Institute of Oceanology, Chinese Academy of Sciences
Article Information
- LI Kaizhi(李开枝), KE Zhixin(柯志新), TAN Yehui(谭烨辉)
- Zooplankton in the Huangyan Atoll, South China Sea: A comparison of community structure between the lagoon and seaward reef slope
- Chinese Journal of Oceanology and Limnology, 36(5): 1671-1680
- http://dx.doi.org/10.1007/s00343-019-7190-5
Article History
- Received Jun. 27, 2017
- accepted in principle Aug. 26, 2017
- accepted for publication Nov. 8, 2018
2 Guangdong Provincial Key Laboratory of Applied Marine Biology, South China Sea Institute of Oceanology, Guangzhou 510301, China;
3 University of Chinese Academy of Sciences, Beijing 100049, China
Zooplankton are an important trophic link between benthic and pelagic food webs in coral reef and atoll lagoon environments (Alldredge and King, 2009); they comprise a food source for many fish, corals, and sessile invertebrates (Alldredge and King, 1977, 2009; Coma et al., 1999; Heidelberg et al., 2004). Zooplankton can also function as biological indicators of pollution, water quality, and eutrophication in coastal or atoll lagoons (Webber et al., 2005; Pagano et al., 2012). Quantitative research on zooplankton community composition and abundance distribution is crucial for understanding the trophic structure and health of coral reef ecosystems. However, very few studies have focused on zooplankton in atoll lagoons, and only a few have concerned the Pacific Ocean (Gerber, 1981; Le Borgne et al., 1989; Carleton and Doherty, 1998; Pagano et al., 2012) and Indian Ocean (Madhupratap et al., 1977; Goswami, 1983; Goswami and Goswami, 1990).
Coral reef and atoll lagoons are productive ecosystems compared with the surrounding ocean (Hatcher, 1997). Some atoll lagoons are highly closed and isolated from the surrounding ocean except for during periods of extreme wave action (Goswami and Goswami, 1990; Carleton and Doherty, 1998; Yin et al., 2011), others are connected to the adjacent ocean by deep passes and/or shallow channels through reef flats (Gerber, 1981; Le Borgne et al., 1989; Pagano et al., 2012; Du et al., 2015). Reef zooplankton assemblages are highly variable in space and time. Studies have shown that zooplankton assemblages vary in species composition and abundance due to the differences in physical and biological parameters between closed and open atolls (Gilmartin, 1958; Carleton and Doherty, 1998; Yin et al., 2011; Pagano et al., 2012; Du et al., 2015). It is generally accepted that lagoonal waters possess unique biota and have a large abundance of zooplankton compared with adjacent oceanic waters in the larger and deeper atolls of the Pacific Ocean (Gerber, 1981; Le Borgne et al., 1989; Carleton and Doherty, 1998). Zooplankton communities vary widely in abundance and species composition between lagoons and surrounding waters in the relatively smaller and shallower atolls of the Indian Ocean (Goswami, 1983; Goswami and Goswami, 1990).
The South China Sea (SCS), in the central Indo Pacific, contains a cluster of coral reefs and atolls over an area of at least 8 000 km2 (Yu, 2012). Fringing and patch reefs in the northern part of SCS have experienced a dramatic decline in coral cover over the past 50 years due to intense anthropogenic activities (Zhao et al., 2012). Conversely, most coral reefs and atolls in the southern and central SCS have received relatively few direct anthropogenic impacts due to their distance from the mainland (Zhao et al., 2013). Only a few studies on zooplankton diversity and community in the atolls located in the central and southern SCS have been sporadically reported. The differences in zooplankton communities between lagoons, reef flats, and seaward slopes are likely due to the relatively high levels of spatial heterogeneity in coral reefs (Yin et al., 2003, 2011; Du et al., 2015). At present, a massive landfilling effort to transform some of the atolls in the SCS into fully-fledged islands has been carried out and may potentially threaten these coral reef ecosystems (Larson, 2015). For example, a brackish species of phytoplankton has been found in the Zhubi Atoll lagoon in the southern SCS as a result of human activities and eutrophication (Shen et al., 2010). How does zooplankton respond to environmental fluctuations in coral reef ecosystems under the influence of natural factors and anthropogenic activities? Until now, relatively few studies have investigated the community structure of zooplankton in the atolls of the central SCS.
In the present study, metazooplankton were sampled from the lagoon and seaward reef slope around Huangyan Atoll, a semi-closed atoll in the central basin of the SCS, to understand the variation of zooplankton community. We hypothesized that the community structure of zooplankton differs between the lagoon and surrounding ocean. This study aims to provide quantitative information on the distribution, composition, and abundance of zooplankton within the lagoon and surrounding waters. It intends to facilitate the assessment of environmental impacts for ecosystem-level management in the atolls of the SCS.
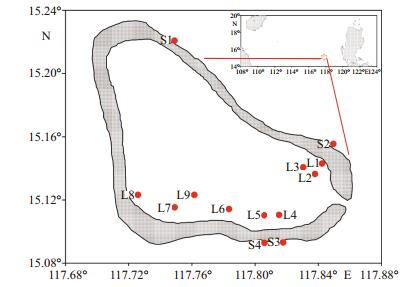
2 MATERIAL AND METHOD 2.1 Field samplingHuangyan Atoll (15°09′N; 117°45′E), also named Democracy Reef or Scarborough Shoal, is located in the eastern part of Zhongsha Islands, in the central SCS. It is almost an isosceles triangular lagoon approximately 15 km wide from the east to the west, and 15 km long from the south to north (Fig. 1). It covers an area, including the inner lagoon, of 150 km2 with a maximum depth of 19.5 m in the central lagoon zone. The width of the reef flat varies from 1 to 3 km around the lagoon. There is a passage (370 m wide and approximately 9–11 m deep) to the southeast between the lagoon and the ocean. To the east of the atoll is the 5 000–6 000 m deep Manila Trench (Huang, 1980). The climate is dominated by the East Asian monsoon system with a southwest monsoon prevailing from May to September, and a northeast monsoon from October to April of the subsequent year. Huangyan Atoll has a micro-tidal regime (approximately 1.2 m); many of the reefs of the atoll are just below water at high tide. Corals are scattered throughout the reef flat and are abundant in the seaward reef slope. The lagoon and the surrounding waters are rich in fisheries and other marine life, which have been exploited by fishing vessels from both China and the Philippines for decades (Shen, 1982). Chen and Yin (1982) previously qualitatively reported species diversity in the Huangyan Atoll.
|
|
Figure 1 Location of Huangyan Atoll and sampling stations in the lagoon (L1–L9) and the seaward slope waters (S1–S4)
Map was modified from |
Between May 25 to May 29 2015, zooplankton samples were collected at nine sites in the semienclosed atoll lagoon and at four leeward sites in the surrounding seaward reef-slope (Fig. 1) with a planktonic net (45 cm diameter, 160 μm mesh opening) towed vertically 1 m above the bottom to the surface. The net mouth was equipped with a flowmeter (Hydro-Bios 438115, Germany) to determine the volume of filtered water in each tow (unit: m3). The trawl winch speed was approximately 0.5–1 m/s. The samples were preserved in 5% formalin-seawater solution for analysis at a later date. All sampling series were completed during the periods of 07:00– 11:00 am.
Temperature and salinity were measured in situ using an YSI 6600 multi-probe sensor (Yellow Springs Instrument Co., OH, USA). Sub-samples for chlorophyll a (Chl a) and dissolved nutrients were also sampled at each station. Chl α concentration was determined fluorometrically after grinding in 90% acetone (Parsons et al., 1984). Water samples for analysis of dissolved nutrients were filtered through 0.7 μm Whatman GF/F filters, and filtrates were immediately frozen at -20℃ in the dark before further processing in the laboratory. Detailed information on the measurement of these samples has been previously documented by Ke et al. (2016).
2.2 Laboratory proceduresIn the laboratory, collections at each station were counted and analyzed for the composition and abundance of zooplankton under a stereoscopic microscope. All specimens were identified to species level, wherever possible, based on the status of taxonomic information currently available (Thompson, 1948; Chen and Zhang, 1965; Chen and Zhang, 1974; Chen and Shen, 1974; Zheng et al., 1984; Gao et al., 2002).
2.3 Data analysisSpecies richness (S) was calculated as the number of taxa observed in a given sample. The similarity (%) in species composition between the lagoon and reef slope was the percentage of the common species to the total species occurring in the lagoon and the reef slope. The Shannon-Wiener diversity index (H') was used to calculate the species diversity (Shannon and Weaver, 1963), and the Pielou evenness index (J) was employed to measure the relative abundances of species in the community (Ōmori and Ikeda, 1984).
A principal components analysis (PCA) was employed to analyze a matrix of the environmental variables including temperature, salinity, Chl a, and dissolved nutrients (SiO32-, NO3-, NO2-, NH4+, dissolved inorganic nitrogen, and PO43-) to identify the differences and groupings in environmental parameters between the sites. A hierarchical cluster analysis was performed on species followed by their abundance ranking from high to low, and their contribution to total abundance above 90%, with an average linkage and Bray-Curtis similarity index. These clusters were superimposed onto two-dimensional (2D) and three-dimensional (3D) plots of the multi-dimensional scaled (nMDS) datasets to explore community structures among sampling stations. A one-way analysis of similarities (ANOSIM) was performed to detect the effect of sampling sites on the composition and abundance distribution of zooplankton. Following the cluster analysis, species with the greatest contribution to the division of samples in a cluster were determined using the similarity percentage program. The similarity percentage (SIMPER) routine was selected to show the percentage contribution of each taxon to the average dissimilarities between the lagoon and reef slope regions. All multivariate analyses were performed on log (x+1) transformed data using the software Primer (v. 6.0, Primer-E, Plymouth, UK) (Clarke and Gorley, 2006).
Differences in environmental parameters and abundances of zooplankton groups between the lagoon and seaward reef slope were determined using the Student's t-test. A difference of P < 0.05 was considered significant. Pearson's correlation analysis was used to determine which environmental parameters may have influenced the distribution of zooplankton abundance (after log-transformation).
|
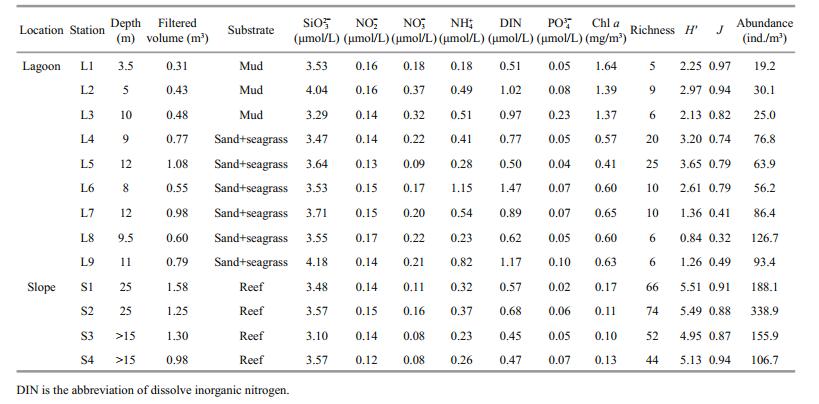
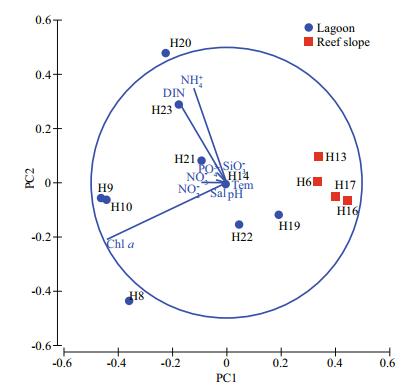
Sea surface temperature and salinity fluctuated between 29.21℃ and 30.77℃, and 33.29 and 33.80 in the study area, respectively. No significant differences were observed in both temperature and salinity in the lagoon and seaward reef slope (Fig. 2). Total Chl a concentration varied widely among stations, ranging from 0.10 mg/m3 to 1.64 mg/m3 (Table 1). Chl a concentration was significantly higher in the lagoon than that in the surrounding waters (P < 0.01), with an average (mean±standard deviation) of 0.87±0.45 mg/m3 and 0.13±0.03 mg/m3, respectively. Chl a concentration exceeded 1.30 mg/m3 at stations H8, H9, and H10. Generally, the concentrations of dissolved nutrients were greater in the lagoon than the reef slope with the exception of silicate (SiO32-) and nitrite (NO2-) (Table 1, Fig. 2). In particular, the NO3- concentration was significantly higher in the lagoon than the outer reef slope (P < 0.05). The PCA showed that the first two components combined accounted for 95.7% of the total variance with eigenvalues of 0.105 and 0.048. All sampling stations showed a clear trend with high Chl a concentration and dissolved nutrients in the lagoon stations, and low Chl a concentrations and dissolved nutrients in the seaward reef slope stations (Fig. 3).

|
| Figure 2 Distribution of temperature, salinity, chlorophyll a, pH and dissolved nutrients (SiO32-, NO3-, NO2-, NH 4+, DIN and PO43-) and their difference in the lagoon and seaward reef slope of the Huangyan Atoll n.s.: not significant, *: P value at the level of 0.05 and **: at the level of 0.01. |
|
| Figure 3 Results from the PCA analysis, showing the principal components 1 and 2 for environmental variables vectors and stations in the lagoon and reef slope of the Huangyan Atoll |
A total of 131 zooplankton taxa were collected during the study. Copepods were the most common with 67 species, followed by planktonic larvae, siphonophores, and chaetognaths (Table 2). Species richness was with 10 species per station in the lagoonal waters than the seaward oceanic waters, which ranged from the lowest value of 44 at station S4 to the highest value of 74 at stations S2, with a mean of 59 species across the whole reef-slope waters (Table 1). Only 31 common species occurred both in the lagoon and reef slope waters, suggesting that there was a difference in the species composition in the two regions to some degree.
|
Shannon-Wiener species diversity ranged from 4.95 to 5.51 with a mean of 5.27±0.27 in the reef slope waters, which was higher than that in the lagoon that had a mean of 2.25±0.95 (P < 0.05) (Table 1). Correspondingly, a high evenness index was also observed in the seaward oceanic waters with an average of 0.90±0.03, and it varied from 0.32 to 0.97 at the lagoonal stations with a mean of 0.70±0.23 (P < 0.05, Table 1).
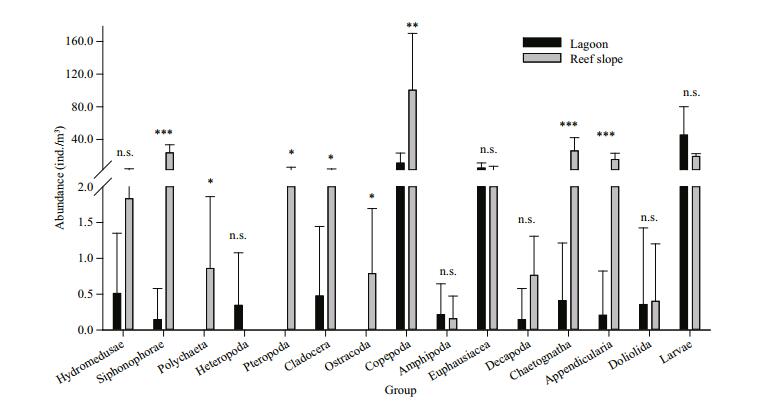
3.2.2 Abundance distributionZooplankton abundance fluctuated between 19.2 ind./m3 and 338.9 ind./m3, and was unevenly distributed in the study area (Table 1, Fig. 4). Average zooplankton abundance was significantly lower in the lagoonal waters than in the seaward oceanic waters (t=-3.664, P < 0.01), with 64.2±35.7 ind./m3 in the former and 197.4±100.0 ind./m3 in the latter. The lowest abundances were recorded at stations L1, L2 and L3 in the lagoon. The abundance of planktonic larvae was higher in the lagoon than the reef slope, although they were abundant in both sampled regions (Fig. 5). Copepods, euphausids, and planktonic larvae accounted for 95.63% of zooplankton abundance in the lagoon. In addition, 93.66% of zooplankton in the reef slope was represented by copepods, chaetognaths, siphonophores, appendicularians, and planktonic larvae. The abundance of siphonophores, copepods, chaetognaths, and appendicularians differed significantly between the lagoon and reef-slope samples (Fig. 5), with the highest abundance in oceanic waters. Moreover, a significant difference was also observed in the abundance of polychaetes, pteropods, cladocerans, and ostracods between the two regions (Fig. 5). Zooplankton abundance was negatively correlated with Chl a concentration (R=-0.708, P < 0.01). No significant correlation was found between zooplankton abundance and other environmental parameters (temperature, salinity and nutrient parameters).

|
| Figure 4 Distribution of zooplankton abundance (ind./m3) in the Huangyan Atoll |
|
| Figure 5 Variation of abundance of zooplankton groups and their difference in the lagoon and seaward reef slope of the Huangyan Atoll n.s.: not significant; *: P value at the level of 0.05; **: at the level of 0.01; ***: at the level of 0.001. |
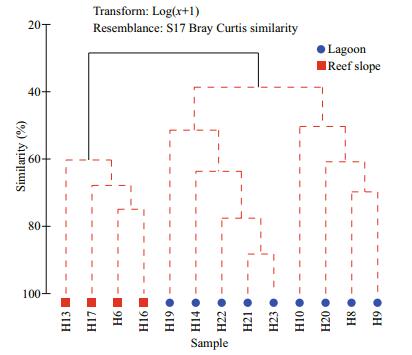
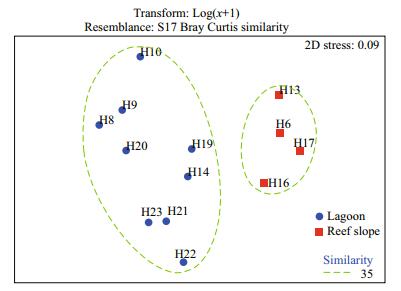
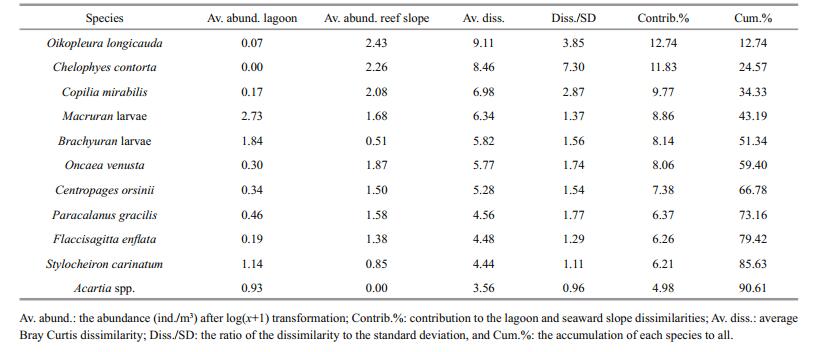
Multivariate analysis of zooplankton abundance revealed that all sampling stations could be clustered into two groups characterized by the lagoon group and the reef-slope group at a similarity level of ~40% (Fig. 6). A 2D ordination of the MDS space confirmed this pattern with a 2D stress value of 0.09 (Fig. 7). The 3D stress value of 0.05 showed little improvement on the 2D value. Results of the ANOSIM analysis on species abundance also revealed a significant difference between the zooplankton assemblages of the two sampling areas (R=0.708, P=0.001). A difference in species composition between the lagoon and reef slope groups was determined at approximately a 90% cutoff level for cumulative dissimilarity (Table 3). The lagoon group had the lowest species diversity due to the dominance of Macruran larvae, Brachyuran larvae, Stylocheiron carinatum, and Acartia spp. Conversely, the oceanic group had the greatest diversity, dominantly characterized by Oikopleura longicauda, Chelophyes contorta, Copilia mirabilis, Oncaea venusta, Macruran larvae, Paracalanus gracilis, Centropages orsinii, and Flaccisagitta enflata.
|
| Figure 6 Cluster analysis using the hierarchical agglomerative method employing group average linking of Bray- Curtis similarities All species abundances were performed on log (x+1) transformed. Black lines connect stations that are statistically distinct groups (P < 0.05). Red lines connect samples that are not statistically unique (P > 0.05). |
|
| Figure 7 Non-multidimensional scaling ordination (nMDS) showing the relative position of stations based on the Bray-Curtis similarity index using log(x+1) transformed species abundance data Contours represent the 35% similarity level among stations. |
|
The predominant feature of zooplankton distribution in the Huangyan Atoll was the higher diversity and abundance of zooplankton on the seaward reef-slope compared with the lagoon. This finding is in accordance with previous investigations of Indo-Pacific atolls that also showed a pronounced difference in the zooplankton community between lagoons and the surrounding sea (Madhupratap et al., 1977; Goswami and Goswami, 1990; Carleton and Doherty, 1998). The abundance and diversity of zooplankton at Huangyan Atoll was greater than that of Taiaro lagoon, French Polunesia (Carleton and Doherty, 1998), but considerably less than in the Indian Ocean (Madhupratap et al., 1977; Goswami and Goswami, 1990), western Pacific (Renon, 1977), and southern SCS (Yin et al., 2011; Du et al., 2015). The Huangyan lagoon community comprised primarily of copepods and meroplankton, and had a low diversity index due to low species richness and dominance by Macruran larvae, Brachyuran larvae, Stylocheiron carinatum and Acartia spp. Oceanic forms such as siphonophores, chaetognaths, and appendicularians, which are usually found in atoll lagoons, were entirely absent (Renon, 1977; Goswami and Goswami, 1990). This may be consistent with the almost complete isolation of Huangyan lagoon from the surrounding ocean. The lack of numerous deep passes between the ocean and lagoon in Huangyan Atoll may explain this indicated restriction in species exchange.
Merozooplankton such as Macruran larvae and Brachyuran larvae constituted an important component of zooplankton in the Huangyan Atoll lagoon, consistent with other atolls (Madhupratap et al., 1977; Goswami and Goswami, 1990; Heidelberg et al., 2004). They contributed 70.63% of the total zooplankton in the lagoon, but only accounted for 9.76% in the seaward reef-slope. These periodic planktonic larvae are possibly transported from oceanic waters outside the atoll into the lagoon or are associated with the reef substrate itself (Alldredge and King, 1977; Heidelberg et al., 2004, 2010; Yin et al., 2011). The zooplankton community in the lagoon was dominated by planktonic larvae similar to most atolls in the Pacific or Indian Oceans (Madhupratap et al., 1977; Goswami and Goswami, 1990; Carleton and Doherty, 1998; Yin et al., 2011; Pagano et al., 2012). The predominance of merozooplankton in atoll lagoons has been attributed to the diverse habitats of coral reef ecosystems, where they are capable of maintaining their position on the reef through active swimming, and by utilizing crevices, caves, and coral heads for protection from predators and currents (Emery, 1968; Alldredge and King, 1977, 2009). The substrate at Huangyan Atoll was characterized by sand, mud, reef and seagrass (Table 1), which resulted in the spatial heterogeneity inside the lagoon. Whether the differences in zooplankton species composition between the lagoon and reef slope are likely relatede to the relatively high levels of spatial heterogeneity at Huangyan Atoll needs to study further.
There was a higher diversity and abundance of zooplankton in the seaward oceanic waters in comparison with lagoonal waters, which can mainly be attributed to the abundance of siphonophores, copepods, chaetognaths, and larvaceans in oceanic waters around Huangyan Atoll. Less pronounced differences also existed in the abundance of polychaetes, pteropods, cladocerans, and ostracods between the lagoon and reef-slope despite low values (Fig. 5). Siphonophores and chaetognaths were generally present in the samples from the oceanic stations but were absent in those from the lagoon (Goswami and Goswami, 1990; Carleton and Doherty, 1998; Heidelberg et al., 2004). At Huangyan Atoll, the abundance of siphonophores, chaetognaths, and appendicularians in the surrounding oceanic waters was approximately 160, 60, and 70 times higher than in the lagoon samples, respectively. It also appears that the occurrence and abundance of different taxa depended on the collection time. For example, harpacticoid copepods and planktonic larvae were more abundant during the night while calanoid copepods, siphonophores, euphausids, and appendicularians were more common in the daytime samples (Yin et al., 2011). They found that zooplankton abundance was 46.2 times in the nighttime than that of the daytime. Nocturnal zooplankton abundance is more pronounced in Indo-Pacific Ocean lagoons due to the clarity of water and high light fluctuations (Alldredge and King, 1977; Goswami and Goswami, 1990; Heidelberg et al., 2004). In the present study, zooplankton samples were always collected during the daytime, which may be one of the reasons to explain the relatively low abundance of merozooplankton and high holoplankton.
Zooplankton distributions are also influenced by wind and water circulation in atoll lagoons (Carleton and Doherty, 1998; Alldredge and King, 2009; Pagano et al., 2012). The period of our sampling was just under the control of the southwestern monsoon. Phytoplankton might have piled up into the lagoon near the lagoon mouth due to the transporting action of the southwestern wind, and thus improve the phytoplankton biomass (Ke et al., 2016). Moreover, there is no information on water circulation available for Huangyan Atoll. Thus, the response of the zooplankton community to these seasonal and spatial variations requires further study.
4.2 Influence of anthropogenic activities on zooplankton communityThere were significant differences in Chl a and nitrate concentrations between the lagoon and oceanic reef-slope. Moreover, zooplankton diversity and abundance significantly differed between the lagoon and oceanic reef-slope. Their distributions were not homogenous in the lagoon. This pattern may have been influenced, not only by the natural characteristics and species-specific behavior, but potentially also by the anthropogenic activities on Huangyan Atoll.
Huangyan Atoll is a productive fishing ground and its lagoon acts as a suitable harbor for fishing vessels from neighboring countries (Shen, 1982). Vessels enter into the lagoon through the southeastern passage of the atoll and usually stay moored nearby. Therefore, waste waters from these moored vessels maybe have contributed to elevated nutrient levels, enhancing phytoplankton biomass. Analysis of variance indicated that NO3- and Chl a concentrations were considerably higher in the lagoon than in the surrounding oceanic waters. Furthermore, micro- phytoplankton seems to be more sensitive to nutrient input than pico-phytoplankton, and they have been shown to be significantly higher in lagoons than in outer reef slope waters (Ke et al., 2016). Metazooplankton appear to be controlled mainly by food resources, as shown in Ahe Atoll lagoon (Pagano et al., 2012). However, a significantly negative correlation between Chl a and zooplankton abundance was observed at Huangyan Atoll in the present study. These results suggest that a bottom-up control of zooplankton does not occur in this atoll lagoon. Coral reefs are often characterized by oligotrophic environments due to a low input of new nutrients and active nutrient recycling, and this also applies for adjacent oceanic waters (Hatcher, 1997). Bottom-up control is relatively common in oligotrophic atoll lagoons, where phytoplankton biomass availability is a limiting factor for the production of upper trophic levels (Calbet et al., 1996). At Huangyan Atoll lagoon, the majority of phytoplankton comprised micro- phytoplankton (Ke et al., 2016), which may be not directly consumed by most zooplankton taxa, especially for planktonic larvae. The uncoupled primary and secondary productions in this atoll lagoon, and the influence on the energy flow and health of coral reef ecosystems warrant further investigation.
5 CONCLUSIONThere were significant differences in Chl a and NO3- concentrations, and zooplankton species composition and abundance between the lagoon and seaward reef-slope of Huangyan Atoll. Meroplankton, mainly comprising Macrura and Brachyura larvae, were dominant in the atoll lagoon. Holoplankton, dominated by copepods, siphonophores, chaetognaths, and appendicularians, represented the zooplankton community at the oceanic reef-slope. These results indicate clear differences between the atoll lagoon and reef slope which are reflective of natural and anthropogenic influences on the structure and distribution of zooplankton communities at Huangyan Atoll.
6 DATA AVAILABILITY STATEMENTAll data generated or analyzed during this study are included in this published article. The datasets during the current study are available from the corresponding author on reasonable request.
7 ACKNOWLEDGMENTThanks are given to Dr. SHI Qi, Dr. TAO Shichen, and LIU Huajian for their assistance in the field investigation.
Alldredge A L, King J M. 1977. Distribution, abundance, and substrate preferences of demersal reef zooplankton at Lizard Island Lagoon, Great Barrier Reef. Marine Biology, 41(4): 317-333.
DOI:10.1007/BF00389098 |
Alldredge A L, King J M. 2009. Near-surface enrichment of zooplankton over a shallow back reef:implications for coral reef food webs. Coral Reefs, 28(4): 895-908.
DOI:10.1007/s00338-009-0534-4 |
Calbet A, Alcaraz M, Saiz E, Estrada M, Trepat I. 1996. Planktonic herbivorous food webs in the catalan Sea (NW Mediterranean):temporal variability and comparison of indices of phyto-zooplankton coupling based on state variables and rate processes. Journal of Plankton Research, 18(12): 2 329-2 347.
DOI:10.1093/plankt/18.12.2329 |
Carleton J H, Doherty P J. 1998. Tropical zooplankton in the highly-enclosed lagoon of Taiaro Atoll (Tuamotu Archipelago, French Polynesia). Coral Reefs, 17(1): 29-35.
DOI:10.1007/s003380050090 |
Chen Q C, Shen J R. 1974. The pelagic copepods of the South China Sea. Ⅱ. Studia Marina Sinica, 9: 125-137.
(in Chinese with English abstract) |
Chen Q C, Yin J Q. 1982. The zooplankton of Huangyan Atoll.In: South China Sea Institute of Oceanology, Chinese Academy of Sciences ed. Symposium on Research Reports on the Sea Area of South China Sea. Science Press, Beijing. p.273-277. (in Chinese)
|
Chen Q C, Zhang S Z. 1974. The pelagic copepods of the South China Sea. I. Studia Marina Sinica, 9: 101-116.
(in Chinese with English abstract) |
Cheng Q C, Zhang S Z. 1965. The planktonic copepods of the Yellow Sea and the East China Sea. I. Calanoida. Studia Marina Sinica, 7: 20-131.
(in Chinese with English abstract) |
Clarke K R, Gorley R N. 2006. PRIMER v6:User Manual/Tutorial. Primer-E, Plymouth.
|
Coma R, Ribes M, Orejas C, Gili J M. 1999. Prey capture by a benthic coral reef hydrozoan. Coral Reefs, 18(2): 141-145.
DOI:10.1007/s003380050168 |
Du F Y, Wang X H, Lin Z J. 2015. The characteristics of summer zooplankton community in the Meiji coral reef, Nansha Islands, South China Sea. Acta Ecologica Sinica, 35(4): 1 014-1 021.
(in Chinese with English abstract) |
Emery A R. 1968. Preliminary observations on coral reef plankton. Limnology and Oceanography, 13(2): 293-303.
DOI:10.4319/lo.1968.13.2.0293 |
Gao S W, Hong H Q, Zhang S M. 2002. Phylum Cnidaria. Science Press, Beijing. p.1-275.
(in Chinese)
|
Gerber R P. 1981. Species composition and abundance of lagoon zooplankton at Eniwetak Atoll, Marshall Islands. Atoll Research Bulletin, 247: 1-22.
DOI:10.5479/si.00775630.247.1 |
Gilmartin M. 1958. Some observations on the lagoon plankton of Eniwetok Atoll. Pacific Science, 12(4): 313-316.
|
Goswami S C, Goswami U. 1990. Diel variation in zooplankton in Minicoy lagoon and Kavarati atoll (Lakshadweep islands). Indian Journal of Marine Sciences, 19: 120-124.
|
Goswami S C. 1983. Production and zooplankton community structure in the lagoon and surrounding sea at Kavaratti Atoll (Lakshadweep). Indian Journal of Marine Sciences, 12: 31-35.
|
Hatcher B G. 1997. Coral reef ecosystems:how much greater is the whole than the sum of the parts?. Coral Reefs, 16(S1): S77-S91.
|
Heidelberg K B, O'Neil K L, Bythell J G, Sebens K P. 2010. Vertical distribution and diel patterns of zooplankton abundance and biomass at Conch Reef, Florida Keys(USA). Journal of Plankton Research, 32(1): 75-91.
DOI:10.1093/plankt/fbp101 |
Heidelberg K B, Sebens K P, Purcell J E. 2004. Composition and sources of near reef zooplankton on a Jamaican forereef along with implications for coral feeding. Coral Reefs, 23(2): 263-276.
|
Huang J S. 1980. Some geological features of the Huangyan Dao (Huangyan Island), South China Sea. Acta Oceanologica Sinica, 2(2): 112-123.
(in Chinese with English abstract) |
Ke Z X, Liu H J, Wang J X, Liu J X, Tan Y H. 2016. Abnormally high phytoplankton biomass near the lagoon mouth in the Huangyan Atoll, South China Sea. Marine Pollution Bulletin, 112(1-2): 123-133.
DOI:10.1016/j.marpolbul.2016.08.028 |
Larson C. 2015. China's island building is destroying reefs. Science, 349(6255): 1 434.
DOI:10.1126/science.349.6255.1434 |
Le Borgne R, Blanchot J, Charpy L. 1989. Zooplankton of Tikehau atoll (Tuamotu archipelago) and its relationship to particulate matter. Marine Biology, 102(3): 341-353.
DOI:10.1007/BF00428486 |
Madhupratap M, Wafar M V M, Haridas P, Narayanan B, Menon P G, Sivadas P. 1977. Comparative studies on the abundance of zooplankton in the surrounding sea and lagoons in the Lakshadweep. Indian Journal of Marine Sciences, 6: 138-141.
|
Ōmori M, Ikeda T. 1984. Methods in Marine Zooplankton Ecology. Wiley, New York. 332p.
|
Pagano M, Sagarra P B, Champalbert G, Bouvy M, Dupuy C, Thomas Y, Charpy L. 2012. Metazooplankton communities in the Ahe atoll lagoon (Tuamotu Archipelago, French Polynesia):spatiotemporal variations and trophic relationships. Marine Pollution Bulletin, 65(10-12): 538-548.
DOI:10.1016/j.marpolbul.2012.01.025 |
Parsons T R, Maita Y, Lalli C M. 1984. A Manual of Chemical and Biological Methods for Sea Water Analysis. Pergamon Press, Oxford.
|
Renon J P. 1977. Zooplancton du lagon de l'atoll de Takapoto(Polynésie Française). Annls Instition Océanography, 53: 217-236.
|
Shannon C E, Weaver W. 1963. The Mathematical Theory of Communication. University of Illinois Press, Urbana. p.144.
|
Shen P P, Tan Y H, Huang L M, Zhang J L, Yin J Q. 2010. Occurrence of brackish water phytoplankton species at a closed coral reef in Nansha Islands, South China Sea. Marine Pollution Bulletin, 60(10): 1 718-1 725.
DOI:10.1016/j.marpolbul.2010.06.028 |
Shen S P. 1982. The ecology of coral reefs on Huangyan Island. In: South China Sea Institute of Oceanology, Chinese Academy of Sciences ed. Symposium on Research Report on the Sea Area of South China Sea.Science Press, Beijing. p.301-308. (in Chinese)
|
Thompson H. 1948. Pelagic Tunicates of Australia. Common wealth Council for Scientific and Industrial Research, Melbourne, Australia.
|
Webber M, Edwards-Myers E, Campbell C, Webber D. 2005. Phytoplankton and zooplankton as indicators of water quality in Discovery Bay, Jamaica. Hydrobiologia, 545(1): 177-193.
DOI:10.1007/s10750-005-2676-x |
Yin J Q, Chen Q C, Tan Y H, Zhang J L. 2003. Zooplanktonic community characteristics in waters of Zhubi coral reef(Nansha Islands) in spring. Journal of Tropical Oceanography, 22(6): 1-8.
(in Chinese with English abstract) |
Yin J Q, Huang L M, Li K Z, Xiong L L. 2011. Species diversity and community structure of zooplankton in the Zhubi Atoll, Nansha Islands, South China Sea. Biodiversity Science, 19(6): 685-695.
(in Chinese with English abstract) |
Yu K F. 2012. Coral reefs in the South China Sea:their response to and records on past environmental changes. Science China Earth Sciences, 55(8): 1 217-1 229.
DOI:10.1007/s11430-012-4449-5 |
Zhao M X, Yu K F, Shi Q, Chen T R, Zhang H L, Chen T G. 2013. Coral communities of the remote atoll reefs in the Nansha Islands, southern South China Sea. Environmental Monitoring and Assessment, 185(9): 7 381-7 392.
DOI:10.1007/s10661-013-3107-5 |
Zhao M X, Yu K F, Zhang Q M, Shi Q, Price G J. 2012. Longterm decline of a fringing coral reef in the northern South China Sea. Journal of Coastal Research, 28(5): 1 088-1 099.
|
Zheng Z, Li S J, Xu Z Z. 1984. Marine Planktology. China Ocean Press, Beijing.
|
 2018, Vol. 36
2018, Vol. 36


