Institute of Oceanology, Chinese Academy of Sciences
Article Information
- DUAN Youjian(段友健), HUO Bin(霍斌), MA Baoshan(马宝珊), YANG Xin(杨鑫), XIE Congxin(谢从新)
- Reproductive biology of Schizopygopsis younghusbandi Regan 1905 (Cyprinidae: Schizothoracinae) in the middle reaches of Yarlung Tsangpo River, China
- Chinese Journal of Oceanology and Limnology, 36(5): 1825-1834
- http://dx.doi.org/10.1007/s00343-018-7115-8
Article History
- Received Jun. 9, 2017
- accepted in principle Jul. 17, 2017
- accepted for publication Aug. 31, 2017
2 College of Fisheries, Huazhong Agricultural University, Wuhan 430070, China;
3 Yangtze River Fisheries Research Institute, Chinese Academy of Fishery Sciences, Wuhan 430223, China;
4 Hunan Fisheries Research Institute, Changsha 410153, China
The Yarlung Tsangpo River originates from a glacier on the northern side of the middle Himalayas in Tibet features cold water temperature with low biological productivity. Protected by Tibetan Buddhism, who was subscribed to the belief that fish are the embodiment of the dragon god, fisheries resource is abundant (Qiu and Chen, 2009). The subfamily Schizothoracinae is the predominant group of endemic fishes living in QinghaiTibetan Plateau (Cao et al., 1981). With immigration from the inland areas caused lifestyle changes, recent developments in the field of fisheries have led to a renewed interest in fishing. Population decline was found for Schizothorax waltoni, Ptychobarbus dipogon, Schizothorax o'connori, Oxygymnocypris stewartii, and Schizopygopsis younghusbandi (Li and Chen, 2009; Qiu and Chen, 2009; Ma et al., 2011; Huo et al., 2012; Duan et al., 2014). Therefore, management concerning the utilization and conservation of fish resources should be established base on biology and ecology.
Schizopygopsis younghusbandi (Cyprinidae: Schizothoracinae) is relatively small Schizothoracinae fishes, distributed only in the middle reaches of Yarlung Tsangpo River (Bureau of Aquatic Products, 1995). Not only are S. younghusbandi important to local commercial fisheries, they are also one of the most ecologically important species to structure their aquatic ecosystems by virtue of high abundance and predatory nature. Chen et al. (2009) and Duan et al. (2014) studied the age and growth of S. younghusbandi by otolith, revealing that it experienced a low growth rate and could live for 18 years old for female. Xu (2011) reported that the duration of the embryonic development needed 295 h at water temperatures of 9.5–11.1℃. Like other Schizothoracinae fishes of the Tibetan Plateau (Ma et al., 2011; Huo et al., 2012), aggregation-spawning was typical characteristics of S. younghusbandi, which groupers were highly predictable and were often caught by commercial fisheries. The investigated found that S. younghusbandi as a favorite species captured from September to December when fisherman targeted them mainly using gill nets and traps. So far, however, available information on biology of S. younghusbandi in the Yarlung Tsangpo River is limited.
Previously, several recent studies investigating reproduction have been carried out on other subfamily Schizothoracinae (Ma et al., 2012; Huo et al., 2013; Zhou et al., 2015), while information on reproductive cycle of S. younghusbandi showed limited attention. Knowledge of reproductive biology, such as spawn season, sexual maturity and fecundity, is vital demographic characteristics essential to understanding of a species' life history and critical component of their management. The aim of the present study was to determine the reproductive biology of S. younghusbandi and answer basic questions including: (1) size and age at maturity of S. younghusbandi; (2) the spawning season and spawning type of the fish, based on monthly proportions of macroscopic gonadal maturity stages, variations in the gonadosomatic index (GSI), and the size distribution of oocytes; and (3) fecundity and relative fecundity of S. younghusbandi, and analyze relationships between length and weight. Water temperature and photoperiod on gonadal development and sexual maturity were also considered.
2 MATERIAL AND METHOD 2.1 Sample collectionBetween August 2008 and August 2009, 719 specimens of S. younghusbandi were caught monthly with floating gillnets (mesh size 7.5 cm) and bottom gillnets (mesh size 6.5 cm) in the Yarlung Tsangbo River (Fig. 1).
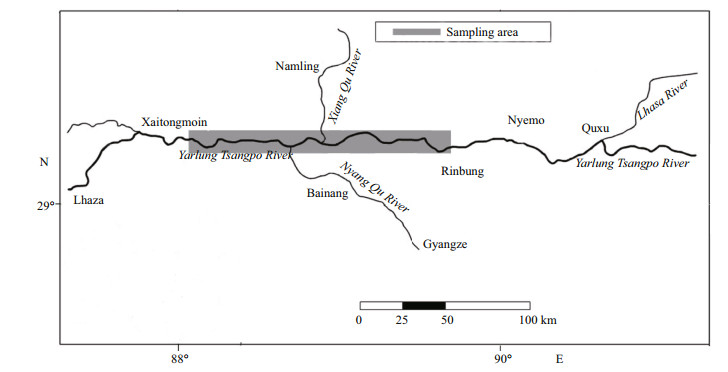
|
| Figure 1 Sampling locations of S. younghusbandi in the Yarlung Tsangpo River during the period 2008–2009 (Zhou et al., 2015) |
Prior to standard length (SL) and body weight (BW) were measured to the nearest 1 mm and 0.1 g, fish was euthanized with MS-222 and eviscerated immediately. The gonads were removed and weighed (to the nearest 0.01 g). The gonad samples were conserved in Bouin's fluid for 48 h and then immersed in 70% ethanol until examination. Fixed gonads were dehydrated in alcohol and embedded in paraffin wax for sectioning. Embedded gonads were sectioned transversely at 7–10 μm thickness and stained with haematoxylin and eosin. Pictures were taken using a Nikon microscope at ×40–400 (Nikon Eclipse 80i photomicroscope).
2.2 Experiments for the estimation of size and age at sexual maturitySize at sexual maturity that defined as the size at which 50% of the population attains sexual maturity (SL50)was performed to assess maturity macroscopically for each sex. In other words, the size at which randomly chosen individual has a 50% chance of being mature (Somerton, 1980). A logistic function to the proportion (P) of mature fish was used to estimate the size with10 mm (SL) size intervals: P=1/{1+exp[-k(SLmid–SL50)]} (Chen and Paloheimo, 1994), where SLmid is the midpoint of the SL class, SL50 is the mean SL at sexual maturity; P is the proportion of mature fish and k is the slope. This logistic function was also performed to describe the possibilities of age at maturity (A50), in which age was determined by otolith. The equation is: P=1/{1+exp[-k(A–A50)]} (Chen and Paloheimo, 1994), where A is the age of fish, A50 is the age at first maturity.
2.3 Experiments for the spawning seasonThe spawning season was determined by monthly variations of the gonadosomatic index (GSI), monthly proportions of the macroscopic maturity stages, and the size distribution of oocytes. The GSI of mature individuals was assessed using the formula: GSI=GW/ BW×100, GW and BW means the gonad weight and the body weight, respectively. The diameter of all oocytes that had started vitellogenesis was measured. The monthly size-frequency distribution of oocytes was photographed (Leica EZ4D dissecting microscope) and measured to the nearest 0.001 mm in diameter (Image Pro Plus 6.0). For each individual, at least 120 oocytes were measured.
2.4 Experiments for the estimation of fecundityIn order to determine fecundity, 69 females were handled by the gravimetric method (Bagenal and Braum, 1978). Portions of each ovary, including anterior, median and posterior, were sampled. Fecundity was calculated as F=n×W/w, where n is the number of oocytes in the subsamples, W is the weight of ovaries, and w is the weight of subsamples. The relative fecundity was determined by the number of vitellogenic oocytes counted per female gram of total female weight (Adebisi, 1987). Regression models were used to describe the relationships between standard length, total weight and absolute fecundity. Data and images were analyzed by means of Microsoft Excel 2003, Adobe Photoshop CS4 and Origin Lab Origin V8.0.
3 RESULT 3.1 Size distributionA total of 719 individuals (527 females, 183 males and 9 undetermined) were collected during the study period (Fig. 2). The overall sex ratio significantly differed from 1:1 (χ2=165.937, d.f.=1, P < 0.05), with 25.5% males and 73.3% females. Length-frequency distributions significantly differed between sexes (Kolmogorov-Smirnov Z=5.198, n1=527, n2=183, P < 0.05).

|
| Figure 2 Size distribution for unsexed, males and females of S. younghusbandi in the Yarlung Tsangpo River during the period 2008–2009 |
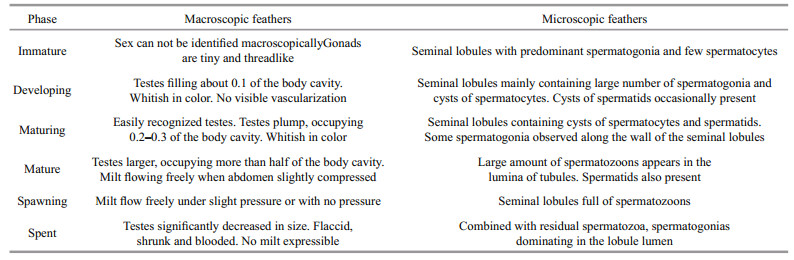
Sex and maturity stage were identified based on macroscopic and microscopic features (Tables 1, 2). The development of gonads was categorized to six phase followed Huo et al. (2013). With regard to macroscopic features, we assigned a gross maturity stage based on the gonads. With regard to histological observation, both ovaries and testes were assigned to six microscopic developmental stages (Figs. 3, 4). The microscopic mature stage was designated when ovaries developed mainly in advanced yolked oocytes (Fig. 3e). Furthermore, vitellogenic oocytes were observed in the spent ovaries (Fig. 3f).
|

|
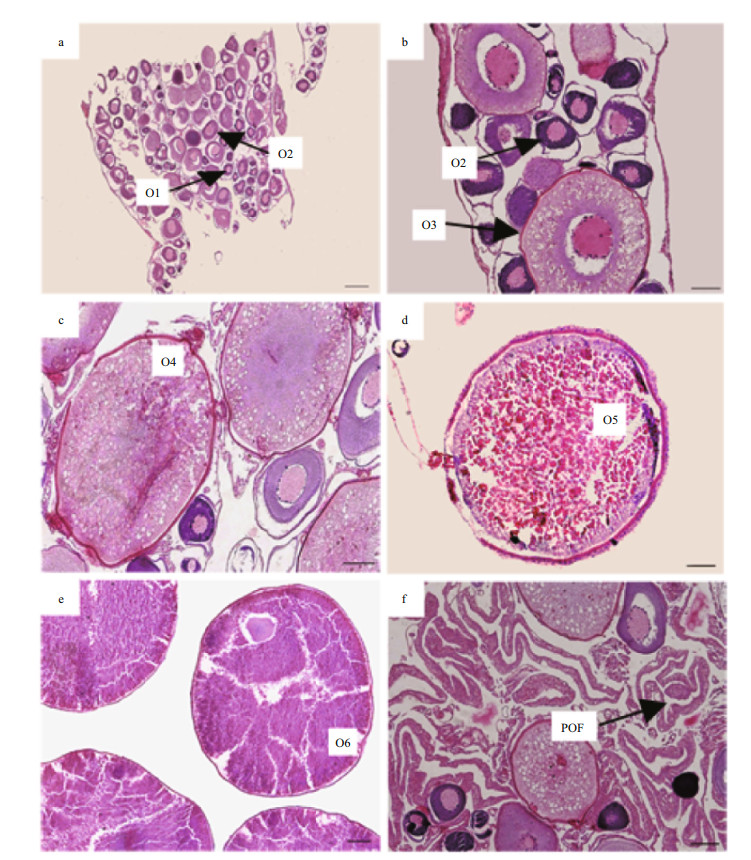
|
| Figure 3 Ovarian development in S. younghusbandi in the Yarlung Tsangpo River during the period 2008–2009 a. immature ovary; b. early developing ovary; c. late developing ovary; d. maturing ovary; e. mature ovary; f. spent ovary. O1: chromatin-nucleolar oocyte; O2: peri-nucleolar oocyte; O3: cortical alveoli oocyte; O4: primary yolk oocyte; O5: second yolk oocyte; O6: tertiary yolk oocyte; ao: atretic oocyte; POF: postovulatory follicles. Scale bar: b, c, d, f: 100 μm; a, e: 200 μm. |
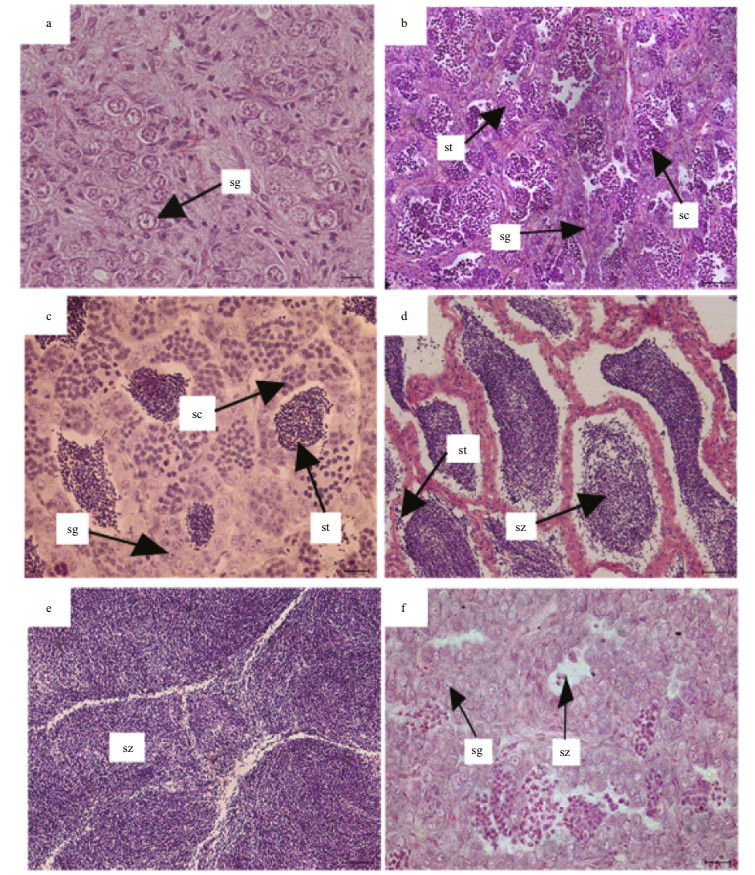
|
| Figure 4 Testes development in S. younghusbandi in the Yarlung Tsangpo River during the period 2008–2009 a. immature testis; b. developing testis; c. maturing testis; d. mature testis; e. spawning testis; f. spent testis. sg: spermatogonia; sc: spermatocytes; st: spermatids; sz: spermatozoa. Scale bar: a, c, f: 20 μm; b, d, e: 50 μm. |
Size (SL50) and age (A50) at 50% maturity were estimated by fitting a logistic function to the proportion (P) of mature fish. Size and age at maturity showed a clear difference between males and females (Figs. 5, 6). Estimated SL50 were 222 mm and 308 mm for males and females, respectively. Estimated A50 are 4.4 years and 7.0 years for males and females, respectively.
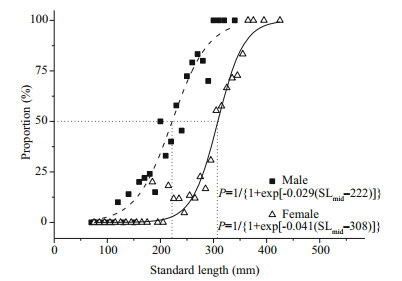
|
| Figure 5 Logistic functions fitted to percent mature by 10 mm standard length intervals of males and females S. younghusbandi in the Yarlung Tsangpo River during the period 2008–2009, showing the mean standard length (SL50) at sexual maturity |
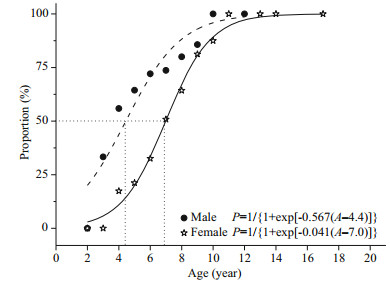
|
| Figure 6 Logistic functions fitted to percent mature by 1 year intervals of males and females S. younghusbandi in the Yarlung Tsangpo River during the period 2008– 2009, showing the mean age (A50) at sexual maturity |
The reproductive cycle can be classified into three phases that contains gonad development, spawning, and resting according to monthly variations of the gonadosomatic index (GSI) (Fig. 7), monthly proportions of the macroscopic maturity stages (Fig. 8), and size distribution of oocytes (Fig. 9).

|
| Figure 7 Monthly gonadosomatic index of males and females S. younghusbandi collected in the Yarlung Tsangpo River during the period 2008–2009 Values are expressed as means and the vertical lines means±standard deviation. |
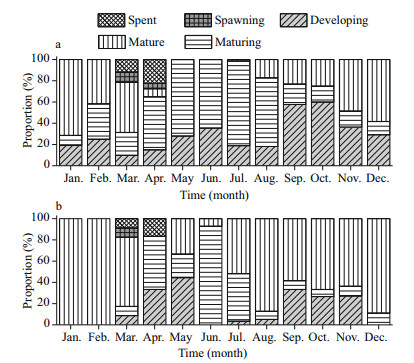
|
| Figure 8 Distribution of macroscopic gonad maturity stages of females (a) and males (b) of S. younghusbandi in the Yarlung Tsangbo River between August 2008 and August 2009 |
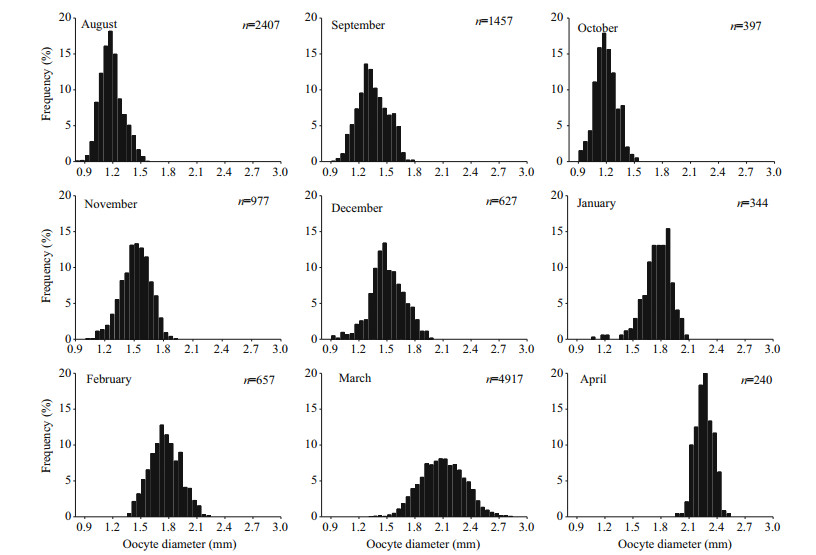
|
| Figure 9 Monthly size-frequency of oocytes for S. younghusbandi in the Yarlung Tsangbo River from July 2008 to April 2009 |
The GSI of S. younghusbandi changed over time (Fig. 7). By November males run a higher level than females and the GSI on the increase for each sex during gonad development phase. With rising water temperature and photoperiod in early spring (Fig. 10), S. younghusbandi showed a high level from January to March. After that, the GSI level of each sex changed substantially and reached at lowest value in May (1.19% for female and 1.20% for male), which remained nearly constant to July.
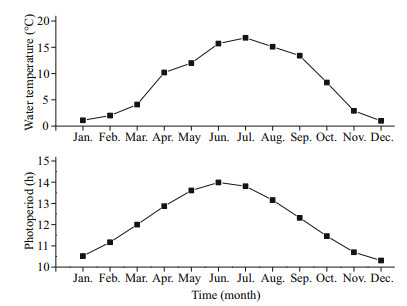
|
| Figure 10 Monthly variation in water temperature and photoperiod in the Yarlung Tsangbo River between August 2008 and August 2009 Values are expressed as means and the vertical lines means±standard deviation. |
According to distribution of macroscopic gonad maturity stages, we concluded that S. younghusbandi might spawn from March to April (Fig. 8). In gonad development phase S. younghusbandi were not found mature proportions exceed 50% until to November for female, while mature proportions mainly occupied for male since early August. The highest level of mature proportions was well synchronized with the rising water temperature and photoperiod at spring (Fig. 10), and both spent stages could be observed in March and April. Gonadal mainly consist of maturing and developing stage in resting phase. Approximately 60% of maturing and developing stage possessed for male, and nearly full occupied for female from May to July.
Egg sizes were measured for mature ovaries from August 2008 to April 2009 (Fig. 9). Oocytes kept growing since yolk began accumulating in August. Prior to the following April, high proportion mature oocytes formed in ovaries with a diameter of > 2.0 mm. Histogram of oocyte diameter showed a unimodal distribution in every month, indicating that there was a high degree of spawning synchronicity in the S. younghusbandi population.
3.5 FecundityThe mean value for absolute fecundity was 18 682 (S.D.=9 038) (ranging from 5 712 to 51 037) eggs for females with standard length from 182 to 393 mm. The relative fecundity was estimated at 57.8 (S.D.=15.2) (ranging from 29.5 to 113.6) eggs/g body weight. A significantly positive correlation was found between absolute fecundity and standard length (Fig. 11a, n=69, R2=0.501), as well as body weight (Fig. 11b, n=69, R2=0.624).
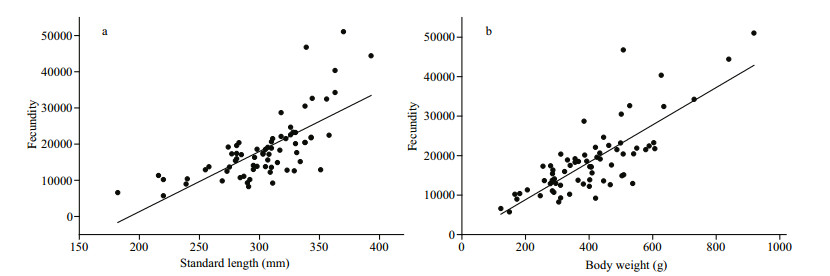
|
| Figure 11 The relationships between ovary weight, standard length and fecundity of S. younghusbandi in the Yarlung Tsangbo River |
The present study was designed to answer basic questions concerning the reproductive biology of S. younghusbandi. Spawning season was determined by the monthly gonadosomatic index and distribution of macroscopic maturity stages in which S. younghusbandi spawns over a short period each year. Our results indicate that life history of S. younghusbandi is typical K-selected traits, which characterize by relatively low fecundity and relatively late sexual maturity (Winemiller, 1992). These reproductive characteristics are in line with those of previous studies in the middle reaches of Yarlung Tsangpo River (Qiu and Chen, 2009; Ma et al., 2012; Huo et al., 2013; Zhou et al., 2015). Fisheries based on K-selected species are more susceptible to growth overfishing and stock depletion (Booth and Buxton, 1997).
Environmental and geographical factors have an influence on fish growth and reproduction, which may lead to reaching at different sizes or at different ages of sexual maturity (Pawson et al., 2000; Yoneda and Wrighte, 2005; Heath et al., 2012). Indeed, our results estimate that the size and age at first sexual maturity of S. younghusbandi are larger and older than values reported for comparable sizes of other cyprinid fishes from plain water (Rutaisire and Booth, 2005; Tarkan, 2006). This phenomenon was also described in other Schizothoracinae fishes in the Yarlung Tsangbo River (Ma et al., 2012; Huo et al., 2013; Zhou et al., 2015). Low water temperature and lack of bait biological in high elevation water could make the fish grow slowly. Environmental characters were also considered as the primary environmental factors to synchronize the endogenous rhythms of spawning in many cyprinid fishes (De Vlaming, 1975; Papoulias et al., 2006). The fish did not spawn until the water temperature and photoperiod began increasing in March, suggesting that water temperature and photoperiod are likely mediated through its impact on the final maturation of oocytes and the initiation of reproductive activities. In addition, unimodal distributions of oocytes suggested that S. younghusbandi population experienced a high degree of spawning synchronicity.
The oocyte growth of spring-spawning freshwater fishes typically occurs following a relatively long phase of gonad quiescence (Peter and Crim, 1979). In contrast with this characteristic, gonadal development was initiated following a relatively short postspawning quiescent period in S. younghusbandi. A possible explanation for this might be that gonadal recrudescence occurs coincident with a period of decreasing photoperiod and water temperatures. This observation may support the hypothesis that short post-spawning quiescent period was advantageous to S. younghusbandi adapt to the rigorous high-elevation plateau river environment.
The S. younghusband, as one of the smallest species from Schizothoracinae fishes in the middle reaches of the Yarlung Tsangpo River, had the lowest values of absolute fecundity, because fecundity tends to increase with fish size and fish body weight increase. (Wootton, 1999; Murua and Saborido-Rey, 2003; Brouwer and Griffiths, 2005). Therefore, it is necessary to eliminate the body size effect in fecundity studies. In this study the relative fecundity of S. younghusbandi has 57.8 eggs/g of fish body weight. Analogously, Ma et al. (2012) found that the relative fecundity of Schizothorax o'connori (Cyprinidae: Schizothoracinae) in the Yarlung Tsangpo River fluctuated from 6.2 to 22.2 eggs/g, with a mean of 14.32 eggs/g of fish body weight. The mean relative fecundity of Oxygymnocypris stewartii in the Yarlung Zangbo River was 25.4 eggs/g of fish body weight (Huo et al., 2013). Zhou et al. (2015) reported that the relative fecundity of Schizothorax waltoni (Cyprinidae: Schizothoracinae) in the Yarlung Tsangpo River with 13.4 eggs/ g of fish body weight. While Hotos et al. (2000) found that the relative fecundity of Liza aurata (Pisces Mugilidae) from a local population in the lagoon of Klisova with a mean of 1 152 eggs/g of fish body weight. The mean relative fecundity of Silver Carp in the Middle Mississippi River was 672 eggs/g of fish body weight (Williamson and Garvey, 2005). Above all, Schizothoracinae fishes represent a lower reproductive potential in the Yarlung Tsangpo River.
Schizopygopsis younghusbandi originated from primitive barbine fishes that date back to the late Tertiary, and evolved into the Qinghai-Tibet plateau fish fauna along the vicariance caused by the uplift of Tibet (Cao et al., 1981; Wu and Tan, 1991; He and Chen, 2006). The isolation made this specialized fish fauna sensitive to anthropogenic activities. Not only anthropogenic disturbance can efficiently remove large mature individuals during spawning aggregations, but could possibly progressively push the fish population towards maturation at smaller length and increase the risk of recruitment failures (Jørgensen, 1990). Thus, knowledge of the reproductive characteristics of this species is fundamental importance to conserving this stock. Our study represents an important step in the understanding of S. younghusbandi's reproductive biology. Based on those characteristics, fishery regulations should focus on the mesh size limit, the fishing closure and the proper fishing methods to prevent overfishing, should be established. Meanwhile, long-term ecological studies are needed to observe the change in size at maturity on the commercial potential of S. younghusbandi in the future.
5 CONCLUSIONSchizopygopsis younghusbandi spawns over a short period each year from March to April, are typically aggregation-spawning with low fecundity and late maturity. Our results indicate that S. younghusbandi may be vulnerable to exploitation in the middle reaches of Yarlung Tsangpo River.
6 DATA AVAILABILITY STATEMENTThe data that support the findings of this study are available from the corresponding author upon reasonable request.
7 ACKNOWLEDGEMENTThanks to ZHOU Xianjun, YANG Xuefeng and HUANG Haiping for helping in sample collection and histological processing. The use of specimens in our research were carried out following the legal requirements "Instructive notions with respect to caring for laboratory animals", which was issued by the Ministry of Science and Technology of the People's Republic of China.
Adebisi A A. 1987. The relationships between the fecundities, gonado-somatic indices and egg sizes of some fishes of Ogun River, Nigeria. Archiv für Hydrobiologie, 111: 151-156.
|
Bagenal T B, Braum E. 1978. Eggs and early life history. In: Bagenal T B ed. Methods for Assessment of Fish Production in Fresh Waters. Blackwell Scientific Publications, Oxford. p.165-201. https://www.afsc.noaa.gov/RACE/recruitment/eltax_rp.php
|
Booth A J, Buxton C D. 1997. Management of the panga Pterogymnus laniarius (Pisces: Sparidae) on the Agulhas Bank, South Africa using per-recruit models. Fisheries Research, 32(1): 1-11.
DOI:10.1016/S0165-7836(97)00045-3 |
Brouwer S L, Griffiths M H. 2005. Reproductive biology of carpenter seabream (Argyrozona argyrozona) (Pisces: Sparidae) in a marine protected area. Fishery Bulletin, 103(2): 258-269.
|
Bureau of Aquatic Products. 1995. Fishes and Fish Resources in Xizang, China. China Agriculture Press, Beijing.
(in Chinese)
|
Cao W X, Chen Y Y, Wu Y F, Zhu S Q. 1981. Origin and evolution of schizothoracine fishes in relation to the upheaval of the Qinghai-Xizang Plateau. In: CAS ed. Studies on the Period, Amplitude and Type of the Uplift of the Qinghai-Xizang Plateau. Science Press, Beijing, p.118-130. (in Chinese)
|
Chen F, Chen Y F, He D K. 2009. Age and growth of Schizopygopsis younghusbandi younghusbandi in the Yarlung Zangbo River in Tibet, China. Environmental Biology of Fishes, 86(1): 155-162.
DOI:10.1007/s10641-008-9370-6 |
Chen Y, Paloheimo J E. 1994. Estimating fish length and age at 50% maturity using a logistic type model. Aquatic Sciences, 56(3): 206-219.
DOI:10.1007/BF00879965 |
De Vlaming V L. 1975. Effects of photoperiod and temperature on gonadal activity in the cyprinid teleost, Notemigonus crysoleucas. The Biological Bulletin, 148(3): 402-415.
DOI:10.2307/1540517 |
Duan Y J, Xie C X, Zhou X J, Ma B S, Huo B. 2014. Age and growth characteristics of Schizopygopsis younghusbandi Regan, 1905 in the Yarlung Tsangpo River in Tibet, China. Journal of Applied Ichthyology, 30(5): 948-954.
DOI:10.1111/jai.2014.30.issue-5 |
He D K, Chen Y Y. 2006. Biogeography and molecular phylogeny of the genus Schizothorax (Teleostei: Cyprinidae) in China inferred from cytochrome b sequence. Journal of Biogeogr, 33(8): 1448-1460.
DOI:10.1111/jbi.2006.33.issue-8 |
Heath M R, Neat F C, Pinnegar J K, Reid D G, Sims D W, Wright P J. 2012. Review of climate change impacts on marine fish and shellfish around the UK and Ireland. Aquatic Conservation: Marine and Freshwater Ecosystems, 22(3): 337-367.
DOI:10.1002/aqc.2244 |
Hotos G N, Avramidou D, Ondrias I. 2000. Reproduction biology of Liza aurata (Risso, 1810), (Pisces Mugilidae) in the lagoon of Klisova (Messolonghi, W. Greece). Fisheries Research, 47(1): 57-67.
DOI:10.1016/S0165-7836(99)00128-9 |
Huo B, Xie C X, Ma B S, Yang X F, Huang H P. 2012. Age and Growth of Oxygymnocypris stewartii (Cyprinidae: Schizothoracinae) in the Yarlung Tsangpo River, Tibet, China. Zoological Studies, 51(2): 185-194.
|
Huo B, Xie C X, Ma B S, Yang X F, Huang H P. 2013. Reproductive biology of Oxygymnocypris stewartii in the Yarlung Zangbo River in Tibet, China. Environmental Biology of Fishes, 96(4): 481-493.
DOI:10.1007/s10641-012-0031-4 |
Jørgensen T. 1990. Long-term changes in age at sexual maturity of Northeast Arctic cod (Gadus morhua L.). ICES Journal of Marine Science, 46(3): 235-248.
DOI:10.1093/icesjms/46.3.235 |
Li X Q, Chen Y F. 2009. Age structure, growth and mortality estimates of an endemic Ptychobarbus dipogon (Regan, 1905) (Cyprinidae: Schizothoracinae) in the Lhasa River, Tibet. Environmental Biology of Fishes, 86(1): 97-105.
DOI:10.1007/s10641-008-9371-5 |
Ma B S, Xie C X, Huo B, Yang X F, Chen S S. 2012. Reproductive biology of Schizothorax o'connori (cyprinidae: schizothoracinae) in the Yarlung Zangbo River, Tibet. Zoological Studies, 51(7): 1 066-1 076.
|
Ma B S, Xie C X, Huo B, Yang X F, Li P. 2011. Age validation, and comparison of otolith, vertebra and opercular bone for estimating age of Schizothorax o'connori in the Yarlung Tsangpo River, Tibet. Environmental Biology of Fishes, 90(2): 159-169.
DOI:10.1007/s10641-010-9727-5 |
Murua H, Saborido-Rey F. 2003. Female reproductive strategies of marine fish species of the North Atlantic. Journal of Northwest Atlantic Fishery Science, 33(1): 23-31.
|
Papoulias D M, Chapman D, Tillitt D E. 2006. Reproductive condition and occurrence of intersex in bighead carp and silver carp in the Missouri River. Hydrobiologia, 571(1): 355-360.
DOI:10.1007/s10750-006-0260-7 |
Pawson M G, Pickett G D, Witthames P R. 2000. The influence of temperature on the onset of first maturity in sea bass. Journal of Fish Biology, 56(2): 319-327.
DOI:10.1111/jfb.2000.56.issue-2 |
Peter R E, Crim L W. 1979. Reproductive endocrinology of fishes: gonadal cycles and gonadotropin in teleosts. Annual Review of Physiology, 41(1): 323-335.
DOI:10.1146/annurev.ph.41.030179.001543 |
Qiu H, Chen Y F. 2009. Age and growth of Schizothorax waltoni in the Yarlung Tsangpo River in Tibet, China. Ichthyological Research, 56(3): 260-265.
DOI:10.1007/s10228-009-0096-z |
Rutaisire J, Booth A J. 2005. Reproductive biology of ningu, Labeo victorianus (Pisces: Cyprinidae), in the Kagera and Sio Rivers, Uganda. Environmental Biology of Fishes, 73(2): 153-162.
DOI:10.1007/s10641-004-5564-8 |
Somerton D A. 1980. A computer technique for estimating the size of sexual maturity in crabs. Canadian Journal of Fisheries and Aquatic Sciences, 37(10): 1 488-1 494.
DOI:10.1139/f80-192 |
Tarkan A S. 2006. Reproductive ecology of two cyprinid fishes in an oligotrophic lake near the southern limits of their distribution range. Ecology of Freshwater Fish, 15(2): 131-138.
DOI:10.1111/eff.2006.15.issue-2 |
Williamson C J, Garvey J E. 2005. Growth, fecundity, and diets of newly established silver carp in the middle Mississippi River. Transactions of the American Fisheries Society, 134(6): 1 423-1 430.
DOI:10.1577/T04-106.1 |
Winemiller K O. 1992. Life-history strategies and the effectiveness of sexual selection. Oikos, 63(2): 318-327.
DOI:10.2307/3545395 |
Wootton R J. 1999. Ecology of Teleost Fishes. 2nd edn. Springer, Netherlands, 392p.
|
Wu Y F, Tan Q J. 1991. Characteristics of the fish-fauna of the characteristics of Qinghai-Xizang plateau and its geological distribution and formation. Acta Zoologica Sinica, 37(2): 135-152.
(in Chinese with English abstract) |
Xu J. 2011. Early development of four Schizothoraeinae fishes in the Yarlung Zangbo River, Tibet. Masterʼs thesis, Huazhong Agricultural Univ., Wuhan, China. (in Chinese with English abstract)
|
Yoneda M, Wright P J. 2005. Effects of varying temperature and food availability on growth and reproduction in firsttime spawning female Atlantic cod. Journal of Fish Biology, 67(5): 1225-1241.
DOI:10.1111/jfb.2005.67.issue-5 |
Zhou X J, Xie C X, Huo B, Duan Y J, Yang X, Ma B S. 2015. Reproductive biology of Schizothorax waltoni (cyprinidae: schizothoracinae) in the Yarlung Zangbo river in Tibet, China. Environmental Biology of Fishes, 98(2): 597-609.
DOI:10.1007/s10641-014-0293-0 |
 2018, Vol. 36
2018, Vol. 36


