aerobic decomposition of algae. Environmental Science and Technology, 5(10): 1023-1031.
DOI:10.1021/es60057a005 |
Jiao N Z, Herndl G J, Hansell D A, Benner R, Kattner G, Wilhelm S W, Kirchman D L, Weinbauer M G, Luo T W, Chen F, Azam F. 2010. Microbial production of recalcitrant dissolved organic matter:long-term carbon storage in the global ocean. Nature Reviews Microbiology, 8: 593-599.
DOI:10.1038/nrmicro2386 |
Lee C. 1992. Controls on organic carbon preservation:the use of stratified water bodies to compare intrinsic rates of decomposition in oxic and anoxic systems. Geochimica et Cosmochimica Acta, 56(8): 3323-3335.
DOI:10.1016/0016-7037(92)90308-6 |
Li W, Ding H, Yang G P, Sui W, Sun L. 2014. Simulated study of lipid degradation in hypoxia sea water. Periodical of Ocean University of China, 44(sup.): 112-129.
(in Chinese) |
Lin J. 2007. Distribution of dissolved organic carbon and particulate organic carbon in the Changjiang estuary and its adjacent area. Master thesis, East China Normal University. (in Chinese)
|
Liu Z G, Xu R, Liu C C, Qin Y T, Cai P. 2012. Characters of hypoxia area off the Yangtze River estuary and its influence. Marine Science Bulletin, 31(5): 588-593.
(in Chinese with English abstract) |
Louda J W, Jie L, Lei L, Winfree M N, Baker E W. 1998. Chlorophyll-a degradation during cellular senescence and death. Organic Geochemistry, 29(5-7): 1233-1251.
DOI:10.1016/S0146-6380(98)00186-7 |
Lü D W, Song Q, Wang X C. 2010a. Decomposition of algal lipids in clay-enriched marine sediment under oxic and anoxic conditions. Chinese Journal of Oceanology and Limnology, 28(1): 131-143.
DOI:10.1007/s00343-010-9226-8 |
Lv X X, Zou L, Sun B W, Wang J T, Sun M Y. 2010b. Variations in lipid yields and compositions of marine microalgae during cell growth and respiration, and within intracellular structures. Journal of Experimental Marine Biology and Ecology, 391(1-2): 73-83.
DOI:10.1016/j.jembe.2010.06.010 |
Mohammady N G. 2004. Total, free and conjugated sterolic forms in three microalgae used in mariculture. Zeitschrift für Naturforschung C, 59(9-10): 619-624.
DOI:10.1515/znc-2004-9-1002 |
Nealson D L, Cox M M. 2012. Lehninger Principles of Biochemistry. 6th edn. W. H. Freeman, New York.
|
Otsuki A, Hanya T. 1972a. Production of dissolved organic matter from dead green algal cells I. Aerobic microbial decomposition. Limnology and Oceanography, 17(2): 248-257.
DOI:10.4319/lo.1972.17.2.0248 |
Otsuki A, Hanya T. 1972b. Production of dissolved organic matter from dead green algal cells Ⅱ. Anaerobic microbial decomposition. Limnology and Oceanography, 17(2): 258-264.
DOI:10.4319/lo.1972.17.2.0258 |
Pan H, Culp R A, Sun M Y. 2017. Influence of physiological states of Emiliania huxleyi cells on their lipids and associated molecular isotopic compositions during microbial degradation. Journal of Experimental Marine Biology and Ecology, 488: 1-9.
DOI:10.1016/j.jembe.2016.12.003 |
Parkes R J, Taylor J. 1983. The relationship between fatty acid distribution and bacterial respiratory types in contemporary marine sediments. Estuarine, Coastal and Shelf Science, 16(2): 173-174.
DOI:10.1016/0272-7714(83)90139-7 |
Popovich C A, Damiani C, Constenla D, Leonardi P I. 2012. Lipid quality of the diatoms Skeletonema costatum and Navicula gregaria from the South Atlantic Coast(Argentina):evaluation of its suitability as biodiesel feedstock. Journal of Applied Phycology, 24(1): 1-10.
DOI:10.1007/s10811-010-9639-y |
Pruski A M, Buscail R, Bourgeois S, Vétion G, Coston-Guarini J G, Rabouille C. 2015. Biogeochemistry of fatty acids in a river-dominated Mediterranean ecosystem (Rhône River prodelta, Gulf of Lions, France):origins and diagenesis. Organic Geochemistry, 83-84: 227-240.
DOI:10.1016/j.orggeochem.2015.04.002 |
Rontani J F, Marchand D. 2000. Photoproducts of phytoplanktonic sterols:a potential source of hydroperoxides in marine sediments?. Organic Geochemistry, 31(2-3): 169-180.
DOI:10.1016/S0146-6380(99)00156-4 |
Rontani J F, Vaultier F, Bonin P. 2014. Biotic and abiotic degradation of marine and terrestrial higher plant material in intertidal surface sediments from Arcachon Bay(France):a lipid approach. Marine Chemistry, 158: 69-79.
DOI:10.1016/j.marchem.2013.11.005 |
Rontani J F, Volkman J K, Prahl F G, Wakeham S G. 2013. Biotic and abiotic degradation of alkenones and implications for U37K' paleoproxy applications:a review. Organic Geochemistry, 59: 95-113.
DOI:10.1016/j.orggeochem.2013.04.005 |
Sánchez S, Martínez M A E, Molina E, de la Casa J A, García F. 1993. The influence of culture media and aeration rate on the growth and fatty-acid content Skeletonema costatum. Process Biochemistry, 28(5): 289-296.
DOI:10.1016/0032-9592(93)85001-V |
Schoch S, Brown J. 1987. The action of chlorophyllase on chlorophyll-protein complexes. Journal of Plant Physiology, 126(4-5): 483-494.
DOI:10.1016/S0176-1617(87)80033-0 |
Servel M O, Claire C, Derrien A, Coiffard L, de RoeckHoltzhauer Y. 1994. Fatty acid composition of some marine microalgae. Phytochemistry, 36(3): 691-693.
DOI:10.1016/S0031-9422(00)89798-8 |
Sun B Y, Liang S K, Wang C Y, Wang X B, Wang X L, Li Y B. 2008. Role of irradiance on the seasonality of Skeletonema costatum cleve blooms in the coastal area in East China Sea. Environmental Science, 29(7): 1849-1854.
(in Chinese with English abstract) |
Sun J, Song S Q. 2009. Phytoplankton growth and microzooplankton herbivory during the spring phytoplankton bloom period in the East China Sea. Acta Ecologica Sinica, 29(12): 6429-6438.
(in Chinese with English abstract) |
Sun M Y, Aller R C, Lee C, Wakeham S G. 2002. Effects of oxygen and redox oscillation on degradation of cellassociated lipids in surficial marine sediments. Geochimica et Cosmochimica Acta, 66(11): 2003-2012.
DOI:10.1016/S0016-7037(02)00830-X |
Sun M Y, Dai J H. 2005. Relative influences of bioturbation and physical mixing on degradation of bloom-derived particulate organic matter:clue from microcosm experiments. Marine Chemistry, 96(3-4): 201-218.
DOI:10.1016/j.marchem.2004.11.003 |
Sun M Y, Shi W, Lee R F. 2000. Lipid-degrading enzyme activities associated with distribution and degradation of fatty acids in the mixing zone of Altamaha estuarine sediments. Organic Geochemistry, 31(9): 889-902.
DOI:10.1016/S0146-6380(00)00051-6 |
Sun M Y, Wakeham S G, Lee C. 1997. Rates and mechanisms of fatty acid degradation in oxic and anoxic coastal marine sediments of Long Island Sound, New York, USA. Geochimica et Cosmochimica Acta, 61(2): 341-355.
DOI:10.1016/S0016-7037(96)00315-8 |
Sun M Y, Wakeham S G. 1998. A study of oxic/anoxic effects on degradation of sterols at the simulated sediment-water interface of coastal sediments. Organic Geochemistry, 28(12): 773-784.
DOI:10.1016/S0146-6380(98)00043-6 |
Sun M Y, Zou L, Dai J H, Ding H, Culp R A, Scranton M I. 2004. Molecular carbon isotopic fractionation of algal lipids during decomposition in natural oxic and anoxic seawaters. Organic Geochemistry, 35(8): 895-908.
DOI:10.1016/j.orggeochem.2004.04.001 |
Suzuki T, Matsuyama Y. 1995. Determination of free fatty acids in marine phytoplankton causing red tides by fluorometric high-performance liquid chromatography. Journal of the American Oil Chemists' Society, 72(10): 1211-1214.
DOI:10.1007/BF02540991 |
Teece M A, Getliff J M, Leftley J W, Parkes R J, Maxwell J R. 1998. Microbial degradation of the marine prymnesiophyte Emiliania huxleyi under oxic and anoxic conditions as a model for early diagenesis:long chain alkadienes, alkenones and alkyl alkenoates. Organic Geochemistry, 29(4): 863-880.
DOI:10.1016/S0146-6380(98)00145-4 |
Urbani R, Magaletti E, Sist P, Cicero A M. 2005. Extracellular carbohydrates released by the marine diatoms Cylindrotheca closterium, Thalassiosira pseudonana and Skeletonema costatum:effect of P-depletion and growth status. Science of the Total Environment, 353(1-3): 300-306.
DOI:10.1016/j.scitotenv.2005.09.026 |
Vieler A, Wilhelm C, Goss R, Süß R, Schiller J. 2007. The lipid composition of the unicellular green alga Chlamydomonas reinhardtii and the diatom Cyclotella meneghiniana investigated by MALDI-TOF MS and TLC. Chemistry and Physics of Lipids, 150(2): 143-155.
DOI:10.1016/j.chemphyslip.2007.06.224 |
Voet D, Voet J G. 2011. Biochemistry. 4th edn. John Wiley and Sons, Inc, Hoboken, NJ.
|
Volkman J K, Farrington J W, Gagosian R B. 1987. Marine and terrigenous lipids in coastal sediments from the Peru upwelling region at 15°S:sterols and triterpene alcohols. Organic Geochemistry, 11(6): 463-477.
DOI:10.1016/0146-6380(87)90003-9 |
Wakeham S G, Beier J A. 1991. Fatty acid and sterol biomarkers as indicators of particulate matter source and alteration processes in the Black Sea. Deep Sea Research Part A. Oceanographic Research Papers, 38(S2): S943-S968.
|
Wakeham S G, Lee C. 1993. Production, transport, and alteration of particulate organic matter in the marine water column. In: Engel M H, Macko S A eds. Organic Geochemistry: Principles and Applications. Springer, Boston, MA. p.145-169.
|
Wang B D, Wei Q S, Chen J F, Xie L P. 2012. Annual cycle of hypoxia off the Changjiang (Yangtze River) estuary. Marine Environmental Research, 77: 1-5.
DOI:10.1016/j.marenvres.2011.12.007 |
Westrich J T, Berner R A. 1984. The role of sedimentary organic matter in bacterial sulfate reduction:the G model tested. Limnology and Oceanography, 29(2): 236-249.
DOI:10.4319/lo.1984.29.2.0236 |
Yang S, Wu R S S, Kong R Y C. 2002. Biodegradation and enzymatic responses in the marine diatom Skeletonema costatum upon exposure to 2, 4-dichlorphenol. Aquatic Toxicology, 59(3-4): 191-200.
DOI:10.1016/S0166-445X(01)00252-1 |
 2018, Vol. 36
2018, Vol. 36