Institute of Oceanology, Chinese Academy of Sciences
Article Information
- GUO Liangliang(郭亮亮), DAI Liangliang(代亮亮), YANG Kai(杨凯), LI Dunhai(李敦海), LI Genbao(李根保)
- Physiological changes of submerged macrophytes in response to a floating filamentous green algae bloom in clear-water conditions
- Chinese Journal of Oceanology and Limnology, 36(5): 1604-1614
- http://dx.doi.org/10.1007/s00343-018-7139-0
Article History
- Received May. 4, 2017
- accepted in principle Jul. 7, 2017
- accepted for publication Dec. 11, 2017
2 University of Chinese Academy of Sciences, Beijing 100049, China;
3 Research Center for Eco-Environmental Sciences, Chinese Academy of Sciences, Beijing 100085, China
Mass filamentous green algae are frequently found in the littoral zones of lakes, ponds, and wetlands (Auer et al., 1982; Irfanullah and Moss, 2005a; Gallego et al., 2014). When filamentous green algae become dominant, they directly or indirectly influence the growth of submerged macrophytes. This can lead to a decrease in, or the disappearance of, the submerged macrophytes (Irfanullah and Moss, 2005a). Some studies have suggested that nutrientrich shallow lakes may be dominated by a particular group, such as epiphytic or filamentous algae or freefloating macrophytes (Morris et al., 2003; Scheffer et al., 2003; Scheffer and van Nes, 2007). Filamentous green algae may be dominant in a clear water state representing an alternative state in high-nutrient shallow temperate lakes (Trochine et al., 2011). But, the overgrowth of filamentous green algae is not conducive to maintain the clear-water state in which submerged macrophytes are the primary producers.
Submerged macrophytes enhance water quality and biodiversity by competing for resources and producing allelochemicals that suppress phytoplankton blooms in shallow lakes. Consequently, the recovery of submerged macrophytes is an important goal for the restoration of eutrophic lakes (Pot and ter Heerdt, 2014; Vanderstukken et al., 2014; Velthuis et al., 2017). Filamentous green algae can affect the growth of submerged macrophytes by shading, allelopathy, mechanical destruction, changing water conditions, and competing for nutrients (Phillips et al., 1978; Simpson and Eaton, 1986; Irfanullah and Moss, 2004). Previous studies have reported that filamentous algae (mainly Cladophora glomerata) did not affect Elodea canadensis when the ratio of E. canadensis biomass to filamentous algae biomass was 1:1, but larger biomasses of decomposing algae limited E. canadensis growth (Tarmanowska, 1995; Pieczyńska and Tarmanowska, 1996). Other studies showed that overgrowth of filamentous green algae resulted in light competition with submerged macrophytes, and strongly inhibited the recovery of Elodea nuttallii (Irfanullah and Moss, 2004, 2005a). In another study, filamentous green algae (mainly Spirogyra sp.) did not influence the number of shoot divisions, shoot length, or growth rate of E. nuttallii by allelopathy under nutrient-rich conditions (Irfanullah and Moss, 2005b). Filamentous green algae such as Cladophora can dislodge from substrates and form a floating mat (Stevenson and Stoermer, 1982; Ólafsson et al., 2013), which provides an anaerobic environment for the growth of bacteria. The growth of Clostridium botulinum in a floating mat was shown to negatively affect water quality (Byappanahalli and Whitman, 2009)
Several studies have focused on the effects of living or decomposing filamentous green algae on submerged macrophytes, but few have investigated the effects of living floating algae mats on submerged macrophytes. To study the potential effects of floating algae on a submerged macrophyte, we selected Cladophora as the floating alga and Elodea as the macrophyte in this experiment. Our objectives were as follows: (1) to determine the effects of different amounts of floating Cladophora on the photosynthetic and antioxidant systems of Elodea; and (2) to determine the amount of floating Cladophora that negatively affects Elodea.
2 MATERIAL AND METHOD 2.1 Growth of test species under field conditionsRestoration of submerged macrophytes in Caohai Lake, a eutrophic lake (102.642°E, 24.982°N; Kunming, China) is one of the main goals of the Major Science and Technology Program for Water Pollution Control and Treatment. Therefore, we constructed different-sized enclosures in the lake to restore submerged macrophytes. The submerged macrophytes (mainly E. nuttallii) initially grew well. However, when the environmental conditions were appropriate, such as water temperature and transparency, Cladophora sp. settled on the E. nuttallii plants and grew rapidly, about a week later, covering the entire enclosure or accumulating in the corner and floating on the surface of the water. After a few days, the submerged macrophytes showed a drastic decrease in growth rate or disappeared.
2.2 Collection of alga and submerged macrophyteCladophora sp. and E. nuttallii were collected from Caohai Lake and cultured outdoors in clean tap water (total nitrogen=1.957±0.053 mg/L, total phosphorus=0.065±0.008 mg/L) for 4 days under natural conditions before the experiment.
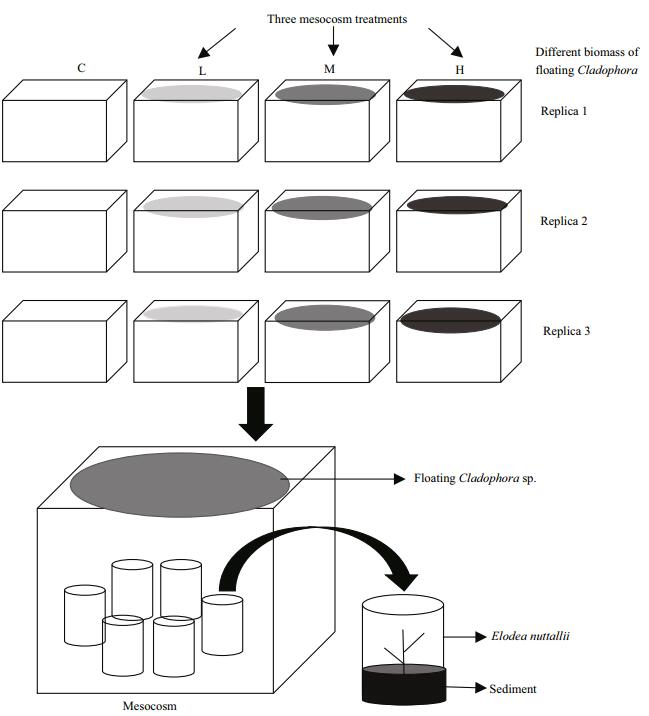
2.3 Experimental designThe experiment was performed from 20th August to 7th September 2014 at the field station near Caohai Lake. Twelve clear acid-washed plastic tanks (length 45 cm×width 32 cm×height 25 cm; volume ca. 36 L each) were used to establish four treatments in triplicate: control, low, middle, high, containing floating Cladophora sp. at 0, 20 g (140 g fresh weight (FW)/m2), 40 g (280 g FW/m2), and 80 g (560 g FW/m2), respectively. The tanks were arranged side by side to ensure that all groups were subjected to the same external conditions. Healthy apical shoots of E. nuttallii (~10 cm length, fully expanded leaves, and without lateral shoots; three per bundle) were weighed and planted in individual plastic pots (12 cm diameter, 8 cm high) containing 7 cm sediment from Caohai Lake (Fig. 1). To ensure that the submerged macrophyte reached a certain biomass and was rooted in the sediment, E. nuttallii plants were cultured for 4 days before adding floating Cladophora sp. into the system. The floating Cladophora sp. were weighed weekly to determine fresh biomass and adjusted to the initial value to maintain the biomass ratio in each treatment. At the end of the experiment, the FW of E. nuttallii in each tank was measured.
|
| Figure 1 Schematic diagram of experimental design C: control, no floating Cladophora sp.; L, M, H: 20, 40, and 80 g (fresh weight) floating Cladophora sp., respectively. |
During the experiment, environmental variables including water temperature, salinity, conductivity, dissolved oxygen, and pH were measured by a multiparameter water quality analyzer (YSI Inc., Yellow Springs, OH, USA) at 12.00 PM every 6 days. The underwater light intensity was measured at a depth of 5 cm using a LI-250 light meter (LI-COR, Lincoln, NE, USA) at 15:00 to 16:00 every 6 days. The percentage of shading was calculated using Eq.1:
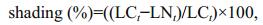 (1)
(1)where LCt (μmol/(m2·s)) and LNt (μmol/(m2·s)) are the underwater light intensities in the control and the treatment at time t, respectively.
2.4.2 Determination of lipid peroxidation and peroxidase and catalase activitiesWeighed plant samples (0.025±0.002 g) were ground in liquid nitrogen and extracted in 5 mL 5% (v/v) trichloroacetic acid. The mixture was centrifuged for 15 min at 13 000×g at 4℃. Then, 2 mL 0.67% thiobarbituric acid was added to 2 mL supernatant, and the mixture was boiled in a 100℃ water bath for 30 min. The mixture was cooled to room temperature, centrifuged for 10 min at 13 000×g, and then the absorbance of the supernatant was measured at 450, 532, and 600 nm. The absorbance values were used to calculate the malonaldehyde (MDA) concentration (Heath and Packer, 1968).
To determine catalase (CAT) and peroxidase (POD) activities, weighed plant samples (0.025± 0.002 g) were ground on ice using a tissue homogenizer and extracted in 5 mL 50 mmol/L potassium phosphate buffer (pH 7.8) containing 10% polyvinyl pyrrolidine (PVP). The extract was centrifuged for 15 min at 13 000×g at 4℃. The supernatant was used to determine CAT (Góth, 1991) and POD (Maehly, 1955) activities, as well as total soluble protein (TSP) content (Bradford, 1976). Activity was expressed as units of CAT/POD activity per milligram TSP. One unit (U) of CAT (POD) activity was defined as an absorbance change of 0.01 units per minute at 240 nm (470 nm) under these conditions.
2.4.3 Extraction and quantification of photosynthetic pigmentsChlorophylls and carotenoids were extracted from leaves (0.025±0.002 g fresh weight) in 25 mL 95% (v/v) ethanol in darkness for 48 h at 4℃. The absorbance of the mixture was determined at 470, 649, and 665 nm and these values were used to calculate the concentrations of chlorophyll a, b, and carotenoids (Lichtenthaler and Buschmann, 2001).
2.4.4 Chlorophyll fluorescenceThe chlorophyll fluorescence parameters of the fourth leaf from the apex were measured using a WATER-PAM (Walz, Effeltrich, Germany) equipped with a Water-EDF fiber optic unit. The efficiency of PSII photochemistry (ΔF/Fm') was calculated as follows: (Fm'-Ft)/Fm'. After the samples were darkadapted for 10 min, the maximum quantum efficiency of photosystem Ⅱ (Fv/Fm) was measured and calculated as follows (Fm-F0)/Fm. Fm' and Fm are the maximal fluorescence from a light-adapted leaf and a darkadapted leaf, respectively; Ft is the instantaneous steady state fluorescence (sometimes called Fs); and F0 is the minimal fluorescence from a dark-adapted leaf (Maxwell and Johnson, 2000; Baker, 2008).
Rapid light-response curves (RLC) were constructed by applying light every 10 s at nine increasing light intensity levels (30, 64, 96, 143, 219, 324, 495, 738, and 1 048 μmol/(m2·s)). The relative electron transport rate (rETR) was calculated as the product of effective quantum yield and light intensity. Relative ETR was plotted against light intensity and fitted with a function from which the light-response curve parameters light utilization efficiency (α) and maximum rETR (rETRmax) were determined. The light saturation parameter (Ek) was calculated as follows: rETRmax/α (Jassby and Platt, 1976). The α, rETRmax, and Ek determined from the RLC reflect the ability of plants to use light efficiently, the maximum photosynthetic rate, and the ability to tolerate high light, respectively (Ralph and Gademann, 2005).
2.5 Data analysisThe MDA content, enzyme activity, photosynthetic pigments, Fv/Fm, and ΔF/Fm' were measured at indicated times during the experiment, and treatment (df=3) and time (df=3) effects were determined using repeated-measures analyses of variance (RMANOVA) with the treatment (control, low, middle, high) as the fixed factor and time as the repeated factor. The sphericity assumption was tested using Mauchly's test, and the degrees of freedom were Greenhouse-Geisser adjusted when the data did not meet the assumption of sphericity. One-way analyses of variance (ANOVA) with Tukey's multiple comparisons were used to detect significant differences in each variable among treatments and time points separately. If heterogeneity of variance was detected, Dunnett's T3 multiple comparisons were used to test the differences. Pearson's correlation analysis was used to test correlations between environmental variables. Paired t-tests were used to test the significance of differences in RLC parameters (α, rETRmax, Ek) between day 6 and day 18. The data were Loge-transformed when necessary. All statistical analyses were performed using SPSS 20.0 (SPSS Inc., Chicago, IL, USA). Significance was accepted at P < 0.05. Data are presented as mean±standard deviation.
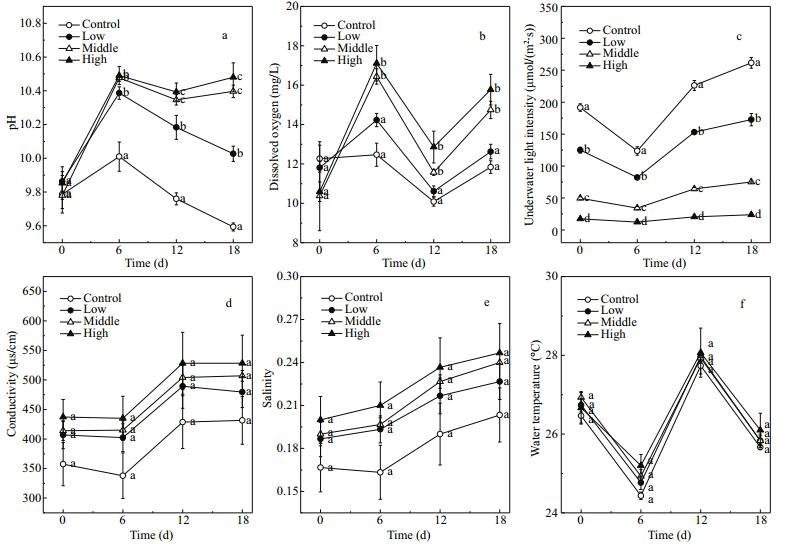
3 RESULT 3.1 Environmental physicochemical parametersDuring the 18-day experiment, the biomass of floating Cladophora sp. decreased in the low treatment, slightly increased in the high treatment and was almost unchanged in middle treatment. Compared with the water in the control, the water in the treatments with Cladophora sp. showed higher pH values (Fig. 2a) and dissolved oxygen concentrations (Fig. 2b). The pH increased during the experiment. On day 18, the average pH of water in the middle and high treatments was 0.80 and 0.89 higher than that in the control, respectively (P < 0.001). The dissolved oxygen concentration tended to fluctuate during the experiment and peaked on day 6 at 16.44±0.48 and 17.11±1.10 mg/L in the middle and high treatments, respectively (F=25.35, P < 0.001). The underwater light intensity decreased substantially as the biomass of floating Cladophora sp. increased. The final percentages of shading in the four groups (Fig. 2c) were as follows: control, 0%; low, 34%±1%; middle, 72%±2%; and high, 90%±1% (P < 0.05).
|
| Figure 2 Changes in pH (a), dissolved oxygen (b), underwater light intensity (c), conductivity (d), salinity (e), and water temperature (f) in control (no floating Cladophora sp.) and low, middle, and high (20 g, 40 g, and 80 g fresh weight floating Cladophora sp., respectively) treatment groups Error bars represent standard deviation (n=3). Different letters indicate significant difference (P<0.05). |
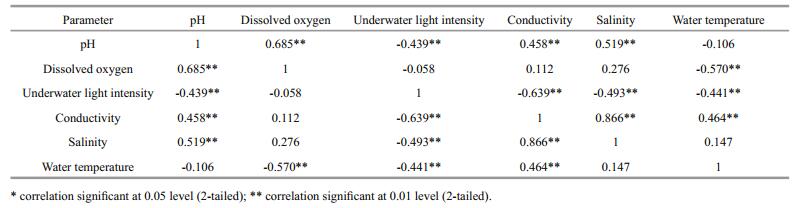
There were no significant differences in conductivity (Fig. 2d), salinity (Fig. 2e), and water temperature (Fig. 2f) among the four treatment groups (P > 0.05). Correlation analyses revealed a negative correlation between dissolved oxygen concentration and water temperature (R=-0.57, P < 0.001), and positive correlations between pH and dissolved oxygen concentration (R=0.69, P < 0.001) and between pH and salinity (R=0.52, P < 0.001) (Table 1).
From day 6 onwards, the MDA content of E. nuttallii differed significantly among the four treatments (F=2.75, P=0.023). At the end of the experiment, the average MDA content in the middle and high treatments was 60.4% and 49.1% higher, respectively, than that in the control (P < 0.05). There was no significant difference in the MDA content of E. nuttallii between the low treatment and the control (P > 0.05) (Fig. 3a), suggesting that greater biomass of floating Cladophora sp. resulted in greater oxidative stress in E. nuttallii.

|
| Figure 3 Changes in malonaldehyde concentration (μmol/g FW) (a), catalase activity (U/mg TSP) (b), and peroxidase activity (c) (U/mg TSP) in Elodea nuttallii Control: no floating Cladophora sp.; low, middle, and high: 20 g, 40 g, and 80 g (fresh weight) floating Cladophora sp., respectively. Error bars represent standard deviation (n=3). Different letters indicate significant difference (P<0.05). |
The presence of floating Cladophora sp. stimulated CAT activity in E. nuttallii, and CAT activity increased over time (F=4.82, P=0.001). The average CAT activity of E. nuttallii in the high treatment was 3.63 times that in the control on day 6 (P=0.011). On day 18, the CAT activity of E. nuttallii in the low, middle, and high treatments was 6.62, 7.44, and 9.73 times that in the control, respectively (P=0.014, P=0.007, P < 0.001, respectively) (Fig. 3b).
The POD activity of E. nuttallii increased over time (F=27.93, P < 0.001), and differed significantly between the high treatment and the other treatments by day 18 (F=9.84, P=0.05). The POD activity in the high treatment was 39.9% higher than that in the control, but did not differ significantly among the control, low, and middle treatments (P > 0.05) (Fig. 3c).
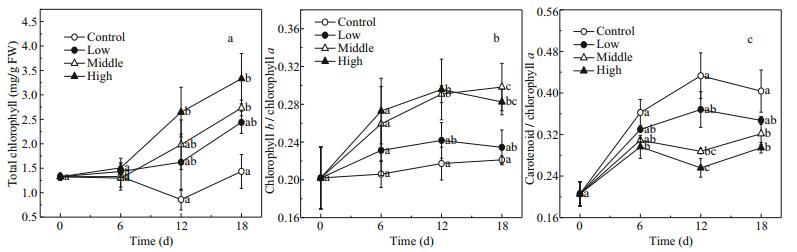
3.3 Photosynthetic pigmentsIn the presence of floating Cladophora sp., the total chlorophyll content (total chl) of E. nuttallii increased over time (F=27.82, P < 0.001) and was proportional to the amount of Cladophora sp. (F=18.73, P=0.001) (Fig. 4a).
|
| Figure 4 Changes in total chlorophyll (mg/g FW) (a), chlorophyll b/chlorophyll a (b) and carotenoids/chlorophyll a (c) in Elodea nuttallii during experiment Control: no floating Cladophora sp.; low, medium, and high: 20 g, 40 g, and 80 g (fresh weight) floating Cladophora sp., respectively. Error bars represent standard deviation (n=3). Different letters indicate significant difference (P<0.05). |
The response of chlorophyll b/chlorophyll a (chl b/chl a) in E. nuttallii to floating Cladophora sp. was similar to that of total chl. The chl b/chl a increased with increasing biomass of floating Cladophora sp. (F=8.10, P=0.008; Fig. 3b). The Cladophora sp. had a strong shading effect, and so E. nuttallii was in a shade-adapted state in the middle and high treatments (Fig. 4b).
The trend in carotenoids/chlorophyll a (car/chl a) was almost opposite to that of total chl and chl b/chl a. The car/chl a decreased with increasing biomass of floating Cladophora sp. (F=21.87, P < 0.001). Between days 6 and 18, the car/chl a in the control (0.40±0.050) was higher than those in the middle and high treatments (0.31±0.020 and 0.28±0.027, respectively), suggesting that E. nuttallii in the control was in a light-protective state (Fig. 4c).
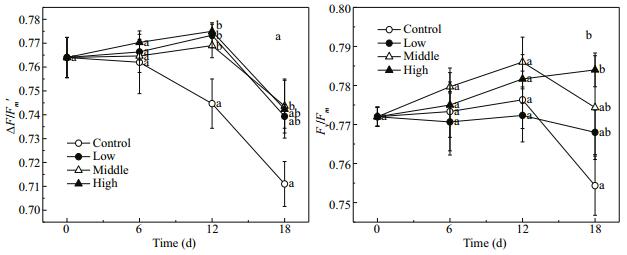
3.4 Chlorophyll fluorescence parametersThe ΔF/Fm' values of E. nuttallii were higher in the treatments with Cladophora sp. than in the control (F=9.60, P=0.005). The ΔF/Fm' first fluctuated over a certain range and then decreased during the experiment (F=31.21, P < 0.001) (Fig. 5a). However, the Fv/Fm value of E. nuttallii was not affected by treatments or time (F=0.96, P=0.466). On day 18, the Fv/Fm value of E. nuttallii was 0.754±0.008 in the control group (Fig. 5b). As indicated by the ΔF/Fm' and Fv/Fm values, E. nuttallii in the four treatment groups maintained photosynthesis during the experiment.
|
| Figure 5 Changes in ΔF/Fm' (a) and F/Fm (b) in Elodea nuttallii during experiment Control: no floating Cladophora sp.; low, medium, high: 20 g, 40 g, and 80 g (fresh weight) floating Cladophora sp., respectively. Error bars represent standard deviation (n=3). Different letters indicate significant difference (P<0.05). |
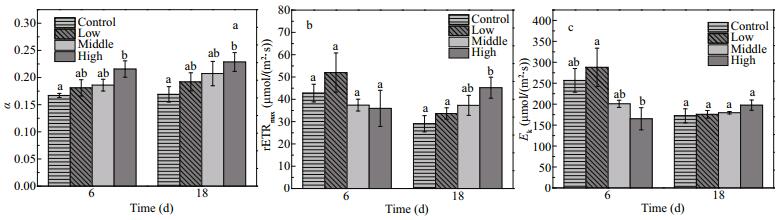
The photosynthetic parameters (α, Ek and rETRmax) of E. nuttallii were influenced by the increasing biomass of floating Cladophora sp. to different extents. The α values of E. nuttallii were higher in the treatments with floating Cladophora sp. than in the control (F=12.855, P=0.002), indicating that E. nuttallii improved its light utilization to adapt to low light intensity (Fig. 6a). The rETRmax and Ek values of E. nuttallii were affected by the amount of Cladophora sp. and time (F=5.84, P=0.021; F=9.68, P=0.005, respectively). The rETRmax value of E. nuttallii was 29.1±4.44 μmol/(m2·s) in the control, lower than that in the high treatment (45.2±5.77 μmol/(m2·s)) on day 18 (P=0.015). (Fig. 6b). The Ek value of E. nuttallii was higher on day 6 when the biomass of floating Cladophora was lower (F=6.62, P=0.015), but there were no differences in Ek among the four treatment groups on day 18 (F=1.96, P=0.20) (Fig. 6c). In the control, the values of rETRmax and Ek for E. nuttallii were lower on day 18 than on day 6 (t=4.91, P=0.039; t=9.74, P=0.010, respectively).
|
| Figure 6 Changes in α (a), rETRmax (μmol/(m2·s)) (b) and Ek (μmol/(m2·s)) (c) in Elodea nuttallii during experiment α: maximum light utilization efficiency; rETRmax (μmol electrons/(m2·s)): maximum relative electron transport rate; Ek (μmol electrons/(m2·s)): light saturation parameter. Control: no floating Cladophora sp.; low, middle, high: 20 g, 40 g, and 80 g (fresh weight) floating Cladophora sp., respectively. Different letters indicate significant difference (P<0.05). |
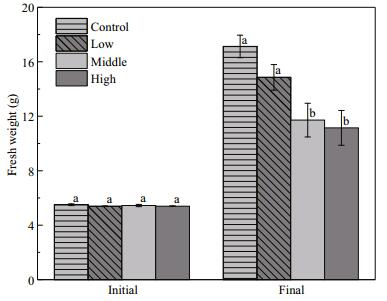
Floating Cladophora sp. negatively affected the growth of E. nuttallii (F=27.39, P < 0.001). In the middle and high Cladophora sp. treatments, the biomass of E. nuttallii was decreased by 30.5% and 38.3%, respectively, compared with that in the control (P < 0.001). However, the low Cladophora sp. treatment did not affect the biomass of E. nuttallii (P=0.083) (Fig. 7).
|
| Figure 7 Fresh weight (g) of Elodea nuttallii at the end of the experiment Control: no floating Cladophora sp.; low, middle, high: 20 g, 40 g, and 80 g (fresh weight) floating Cladophora sp., respectively. Different letters indicate significant difference (P<0.05). |
The effects of floating Cladophora sp. on the submerged macrophyte E. nuttallii were rather complex, and included positive and negative effects. The percentage of shading, dissolved oxygen, and pH value increased with increasing biomass of Cladophora sp., while dissolved oxygen and pH values fluctuated in a small range in the control. During the day, the floating Cladophora sp. caused the dissolved oxygen concentration increasing and carbon dioxide concentration decreasing in the water column through photosynthesis. Decreased carbon dioxide concentration in turn raised the pH value in the water. Conversely, the floating Cladophora sp. were only respiring at night, decreasing dissolved oxygen concentration in the water column and producing carbon dioxide that lowered pH level. The dissolved oxygen concentration decreased with increasing water temperature, which implied that high water temperature negatively affected the photosynthesis of both E. nuttallii and Cladophora sp. Netten et al. (2010) found that the free-floating macrophyte Salvinia natans showed increased growth with an increase in water temperature, and it outcompeted E. nuttallii to take advantage of the environmental change (Netten et al., 2010). However, the water temperature was not the main factor limiting the growth of E. nuttallii in our experiment. Several studies have shown that E. nuttallii has strong phenotypic plasticity to adapt to changing conditions (Ozimek et al., 1993; Baldy et al., 2015). Velthuis et al. (2017) reported that the aboveground biomass of E. nuttallii showed no difference between 10℃ and 25℃, but the C:N ratio was lower at 25℃ (Velthuis et al., 2017). The conductivity and salinity of each group increased over time, but did not differ significantly among the four groups. Aquatic plants can produce secondary metabolites such as organic acids and terpenoids (Irfanullah and Moss, 2005b; Gao et al., 2014; Wang et al., 2014), which might explain this phenomenon.
Larger biomasses of Cladophora sp. at the water surface resulted in increased shading, and the total chlorophyll content and chl b/chl a of E. nuttallii were positively correlated with the degree of shading. These results suggest that the decrease in light intensity led to greater chlorophyll accumulation to produce sufficient energy for plant growth. An increase in the relative chlorophyll b content in leaves has been shown to be beneficial for absorbing shorter wavelengths (Lichtenthaler et al., 1981). The high total chlorophyll content of E. nuttallii increased the values of several photosynthetic parameters (including ΔF/Fm' and the RLC parameters). Some studies have shown that the total chlorophyll content affects the efficiency of photosystem Ⅱ at the genetic level (Vijayalakshmi et al., 2010; Czyczyło-Mysza et al., 2013). Under shaded conditions, the E. nuttallii leaf cells showed an accelerated electron transfer rate and had a higher proportion of open reaction centers to increase their photosynthetic activity. These are strategies that allow plants to tolerate short-term low light intensity (Ralph and Gademann, 2005).
In a previous study, the chlorophyll fluorescence of a submerged macrophyte remained high under prolonged shading (30 d) (Lu et al., 2012). However, we observed a slight decrease in the ΔF/Fm' and Fv/Fm values of E. nuttallii from day 12 to day 18. These decreases were related to the high pH values and dissolved oxygen concentration. Simpson et al. (1980), Simpson and Eaton (1986) found that high O2, high pH, and low CO2 conditions led to major decreases in the photosynthetic activity of E. canadensis (Simpson et al., 1980; Simpson and Eaton, 1986). In the control, the chlorophyll fluorescence parameters (ΔF/Fm', Fv/Fm, rETRmax, and Ek) of E. nuttallii decreased and the car/chl a increased as a result of the high light intensity. Hussner et al. (2010) found that the effective quantum yield of photosystem Ⅱ in E. nuttallii decreased when photosynthetically active radiation was higher than 100 μmol/(m2·s) (Hussner et al., 2010). In addition, the large amount of UV radiation decreased the photosynthetic efficiency of E. nuttallii at a field station located at 1 950 m above sea level (Chen et al., 2012). Since the photosynthetic activity of E. nuttallii was higher in the presence Cladophora sp. than in its absence, we suggest that the presence of floating Cladophora sp. had a stimulating effect on the photosynthetic system of E. nuttallii.
The impact of floating macrophytes appears to be more complex than just simple physical shading (Lu et al., 2012). Drastic changes in the external environment in the presence of floating Cladophora sp. caused an increase in free radicals, increased MDA content, and increased antioxidant enzyme activities in E. nuttallii leaves. In the present experiment, POD activity increased significantly by day 18, whereas CAT activity increased throughout the experiment, suggesting that it was more sensitive than POD activity to the ROS induced by the presence of Cladophora sp. Butow et al. (1994) found that CAT activity remained unchanged within the range of pH 6 to pH 10 in in vitro and in vivo conditions, and that high pO2/pCO2 increased CAT activity (Butow et al., 1994). Zhang et al. (2010) and Song et al. (2017) found that low light intensity resulted in increased POD and SOD activities in plants (Zhang et al., 2010; Song et al., 2017). We did not observe large changes in POD activity in our experiment, indicating that shading was not the reason for the increased enzymes activities. Instead, the high dissolved oxygen concentration and pH values were the main contributors to oxidative stress.
Elodea nuttallii can adapt to low light conditions, but very low light is not conducive to the growth of E. nuttallii over a long period (Li et al., 2015; Zefferman, 2015). The light intensity and UV radiation were high at the experimental site; therefore, shading protected/promoted photosynthetic activity during this 18-day experiment. Although photosynthesis is one of the main factors affecting plant growth, there was no direct correlation between the growth rate and photosynthetic rate. The growth rate of E. nuttallii was likely influenced by other factors, for example, water level fluctuations, life-cycle, and competitive ability (Korner, 1991; Dollerup et al., 2013; Grudnik et al., 2014; Wang et al., 2016). In the present study, the increasing concentration of dissolved oxygen and pH value were the main factors affecting the biomass of E. nuttalllii via their negative effects on oxidative stress and enzyme activities. The increased antioxidant enzyme activities consumed energy, which meant that less energy was available for biomass accumulation and reproduction of E. nuttallii. Thus, E. nuttallii had a low biomass in the high Cladophora sp. treatments (280 and 560 g FW/m2). In addition, the high pH decreased the amount of dissolved inorganic carbon (Choo et al., 2004; Falkowski and Raven, 2013), negatively affecting the growth of E. nuttallii.
5 CONCLUSIONFloating Cladophora sp. changed the water environment by shading and increasing the dissolved oxygen concentration and pH value. Shading promoted the photosynthetic activity of E. nuttallii, while the increases in pH value and dissolved oxygen concentration reduced photosynthetic activity and increased the antioxidant enzyme activity of E. nuttallii. These changes resulted in decreased biomass of E. nuttallii in the presence of floating Cladophora sp. The results indicated that the indirect effects of floating Cladophora sp. on water quality may be more important than the direct effects of shading. Consequently, in the ecological restoration of submerged macrophytes, managers should pay more attention to changes in water quality than to shading by floating Cladophora sp.
6 DATA AVAILABILITY STATEMENTThe datasets obtained in this study are available from the corresponding author on request.
7 ACKNOWLEDGEMENTWe thank Jennifer Smith, PhD, from Liwen Bianji, Edanz Group China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Auer M T, Canale R P, Grundler H C, Matsuoka Y. 1982. Ecological studies and mathematical modeling of Cladophora in Lake Huron: 1. Program description and field monitoring of growth dynamics. Journal of Great Lakes Research, 8(1): 73-83.
|
Baker N R. 2008. Chlorophyll fluorescence: a probe of photosynthesis in vivo. Annual Review of Plant Biology, 59(1): 89-113.
DOI:10.1146/annurev.arplant.59.032607.092759 |
Baldy V, Thiebaut G, Fernandez C, Sagova-Mareckova M, Korboulewsky N, Monnier Y, Perez T, Trémolieres M. 2015. Experimental assessment of the water quality influence on the phosphorus uptake of an invasive aquatic plant: biological responses throughout its phenological stage. PLoS One, 10(3): e0118844.
DOI:10.1371/journal.pone.0118844 |
Bradford M M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72(1-2): 248-254.
DOI:10.1016/0003-2697(76)90527-3 |
Butow B, Wynne D, Tel-Or E. 1994. Response of catalase activity to environmental stress in the freshwater dinoflagellate Peridinium gatunense. Journal of Phycology, 30(1): 17-22.
DOI:10.1111/j.0022-3646.1994.00017.x |
Byappanahalli M N, Whitman R L. 2009. Clostridium botulinum type E occurs and grows in the alga Cladophora glomerata. Canadian Journal of Fisheries and Aquatic Sciences, 66(6): 879-882.
DOI:10.1139/F09-052 |
Chen L Z, Xie M, Bi Y H, Wang G H, Deng S Q, Liu Y D. 2012. The combined effects of UV-B radiation and herbicides on photosynthesis, antioxidant enzymes and DNA damage in two bloom-forming cyanobacteria. Ecotoxicology and Environmental Safety, 80: 224-230.
DOI:10.1016/j.ecoenv.2012.03.007 |
Choo K S, Snoeijs P, Pedersén M. 2004. Oxidative stress tolerance in the filamentous green algae Cladophora glomerata and Enteromorpha ahlneriana. Journal of Experimental Marine Biology and Ecology, 298(1): 111-123.
DOI:10.1016/j.jembe.2003.08.007 |
Czyczyło-Mysza I, Tyrka M, Marcińska I, Skrzypek E, Karbarz M, Dziurka M, Hura T, Dziurka K, Quarrie S A. 2013. Quantitative trait loci for leaf chlorophyll fluorescence parameters, chlorophyll and carotenoid contents in relation to biomass and yield in bread wheat and their chromosome deletion bin assignments. Molecular Breeding, 32(1): 189-210.
DOI:10.1007/s11032-013-9862-8 |
Dollerup K, Riis T, Clayton J S. 2013. Do patterns of establishment support invasive status of five aquatic plants in New Zealand?. Journal of Aquatic Plant Management, 51: 1-6.
|
Falkowski P G, Raven J A. 2013. Aquatic Photosynthesis. Princeton University Press, Princeton.
|
Gallego I, Davidson T A, Jeppesen E, Pérez-Martínez C, Fuentes-Rodríguez F, Juan M, Casas J J. 2014. Disturbance from pond management obscures local and regional drivers of assemblages of primary producers. Freshwater Biology, 59(7): 1406-1422.
DOI:10.1111/fwb.2014.59.issue-7 |
Gao Y N, Zhang L P, Liu B Y, Zhang Y Y, Wu Z B. 2014. Research on Allelochemicals with material properties exuded by submerged freshwater Macrophyte Elodea nuttallii. Advanced Materials Research, 1023: 71-74.
DOI:10.4028/www.scientific.net/AMR.1023 |
Góth L. 1991. A simple method for determination of serum catalase activity and revision of reference range. Clinica Chimica Acta, 196(1-2): 143-151.
|
Grudnik Z M, Jelenko I, Germ M. 2014. Influence of abiotic factors on invasive behaviour of alien species Elodea nuttallii in the Drava River (Slovenia). Ann. Limnol.-Int. J. Lim., 50(1): 1-8.
DOI:10.1051/limn/2013065 |
Heath R L, Packer L. 1968. Photoperoxidation in isolated chloroplasts: Ⅰ. Kinetics and stoichiometry of fatty acid peroxidation. Archives of Biochemistry and Biophysics, 125(1): 189-198.
DOI:10.1016/0003-9861(68)90654-1 |
Hussner A, Hoelken H P, Jahns P. 2010. Low light acclimated submerged freshwater plants show a pronounced sensitivity to increasing irradiances. Aquatic Botany, 93(1): 17-24.
DOI:10.1016/j.aquabot.2010.02.003 |
Irfanullah H M, Moss B. 2004. Factors influencing the return of submerged plants to a clear-water, shallow temperate lake. Aquatic Botany, 80(3): 177-191.
DOI:10.1016/j.aquabot.2004.07.010 |
Irfanullah H M, Moss B. 2005a. A filamentous green algaedominated temperate shallow lake: variations on the theme of clear-water stable states?. Archiv für Hydrobiologie, 163(1): 25-47.
DOI:10.1127/0003-9136/2005/0163-0025 |
Irfanullah H M, Moss B. 2005b. Allelopathy of filamentous green algae. Hydrobiologia, 543(1): 169-179.
DOI:10.1007/s10750-004-6955-8 |
Jassby A D, Platt T. 1976. Mathematical formulation of the relationship between photosynthesis and light for phytoplankton. Limnology and Oceanography, 21(4): 540-547.
DOI:10.4319/lo.1976.21.4.0540 |
Korner C. 1991. Some often overlooked plant characteristics as determinants of plant growth: a reconsideration. Functional Ecology, 5(2): 162-173.
DOI:10.2307/2389254 |
Li H L, Wang Y Y, Zhang Q, Wang P, Zhang M X, Yu F H. 2015. Vegetative propagule pressure and water depth affect biomass and evenness of submerged macrophyte communities. PLoS One, 10(11): e0142586.
DOI:10.1371/journal.pone.0142586 |
Lichtenthaler H K, Buschmann C, Döll M, Fietz H J, Bach T, Kozel U, Meier D, Rahmsdorf U. 1981. Photosynthetic activity, chloroplast ultrastructure, and leaf characteristics of high-light and low-light plants and of sun and shade leaves. Photosynthesis Research, 2(2): 115-141.
DOI:10.1007/BF00028752 |
Lichtenthaler H K, Buschmann C. 2001. Chlorophylls and carotenoids: measurement and characterization by UVVIS spectroscopy. In: Current Protocols in Food Analytical Chemistry. John Wiley and Sons, Inc., New York.
|
Lu J, Wang Z X, Xing W, Liu G H. 2012. Effects of substrate and shading on the growth of two submerged macrophytes. Hydrobiologia, 700(1): 157-167.
|
Maehly A C. 1955. Plant peroxidase. Methods in Enzymology, 2: 801-813.
DOI:10.1016/S0076-6879(55)02307-0 |
Maxwell K, Johnson G N. 2000. Chlorophyll fluorescence—a practical guide. Journal of Experimental Botany, 51(345): 659-668.
DOI:10.1093/jexbot/51.345.659 |
Morris K, Bailey P C, Boon P I, Hughes L. 2003. Alternative stable states in the aquatic vegetation of shallow urban lakes. Ⅱ. Catastrophic loss of aquatic plants consequent to nutrient enrichment. Marine and Freshwater Research, 54(3): 201-215.
DOI:10.1071/MF02003 |
Netten J J C, Arts G H P, Gylstra R, van Nes E H, Scheffer M, Roijackers R M. 2010. Effect of temperature and nutrients on the competition between free-floating Salvinia natans and submerged Elodea nuttallii in mesocosms. Fundamental and Applied Limnology, 177(2): 125-132.
DOI:10.1127/1863-9135/2010/0177-0125 |
Ólafsson E, Aarnio K, Bonsdorff E, Arroyo N L. 2013. Fauna of the green alga Cladophora glomerata in the Baltic Sea: density, diversity, and algal decomposition stage. Marine Biology, 160(9): 2353-2362.
DOI:10.1007/s00227-013-2229-1 |
Ozimek T, van Donk E, Gulati R D. 1993. Growth and nutrient uptake by two species of Elodea in experimental conditions and their role in nutrient accumulation in a macrophyte-dominated lake. Hydrobiologia, 251(1-3): 13-18.
DOI:10.1007/BF00007159 |
Phillips G L, Eminson D, Moss B. 1978. A mechanism to account for macrophyte decline in progressively eutrophicated freshwaters. Aquatic Botany, 4: 103-126.
DOI:10.1016/0304-3770(78)90012-8 |
Pieczyńska E, Tarmanowska A. 1996. Effect of decomposing filamentous algae on the growth of Elodea canadensis Michx. (a laboratory experiment). Aquatic Botany, 54(4): 313-319.
DOI:10.1016/0304-3770(96)01034-0 |
Pot R, ter Heerdt G N J. 2014. Succession dynamics of aquatic lake vegetation after restoration measures: increased stability after 6 years of development. Hydrobiologia, 737(1): 333-345.
|
Ralph P J, Gademann R. 2005. Rapid light curves: a powerful tool to assess photosynthetic activity. Aquatic Botany, 82(3): 222-237.
DOI:10.1016/j.aquabot.2005.02.006 |
Scheffer M, Szabó S, Gragnani A, Van Nes E H, Rinaldi S, Kautsky N, Norberg J, Roijackers R M M, Franken R J M. 2003. Floating plant dominance as a stable state. Proceedings of the National Academy of Sciences of the United States of America, 100(7): 4040-4045.
DOI:10.1073/pnas.0737918100 |
Scheffer M, van Nes E H. 2007. Shallow lakes theory revisited: various alternative regimes driven by climate, nutrients, depth and lake size. Hydrobiologia, 584(1): 455-466.
DOI:10.1007/s10750-007-0616-7 |
Simpson P S, Eaton J W, Hardwick K. 1980. The influence of environmental factors on apparent photosynthesis and respiration of the submersed macrophyte Elodea canadensis. Plant, Cell & Environment, 3(6): 415-423.
|
Simpson P S, Eaton J W. 1986. Comparative studies of the photosynthesis of the submerged macrophyte Elodea canadensis and the filamentous algae Cladophora glomerata and Spirogyra sp. Aquatic Botany, 24(1): 1-12.
DOI:10.1016/0304-3770(86)90112-9 |
Song X X, Wang Z, Xiao B D, Li E H, Wang X L. 2017. Growth of Potamogeton crispus L. from turions in darkness: implications for restoring submerged plants in eutrophic lakes. Ecological Engineering, 101: 255-260.
DOI:10.1016/j.ecoleng.2017.01.035 |
Stevenson R J, Stoermer E F. 1982. Seasonal abundance patterns of diatoms on Cladophora in Lake Huron. Journal of Great Lakes Research, 8(1): 169-183.
DOI:10.1016/S0380-1330(82)71955-0 |
Tarmanowska A. 1995. Laboratory studies on the influence of living and decomposing filamentous algae on the growth of Elodea canadensis Michx. Acta Botanica Gallica, 142(6): 685-692.
DOI:10.1080/12538078.1995.10515293 |
Trochine C, Guerrieri M, Liboriussen L, Meerhoff M, Lauridsen T L, Søndergaard M, Jeppesen E. 2011. Filamentous green algae inhibit phytoplankton with enhanced effects when lakes get warmer. Freshwater Biology, 56(3): 541-553.
DOI:10.1111/fwb.2011.56.issue-3 |
Vanderstukken M, Declerck S A J, Decaestecker E, Muylaert K. 2014. Long-term allelopathic control of phytoplankton by the submerged macrophyte Elodea nuttallii. Freshwater Biology, 59(5): 930-941.
DOI:10.1111/fwb.12316 |
Velthuis M, van Deelen E, van Donk E, Zhang P Y, Bakker E S. 2017. Impact of temperature and nutrients on carbon: nutrient tissue stoichiometry of submerged aquatic plants: an experiment and meta-analysis. Frontiers in Plant Science, 8: 655.
DOI:10.3389/fpls.2017.00655 |
Vijayalakshmi K, Fritz A K, Paulsen G M, Bai G H, Pandravada S, Gill B S. 2010. Modeling and mapping QTL for senescence-related traits in winter wheat under high temperature. Molecular Breeding, 26(2): 163-175.
DOI:10.1007/s11032-009-9366-8 |
Wang H Q, Zhu H J, Zhang L Y, Xue W J, Yuan B. 2014. Identification of antialgal compounds from the aquatic plant Elodea nuttallii. Allelopathy Journal, 34(2): 207-213.
|
Wang M Z, Liu Z Y, Luo F L, Lei G C, Li H L. 2016. Do amplitudes of water level fluctuations affect the growth and community structure of submerged macrophytes?. PLoS One, 11(1): e0146528.
DOI:10.1371/journal.pone.0146528 |
Zefferman E P. 2015. Experimental tests of priority effects and light availability on relative performance of Myriophyllum spicatum and Elodea nuttallii propagules in artificial stream channels. PLoS One, 10(3): e0120248.
DOI:10.1371/journal.pone.0120248 |
Zhang M, Cao T, Ni L Y, Xie P, Li Z Q. 2010. Carbon, nitrogen and antioxidant enzyme responses of Potamogeton crispus to both low light and high nutrient stresses. Environmental and Experimental Botany, 68(1): 44-50.
DOI:10.1016/j.envexpbot.2009.09.003 |
 2018, Vol. 36
2018, Vol. 36