Institute of Oceanology, Chinese Academy of Sciences
Article Information
- HUO Zhongming(霍忠明), MENG Xiangyu(孟祥宇), Md. Golam RBBANI, CAO Weinan(曹纬楠), WU Qidi(吴启迪), LI Ying(李莹), WANG Jingtian(王靖天), YUAN Hongmei(袁洪梅), YANG Feng(杨凤), YAN Xiwu(闫喜武)
- Seawater acidification affects the immune enzyme activities of the Manila clam Ruditapes philippinarum
- Chinese Journal of Oceanology and Limnology, 36(5): 1688-1696
- http://dx.doi.org/10.1007/s00343-019-7196-z
Article History
- Received Jul. 1, 2017
- accepted in principle Sep. 6, 2017
- accepted for publication Nov. 13, 2018
Seawater acidification refers to the reduced pH in oceans that results from increased anthropogenic carbon dioxide (CO2) emissions (Caldeira and Wickett, 2003; Feely et al., 2004; Sabine et al., 2004). Many experimental results have confirmed that ocean acidification leads to changes in marine carbonate systems, which have direct effects on physiological and immune functions of marine bivalves such as respiration, metabolism, reproduction, biological mineralization, energy budget, and hemocyte parameters (Ding et al., 2012; Wang et al., 2015; Sui et al., 2016a, b; Hu et al., 2017). For example, sperm motility of the clam Tegillarca granosa was decreased and sperm half-life was shortened under low pH conditions (Zhao et al., 2012). In addition, the fertilization rate (sperm:egg ratio 10:1) of T. granosa after acidification was significantly lower than that of the control group. Caldeira and Wickett (2003) reported devastating damage to marine species, especially the formation of shells, caused by ocean acidification. Talmage (2011) showed that the calcification ability of bivalve larvae was weakened at high concentrations of CO2. Wang et al. (2015) indicated that physiological energetics of juvenile Mytilus coruscus are able to acclimate to CO2 acidification with little physiological effect. To date, however, the effect of seawater acidification on the immune functions of shellfish has not been studied in detail. A few studies confirmed that the immune system function of shellfish can be damaged when the seawater pH is lower than the normal value (Michaelidis et al., 2005; Fan et al., 2006). The increasing degree of acidification of sea water also likely affects the immune function of shellfish (Huang et al., 2016; Wu et al., 2016; Sui et al., 2017).
The Manila clam is an ecologically and commercially important bivalve found along the west coast of North America, Europe, and throughout Asia. It has become one of the most important aquaculture species because of its fast growth, low mobility, strong adaptability, and short growth cycle. In coastal areas of southern and northern China, R. philippinarum aquaculture has produced good economic and social benefits. Studies of the Manila clam have documented sensitivity to several stressors, including temperature, salinity, pathogens, and food availability (Paillard, 2004; Inoue et al., 2007; Yan et al., 2009), but few studies have investigated the impact of ocean acidification (Xu et al., 2016). The present study investigated the effects of seawater acidification on immune enzyme activities of the Manila clam. The results provide valuable information about the immune response of R. philippinarum to reduced water pH and insights for future research investigating exposure of bivalves to elevated CO2 conditions.
2 MATERIAL AND METHOD 2.1 MaterialEight hundred and ten R. philippinarum specimens (33.6±1.8 mm shell length) were collected from the intertidal shores of Liangshui Bay (39°04′14.41″N, 122°01′47.70″E), Liaodong Peninsula, Northeast China, in May 2015. Clams were transported to an aquarium facility where they were allowed to acclimate to laboratory conditions for 2 weeks prior to experimentation. Clams were held in recirculating seawater that mimicked natural conditions. Temperature, salinity, pH, and light: dark cycles were maintained at constants of 9.0±0.31℃, 32.12±0.08, 8.1±0.05, and 12 h:12 h, respectively.
2.2 Experimental setupTo control the seawater pH, nine recirculating systems were established. Each system contained 100 L of seawater and had three chambers (filter, temperature controlled, and gas mixing). CO2 gas was used in the gas chamber of these systems to control pH. Gas flow was adjusted using a mass flow controller. After 36 h, the systems reached a stable state. The experiment included three systems each for pH 7.4, pH 7.7, and pH 8.0; the latter, which served as the control, was bubbled with ambient air only (Xu et al., 2016). Ninety clams were kept in a sand substrate in each of the nine tanks for 3 months under the three pH conditions. Clams were fed twice daily with an equal mixture of the microalgae Chaetoceros muelleri, Nitzschia closterium, Chlorella vulgaris, and Isochrysis galbana to fulfill their nutritional requirements.Aconstant food supply (20 000 cells/mL) was maintained over the duration of the experiment. Over the following 90 days, clams were subjected to increasing temperature regimes. In all temperature was simultaneously increased ca. 0.3℃ per day from 9 to 25℃. clams were maintained at 25℃ for 40 days.
2.3 MeasurementAt the end of the experiment, three individuals from each of three replicate tanks of each experimental group were selected to collect hemocyte and gill tissue for immune activity analyses. Mean values were used in data analysis to avoid pseudoreplication.
Throughout the duration of the experiment, constant low pH conditions were maintained. Dissolved oxygen content (DO), Temperature, salinity, and pH were recorded every day. TA was measured using an alkalinity titrator. Partial pressure of CO2 in seawater (pCO2), dissolved inorganic carbon (DIC), and CaCO3 saturation state for aragonite (Ωara) and calcite (Ωcal) were calculated on the basis of the pH, temperature, TA, and salinity of the seawater, following the method of Wang et al. (2015).
2.3.1 Measurement of cell membrane stability of clamsTo measure cell membrane stability, a 50-μL aliquot of hemocytes was collected in an enzyme- linked immunosorbent assay (ELISA) plate and incubated at 4℃ for 45 min. The sample was washed twice with normal saline that did not adsorb hemocyte cells (100 μL). Next, 200 μL of 0.004% neutral red solution were added, and the mixture was incubated at 24℃ for 3 h. The excess dye was washed off with normal saline, and the sample was added to the enzyme-labeled plate to mix with 200 μL of alcohol with pH adjusted corresponding to the experimental groups. The ELISA plate then was placed into the enzyme standard micropipette reader (FluorMax 2000, Shanpu Biological Technology Co. Ltd., Shanghai, China) to read the value at optical density at 550 (OD550).
2.3.2 Phagocytic activity of hemocyte cellsTo measure phagocytic activity, 50 μL samples of Manila clam hemocytes were placed into an ELISA plate and incubated at 4℃ for 1 h. Non-adherent hemocyte cells were washed away with two 100 μL washes of physiological saline. After adding 50 μL (50×107/mL) of neutral red dyed yeast suspension (Xiuming Biotechnology Co. Ltd., Shanghai, China) at 20℃ for 30 min, 100 μL of 7-Benzoyloxy-4- trifluoromethyl coumarin were added to terminate the reaction. Excess yeast particles were washed away with normal saline, and the sample was mixed with 100 μL of alcohol with pH adjusted corresponding to the experimental groups. The plate was placed in the microplate reader (Molecular Devices, Sunnyvale, California, USA) and readings were taken at OD550. Phagocytic activity was expressed as the number of yeast particles per mg of protein.
2.3.3 Total protein content in the hemocytes and gill tissueThe total protein content of the gill tissue and hemocytes was determined using the Coomassie brilliant blue method. Gill tissue was weighed (g) and mixed with physiological saline (mL) at a volume ratio of 1:9, and the sample was ground using a mortar and pestle. The sample was placed in a centrifuge tube and centrifuged for 10 min at 3 000 r/min. The supernatant was used as the experimental sample. Absorbance value was measured at room temperature (18℃) at the wavelength of 595 nm (1 cm light transmittance path). Total protein content was calculated as follows:

where Cs is the concentration of the standard, which was 0.563 g/L.
2.3.4 Determination of lysozyme (LSZ) contentThe LSZ content of the gill tissue was determined using a LSZ determination kit following the instructions provided by the manufacturer (Jian Cheng Bio-engineering Institute, Nanjing, China). The contents of each tube were mixed well and kept at 37℃ in a water bath for 15 min, then immediately transferred to an ice water bath (< 0℃) for 3 min. The absorbance value of each tube was measured at room temperature (18℃) at the wavelength of 530 nm (1 cm light transmittance path). LSZ content (μg/mL) was calculated as follows:

This analysis was conducted following the instructions provided in the ATPase determination kit (Jian Cheng Bio-engineering Institute). The sample was mixed well and kept at 45℃ in a water bath for 20 min after all steps were completed, followed by immediate cooling to room temperature. The absorbance value of each tube was measured at the wavelength of 660 nm (1 cm light transmittance path). ATPase enzyme activity was calculated using the following formula:

Samples were processed following the instructions provided in the CAT determination kit (Jian Cheng Bio-engineering Institute, Nanjing). The mixture in each tube was shaken and the absorbance value of each tube was measured at room temperature at the wavelength of 405 nm (0.5 cm light transmittance path). CAT activity was calculated using the following formula:

where ACAT is the activity of CAT in the tissue, Ctest is the protein concentration of the sample tested, and W is the amount of the sample (mg).
2.3.7 Statistical analysisThe objective of this study was to examine the immune enzyme activities of Manila clam reared at different pH values. All analyses were performed using the Statistical Package for R statistical software. The potential effects of pH on immune enzyme activities were analyzed using one-way analysis of variance. Significant effect was tested using Tukey's honest significant difference (HSD) test. Significance level for all analyses was set at P < 0.05. Measurements are reported as the mean (of three replicates)±95% confidence limit as implemented in R.
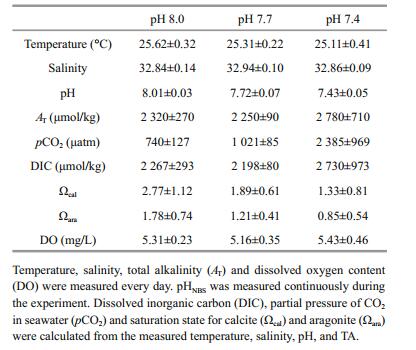
3 RESULT 3.1 Water chemistryCarbonate chemistry parameters of seawater within each treatment are summarized in Table 1. The desired temperature and pH levels were successfully reached within each treatment. Salinity was maintained at 32.88±0.5, and dissolved oxygen remained at approximately 5 mg/L.
|
Survival rates of Manila clams at different temperatures on day 90 are shown in Fig. 1. Results showed that the survival rate was not significantly different from pH 7.4 to 8.0.

|
| Figure 1 Survival rate of Manila clams under different pH conditions The error bars represent standard deviation, the same meaning as follows. |
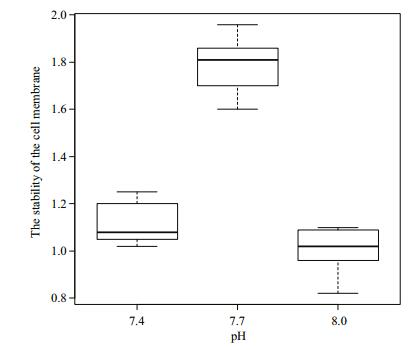
Figure 2 show the stability of the cell membrane of R. philippinarum under different pH conditions. As pH decreased from 8.0, the stability of the cell membrane increased to its peak value at pH 7.7 and then decreased at pH 7.4. Cell membrane stability did not differ significantly (P > 0.05) between pH 8 and 7.4, but the difference was significant between pH 7.7 and the other two pH values (P < 0.05).
|
| Figure 2 Stability of the cell membrane of Manila clams under different pH conditions The error bars represent standard deviation. |
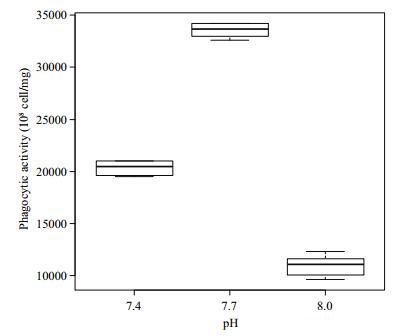
Differences in the phagocytic activity of Manila clam hemocyte cells under different pH conditions are shown in Fig. 3. Phagocytic activity first increased as pH decreased from 8.0 to 7.7, where maximum activity was reached, and then it decreased at pH 7.4. Significant differences in the phagocytosis of the hemocyte cells were detected among the three pH groups (P < 0.05).
|
| Figure 3 Phagocytic activity of Manila clams under different pH conditions |
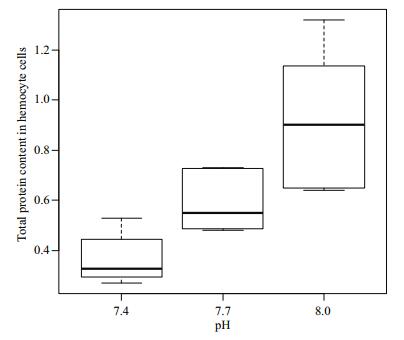
Figure 4 show the total protein content in the hemocyte cells of R. philippinarum under different pH conditions. The protein content in the hemocytes decreased with decreasing pH. There was no significant difference (P > 0.05) in total protein content between the pH 7.4 and 7.7 groups, but the content was significantly higher in the pH 8.0 treatment compared to the other two groups (P < 0.05).
|
| Figure 4 Change of total protein content in hemocyte cells under different pH conditions |
The total protein content in the gill tissue of Manila clams under different pH conditions is shown in Fig. 5. The content of total protein in gill tissue did not differ significantly (P > 0.05) when pH was 8 and 7.7.
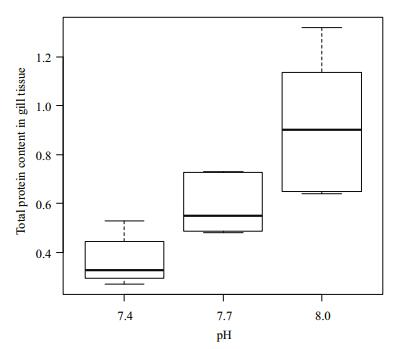
|
| Figure 5 Change of total protein content in gill tissue under different pH conditions |
The activity of LSZ in the hemocytes of R. philippinarum under different pH conditions is shown in Fig. 6. The LSZ activity in the hemocytes increased with the decrease of pH, with the maximum value reached at pH 7.4. The value differed significantly among the three treatment groups (P < 0.05).
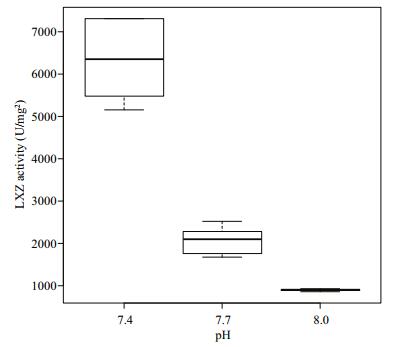
|
| Figure 6 Change of lysozyme activity in hemocytes under different pH conditions |
Figure 7 show the ATPase activity in the gill tissue of Manila clams in different pH environments. The ATPase activity at pH 7.4 was significantly higher (P < 0.05) than that in the other two groups, and no significant difference was observed between the pH 7.7 and 8.0 groups (P > 0.05).
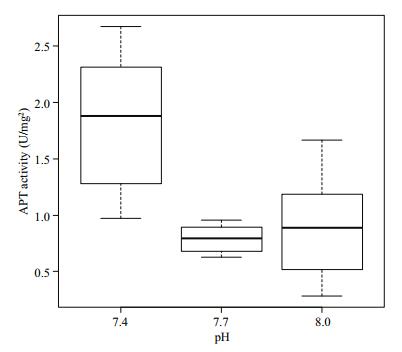
|
| Figure 7 Change of ATP activity in gill tissue under different pH conditions |
The CAT activity in the gill tissue of R. philippinarum under different pH conditions is shown in Fig. 8. CAT activity changed from pH 8.0 to 7.4. The highest value occurred at 7.7, and then it decreased at pH 7.4. CAT activity differed significantly (P > 0.05) among the three experimental groups.
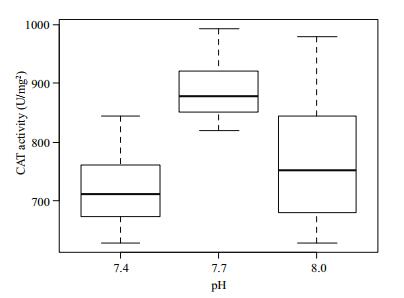
|
| Figure 8 Change of CAT activity in gill tissues under different pH conditions |
Sea water acidification has numerous effects on marine organisms, including plankton, fish, and bivalves (Matozzo et al., 2012; De Souza et al., 2014; Li et al., 2014; Meseck et al., 2016; Clements and Hunt, 2017; Taucher et al., 2017). Marine bivalves need to acclimate to their environment to avoid adverse effects on reproduction, the immune system, metabolism, growth, and survival (Sui et al., 2016a, b). In this respect, phagocytosis and the enzymes related to phagocytosis are important immune mechanisms. The results of the current study show that seawater acidification has an impact on the immune enzymes of R. philippinarum.
The results of the experiment revealed differences in cell membrane stability and phagocytosis of hemocytes in Manila clams reared under different pH conditions. Both factors first increased and then decreased with the decrease of pH, with maximum values occurring at pH 7.7, and both were higher in the pH 7.4 and 7.7 groups than in the pH 8.0 (control group) treatment. Seawater acidification affects cell membrane stability through intracellular ions and the acid-base status. Molluscs depend on an ion exchange mechanism to exchange H+ or HCO3- across cell membranes using protein carriers to minimize the effect of ocean acidification (Pörtner, 1996, 2004; Marchant et al., 2010; Parker et al., 2013). Feng (1988) showed that when foreign materials invade a living organism, their identification and adsorption are determined by the stability of the cell membrane and phagocytosis by hemocytes. Similar results were reported for the phagocytic activity of Mytilus edulis (Bibby et al., 2008; Sun et al., 2017). The results of the current study indicate that a moderate reduction in the pH of sea water will have a certain stimulating effect on the stability of the cell membrane and phagocytosis of R. philippinarum.
LSZ content is one of the most important physiological indexes that can be used to assess the non-specific immune function of animals. It is an important component in the extracellular killing of microorganisms by bivalve hemocytes, as it breaks down the bacterial cell wall by dissolving cell wall peptides (Cheng and Rodrick, 1975; Winston et al., 1996; Liu et al., 2003). The role of LSZ originally was to help digest food, but as the environment changed and natural selection occurred, LSZ gradually developed as a blood-borne immune function in shellfish. Bibby et al. (2008) reported that a reduction of LSZ activity involves in the disruption of cellular pathways (Bibby et al., 2008). Matozzo et al. (2012) reported that decreased pH resulted in significantly lower LSZ activity in Chamelea gallina and Mytilus galloprovincialis. However, the results of the current study showed that LSZ content increased with decreased pH, with the maximum level at pH 7.4. This finding indicates that reduction of the pH of the sea water can increase the content of LSZ. Increased LSZ content helps the hemolymph to more actively defend against invaders.
The main role of ATPase is to catalyze the decomposition of ATP into ADP and phosphate ions to release energy. However, in aquatic organisms, Na+ K+-ATPase plays an important role in the regulation of osmotic homeostasis (Yadwad et al., 1990) by affecting active electrical transport across the gills (Parvez et al., 2006). Mg2+-ATPase plays an important role in ionic transport and oxidative phosphorylation and is responsible for the transepithelial regulation of Mg2+ ions (Parvez et al., 2006). Ca2+-ATPase also plays a significant role in removing excessive calcium ions from the cytoplasm to maintain low Ca2+ levels (Saxena et al., 2000). Thus, in some species changing pH had no effect on activity of Na+K+-ATPase (Seidelin et al., 2001), whereas in others increased Na+K+-ATPase activity was observed (Ishimatsu et al., 2005; Deigweiher et al., 2008). Increased ATPase activity with decreasing pH was observed in marine fishes such as cod (Melzner et al., 2009), whereas no significant change was detected in oysters (Lannig et al., 2010) and decreased activities were observed in the mussel M. galloprovincialis (Michaelidis et al., 2005). In the present study, ATPase activity of the Manila clam first increased as pH decreased, with the highest value at pH 7.7, and then it decreased to the lowest value at 7.4. As the pH decreased, more energy was used for acid-base regulation, resulting in elevated ATPase enzyme activities. However, an excessive decrease in pH along with long-term exposure induces metabolic depression (Barnhart, 1989; Rees and Hand, 1990) and decreased ATPase activities.
CAT exists in a wide variety of organisms and plays an important role in the process of removal of bacteria, pathogens, and toxic chemicals. The function of CAT was reported in shellfish such as M. edulis (Lin et al., 2000), Sanguinolaria sp. and Sanguinolaria diphos (Sun et al., 2008), Ostrea gigas and T. granosa (Xiao et al., 2003). These species use CAT to convert hydrogen peroxide into water (Estrada et al., 2007), which is considered to be an adaptive reaction of the organism to overcome stress derived from xenobiotics (Geracitano et al., 2004). In response to toxic chemicals, CAT activity increases to reinforce the catabolic rate and/or to directly inhibit the enzymes (Viarengo et al., 2007; Qiu et al., 2013). In the current study, R. philippinarum exposed to different pH levels exhibited different CAT activities. As the pH decreased from 8.0 for the control group, CAT activity increased at pH 7.7 and then decreased at pH 7.4. Similar results were observed for the CAT activity of the scallop Chlamys farreri (Fan et al., 2006). Generally, a change in CAT activity helps the organism to neutralize the effect of harmful toxic chemicals and defend against disease. In the current study, CAT was effectively stimulated and defended against disease or toxic chemicals at pH 7.7, but at pH 7.4 the immune activity may have been inhibited, make the Manila clams more susceptible to invaders and toxic chemicals (Viarengo et al., 2007; Qiu et al., 2013).
5 CONCLUSIONIn conclusion, reduction in pH resulted in changes in several immune enzymes that play important roles in the Manila clam's ability to tolerate elevated pCO2 conditions. These changes in immune enzyme levels potentially affected the immune capacity of R. philippinarum. Results of this study provide valuable information about the immune response of R. philippinarum to reduced water pH and insights for future research investigating exposure of bivalves to elevated CO2 conditions. Future studies should consider seawater acidification effects on the immune genes of the Manila clam R. philippinarum.
6 DATA AVAILABILITY STATEMENTThe datasets generated and/or analyzed during the current study are available from the corresponding author upon request.
7 ACKNOWLEDGMENTWe thank the anonymous reviewers for their helpful comments on this work.
Barnhart M C. 1989. Respiratory acidosis and metabolic depression in dormant invertebrates. In: Malan A, Canguilhem B eds. Living in the Cold, Ⅱ. John Libbey Eurotext, London. p.321-331.
|
Bibby R, Widdicombe S, Parry H, Spicer J, Pipe R. 2008. Effects of ocean acidification on the immune response of the blue mussel Mytilus edulis. Aquatic Biology, 2: 67-74.
DOI:10.3354/ab00037 |
Caldeira K, Wickett M E. 2003. Oceanography:anthropogenic carbon and ocean pH. Nature, 425(6956): 365.
DOI:10.1038/425365a |
Cheng T C, Rodrick G E. 1975. Lysosomal and other enzymes in the hemolymph of Crassostrea virginica and Mercenaria mercenaria. Comparative Biochemistry and Physiology Part B:Comparative Biochemistry, 52(3): 443-447.
DOI:10.1016/0305-0491(75)90159-5 |
Clements J C, Hunt H L. 2017. Effects of CO2-driven sediment acidification on infaunal marine bivalves:a synthesis. Marine Pollution Bulletin, 117(1-2): 6-16.
DOI:10.1016/j.marpolbul.2017.01.053 |
De Souza B K, Jutfelt F, Kling P, Förlin L, Sturve J. 2014. Effects of increased CO2 on fish gill and plasma proteome. PLoS One, 9(7): e102901.
DOI:10.1371/journal.pone.0102901 |
Deigweiher K, Koschnick N, Pörtner H O, Lucassen M. 2008. Acclimation of ion regulatory capacities in gills of marine fish under environmental hypercapnia. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 295(5): R1 660-R1 670.
DOI:10.1152/ajpregu.90403.2008 |
Ding Z K, Li H H, Xu Y Q. 2012. Effect of ocean acidification on respiratory metabolism of marine living things. Feed Industry, 33(20): 15-17.
(in Chinese with English abstract) |
Estrada N, De Jesús Romero M, Campa-Córdova A, Luna A, Ascencio F. 2007. Effects of the toxic dinoflagellate, Gymnodinium catenatum on hydrolytic and antioxidant enzymes, in tissues of the giant lions-paw scallop Nodipecten subnodosus. Comparative Biochemistry and Physiology Part C:Toxicology & Pharmacology, 146(4): 502-510.
|
Fan Z J, Yang A G, Liu Z H, Dai J X, Dong Y H, Ren J F. 2006. Effect of pH on the immune factors of Chlamys farreri. Journal of Fishery Sciences of China, 13(4): 650-654.
(in Chinese with English abstract) |
Feely R A, Sabine C L, Lee K, Berelson W, Kleypas J, Fabry V J, Millero F J. 2004. Impact of anthropogenic CO2 on the CaCO3 system in the oceans. Science, 305(5682): 362-366.
DOI:10.1126/science.1097329 |
Feng S Y. 1988. Cellular defense mechanism of oysters and mussels. Management of Asian Reservoir Fisheries, 18: 153-168.
|
Geracitano L A, Monserrat J M, Bianchini A. 2004. Oxidative stress in Laeonereis acuta (Polychaeta, Nereididae):environmental and seasonal effects. Marine Environmental Research, 58(2-5): 625-630.
DOI:10.1016/j.marenvres.2004.03.053 |
Hu M H, Lin D H, Shang Y Y, Hu Y, Lu W Q, Huang X Z, Ning K, Chen Y M, Wang Y J. 2017. CO2-induced pH reduction increases physiological toxicity of nano-TiO2 in the mussel Mytilus coruscus. Scientific Reports, 7: 40 015.
DOI:10.1038/SREP40015 |
Huang X Z, Lin D H, Ning K, Sui Y M, Hu M H, Lu W Q, Wang Y J. 2016. Hemocyte responses of the thick shell mussel Mytilus coruscus exposed to nano-TiO2 and seawater acidification. Aquatic Toxicology, 180: 1-10.
DOI:10.1016/j.aquatox.2016.09.008 |
Inoue S, Oshima Y, Usuki H, Hamaguchi M, Hanamura Y, Kai N, Shimasaki Y, Honjo T. 2007. Effect of tributyltin on veliger larvae of the Manila clam, Ruditapes philippinarum. Chemosphere, 66(7): 1 353-1 357.
DOI:10.1016/j.chemosphere.2006.06.052 |
Ishimatsu A, Hayashi M, Lee K S, Kikkawa T, Kita J. 2005. Physiological effects on fishes in a high-CO2 world. Journal of Geophysical Research:Oceans, 110(C9): C09S09.
|
Lannig G, Eilers S, Pörtner H O, Sokolova I M, Bock C. 2010. Impact of ocean acidification on energy metabolism of oyster, Crassostrea gigas-changes in metabolic pathways and thermal response. Marine Drugs, 8(8): 2 318-2 339.
DOI:10.3390/md8082318 |
Li J Q, Jiang Z J, Zhang J H, Mao Y Z, Bian D P, Fang J G. 2014. The potential of ocean acidification on suppressing larval development in the Pacific oyster Crassostrea gigas and blood cockle Arca inflata Reeve. Chinese Journal of Oceanology and Limnology, 32(6): 1 307-1 313.
DOI:10.1007/s00343-014-3317-x |
Lin S Q, Lan R F, Yu P, Cheng W. 2000. Purification and partial characteristics of the catalase from Perna viridis. Food Science, 21(11): 22-24.
|
Liu Z H, Mou H J, Wang Q Y. 2003. Research progress of immune related enzymes in mollusca. Marine Fisheries Research, 24(3): 86-90.
(in Chinese with English abstract) |
Marchant H K, Calosi P, Spicer J I. 2010. Short-term exposure to hypercapnia does not compromise feeding, acid-base balance or respiration of Patella vulgata but surprisingly is accompanied by radula damage. Journal of the Marine Biological Association of the United Kingdom, 90(7): 1 379-1 384.
DOI:10.1017/S0025315410000457 |
Matozzo V, Chinellato A, Munari M, Finos L, Bressan M, Marin M G. 2012. First evidence of immunomodulation in bivalves under seawater acidification and increased temperature. PLoS One, 7(3): e33820.
DOI:10.1371/journal.pone.0033820 |
Melzner F, Göbel S, Langenbuch M, Gutowska M A, Pörtner H O, Lucassen M. 2009. Swimming performance in Atlantic Cod (Gadus morhua) following long-term (4-12 months) acclimation to elevated seawater Pco2. Aquatic Toxicology, 92(1): 30-37.
DOI:10.1016/j.aquatox.2008.12.011 |
Meseck S L, Alix J H, Swiney K M, Long W C, Wikfors G H, Foy R J. 2016. Ocean acidification affects hemocyte physiology in the tanner crab (Chionoecetes bairdi). PLoS One, 11(2): e0148477.
DOI:10.1371/journal.pone.0148477 |
Michaelidis B, Ouzounis C, Paleras A, Pörtner H O. 2005. Effects of long-term moderate hypercapnia on acid-base balance and growth rate in marine mussels Mytilus galloprovincialis. Marine Ecology Progress Series, 293: 109-118.
DOI:10.3354/meps293109 |
Paillard C. 2004. A short-review of brown ring disease, a vibriosis affecting clams, Ruditapes philippinarum and Ruditapes decussatus. Aquatic Living Resources, 17(4): 467-475.
DOI:10.1051/alr:2004053 |
Parker L M, Ross P M, O'Connor W A, Pöertner H O, Scanes E, Wright J M. 2013. Predicting the response of molluscs to the impact of ocean acidification. Biology, 2(2): 651-692.
DOI:10.3390/biology2020651 |
Parvez S, Sayeed I, Raisuddin S. 2006. Decreased gill ATPase activities in the freshwater fish Channa punctata (Bloch) exposed to a diluted paper mill effluent. Ecotoxicology and Environmental Safety, 65(1): 62-66.
DOI:10.1016/j.ecoenv.2005.07.010 |
Pörtner H O, Finke E, Lee P G. 1996. Metabolic and energy correlates of intracellular pH in progressive fatigue of squid (L. brevis) mantle muscle. The American Journal of Physiology, 271(5): R1 403-R1 414.
|
Pörtner H O, Langenbuch M, Reipschläger A. 2004. Biological impact of elevated ocean CO2 concentrations:lessons from Animal Physiology and Earth History. Journal of Oceanography, 60(4): 705-718.
DOI:10.1007/s10872-004-5763-0 |
Qiu J B, Ma F F, Fan H, Li A F. 2013. Effects of feeding Alexandrium tamarense, a paralytic shellfish toxin producer, on antioxidant enzymes in scallops(Patinopecten yessoensis) and mussels (Mytilus galloprovincialis). Aquaculture, 396-399: 76-81.
DOI:10.1016/j.aquaculture.2013.02.040 |
Rees B B, Hand S C. 1990. Heat dissipation, gas exchange and acid-base status in the land snail Oreohelix during shortterm estivation. Journal of Experimental Biology, 152: 77-92.
|
Sabine C L, Feely R A, Gruber N, Key R M, Lee K, Bullister J L, Wanninkhof R, Wong C S, Wallace D W R, Tilbrook B, Millero F J, Peng T H, Kozyr A, Ono T, Rios A F. 2004. The oceanic sink for anthropogenic CO2. Science, 305(5682): 367-371.
DOI:10.1126/science.1097403 |
Saxena T B, Zachariassen K E, Jørgensen L. 2000. Effects of ethoxyquin on the blood composition of turbot, Scophthalmus maximus L. Comparative Biochemistry and Physiology Part C:Pharmacology, Toxicology and Endocrinology, 127(1): 1-9.
DOI:10.1016/S0742-8413(00)00131-6 |
Seidelin M, Brauner C J, Jensen F B, Madsen S S. 2001. Vacuolar-type H+-ATPase and Na+, K+-ATPase expression in gills of Atlantic salmon (Salmo salar) during isolated and combined exposure to hyperoxia and hypercapnia in fresh water. Zoological Science, 18(9): 1 199-1 205.
DOI:10.2108/zsj.18.1199 |
Sui Y M, Hu M H, Shang Y Y, Wu F L, Huang X Z, Dupont S, Storch D, Pörtner H O, Li J L, Lu W Q, Wang Y J. 2017. Antioxidant response of the hard shelled mussel Mytilus coruscus exposed to reduced pH and oxygen concentration. Ecotoxicology and Environmental Safety, 137: 94-102.
DOI:10.1016/j.ecoenv.2016.11.023 |
Sui Y M, Kong H, Huang X Z, Dupont S, Hu M H, Storch D, Pörtner H O, Lu W Q, Wang Y J. 2016b. Combined effects of short-term exposure to elevated CO2 and decreased O2 on the physiology and energy budget of the thick shell mussel Mytilus coruscus. Chemosphere, 155: 207-216.
DOI:10.1016/j.chemosphere.2016.04.054 |
Sui Y M, Kong H, Shang Y Y, Huang X Z, Wu F L, Hu M H, Lin D H, Lu W Q, Wang Y J. 2016a. Effects of short-term hypoxia and seawater acidification on hemocyte responses of the mussel Mytilus coruscus. Marine Pollution Bulletin, 108(1-2): 46-52.
DOI:10.1016/j.marpolbul.2016.05.001 |
Sun T L, Tang X X, Jiang Y S, Wang Y. 2017. Seawater acidification induced immune function changes of haemocytes in Mytilus edulis:a comparative study of CO2 and HCl enrichment. Scientific Reports, 7: 41 488.
DOI:10.1038/srep41488 |
Sun Y Y, Gao R C, Wen Y M, Chen N, Wang S. 2008. Effects of temperature on hydrolase and antioxidase activities in liver of clam Hiatula chinensis and H. diphos. Fisheries Science, 27(10): 543-544.
(in Chinese with English abstract) |
Talmage S C. 2011. The Effects of Elevated Carbon Dioxide Concentrations on the Early Life History of Bivalve Shellfish. The Graduate School, Stony Brook University: Stony Brook, NY.
|
Taucher J, Haunost M, Boxhammer T, Bach L T, Algueró-Muñiz M, Riebesell U. 2017. Influence of ocean acidification on plankton community structure during a winter-to-summer succession:an imaging approach indicates that copepods can benefit from elevated CO2 via indirect food web effects. PLoS One, 12(2): e0169737.
DOI:10.1371/journal.pone.0169737 |
Viarengo A, Lowe D, Bolognesi C, Fabbri E, Koehler A. 2007. The use of biomarkers in biomonitoring:a 2-tier approach assessing the level of pollutant-induced stress syndrome in sentinel organisms. Comparative Biochemistry and Physiology Part C:Toxicology & Pharmacology, 146(3): 281-300.
|
Wang Y J, Li L S, Hu M H, Lu W Q. 2015. Physiological energetics of the thick shell mussel Mytilus coruscus exposed to seawater acidification and thermal stress. Science of the Total Environment, 514: 261-272.
DOI:10.1016/j.scitotenv.2015.01.092 |
Winston G W, Moore M N, Kirchin M A, Soverchia C. 1996. Production of reactive oxygen species by Hemocytes from the marine mussel, Mytilus edulis:lysosomal localization and effect of xenobiotics. Comparative Biochemistry and Physiology Part C:Pharmacology, Toxicology and Endocrinology, 113(2): 221-229.
DOI:10.1016/0742-8413(95)02091-8 |
Wu F L, Lu W Q, Shang Y Y, Kong H, Li L S, Sui Y M, Hu M H, Wang Y J. 2016. Combined effects of seawater acidification and high temperature on hemocyte parameters in the thick shell mussel Mytilus coruscus. Fish & Shellfish Immunology, 56: 554-562.
|
Xiao X, Deng R P, Chen Z L, Han Y L. 2003. Characteristics study on Oyster catalase and Tegillarca granosa catalase. Food Science, 24(9): 32-34.
(in Chinese with English abstract) |
Xu X, Yang F, Zhao L Q, Yan X W. 2016. Seawater acidification affects the physiological energetics and spawning capacity of the Manila clam Ruditapes philippinarum during gonadal maturation. Comparative Biochemistry and Physiology Part A:Molecular & Integrative Physiology, 196: 20-29.
|
Yadwad V B, Kallapur V L, Basalingappa S. 1990. Inhibition of gill Na+K+-ATPase activity in dragonfly larva, Pantala flavesens, by endosulfan. Bulletin of Environmental Contamination and Toxicology, 44(4): 585-589.
DOI:10.1007/BF01700880 |
Yan X W, Zhang Y H, Huo Z M, Yang F, Zhang G F. 2009. Effects of starvation on larval growth, survival, and metamorphosis of Manila clam Ruditapes philippinarum. Acta Ecologica Sinica, 29(6): 327-334.
DOI:10.1016/j.chnaes.2009.09.012 |
Zhao X G, Chai X L, Xiao G Q, Sun C S, Liu G X. 2012.Effects of ocean acidification on intertidal shellfish fertilization research. In: Proceedings of 2012 Abstracts of the 2012 Annual Meeting of China Environmental Science Society Marine Environmental Protection Specialized Committee. Chinese Society for Oceanology and Limnology, Chinese Society for Environmental Sciences, Yanji. (in Chinese)
|
 2018, Vol. 36
2018, Vol. 36


