Institute of Oceanology, Chinese Academy of Sciences
Article Information
- LI Zhongbao(黎中宝), YANG Huan(杨欢), SHANGGUAN Jingbo(上官静波), CHEN Qiang(陈强), LI Wenjing(李文静), LU Jing(卢静)
- Growth performance, digestive enzyme activities and serum nonspecific immunity of the red tilapia (Oreochromis mossambicus×Oreochromis niloticus) fed diets supplemented with ultrafine powder of Enteromopha prolifera
- Chinese Journal of Oceanology and Limnology, 36(5): 1843-1850
- http://dx.doi.org/10.1007/s00343-018-7142-5
Article History
- Received Jun. 11, 2017
- accepted in principle Aug. 14, 2017
- accepted for publication Sep. 14, 2017
2 Fujian Provincial Key Laboratory of Marine Fishery Resources and Eco-Environment, Xiamen 361021, China
The hybrid red tilapia, Oreochromis mossambicus × Oreochromis niloticus, is a popular aquaculture species in China. The species exhibits excellent characteristics for aquaculture, such as high tolerance to harsh environmental conditions, easy reproduction, fast growth and high quality meat (Chiu et al., 2013). The red tilapia is considered having high potentials for further production expansion because of its strong market demands and high adaptive capacity (Romana-Eguia and Eguia, 1999). Past studies on the red tilapia have focused more on the strain variations in growth and other important traits, such as fecundity, feed conversion efficiency, red color inheritance, and reproduction under high salinity or temperature conditions (e.g. Koren et al., 1994; Richter et al., 2002) and there was relatively few studies investigated nutrition requirement, digestive physiology and immune responses of the red tilapia.
Enteromorpha prolifera is a large green macroalgae belonging to the family Ulvaceae (Zeng et al., 1962; Hiraoka et al., 2003; Yao, 2011). It is a commercial important species (Aguilera-Morales et al., 2005) and distributes globally on rock and gravel between the intertidal to the upper subtidal zones (Hiraoka et al., 2003). In recent years, marine eutrophication in China has often led to blooms of E. prolifera in summer (as known as green tides), blanketing coastal surface. The algal blooms threatens marine environment and the local governments had to organize costly clean up. Although when forms green tides, E. prolifera is a threat to the environment, the algae actually contains high protein and dietary fiber, as well as rich in polysaccharide and minerals (Aguilera-Morales et al., 2005) and has been proved as a good feed supplement for livestock (Michalak and Chojnaka, 2009).
Additionally, given that the sub-therapeutic antibiotics were forbidden as growth-promoters in the European Union in 2006 (Regulation1831/2003/EC), consumer demands on eco-friendly farming practices and safe food products have increased significantly (Rawling et al., 2009). As the result, additive alternative to traditional antibiotics in aquaculture feeds, particularly those sourced from plants, have received much attention recently (Bricknell and Dalmo, 2005). Research have indicated high potential of phytogenic substances in fish diets as they can act as the alternatives for antibiotics and growth promoters (Jian and Wu, 2003) as well as enhance storage quality and antioxidant properties of fish fillet (Gatlin et al., 1992). In fact, in recent years, the rapid expansion of intensive aquaculture of tilapia in China and Southeast Asia has led to frequent outbreak of diseases, consequently antibiotics are often used for disease prevention and control. However, it has been questioned that resistant bacterial populations could be induced from the use of antibiotics, and more serious is the unpredictable long-term effects on public health. Hence, green feed additives, such as marine macroalgae, have been viewed as potential dietary alternatives for improving the immune responses, disease resistance and growth performance of cultured fish (Wassef et al., 2009, 2013).
There is limited information available on the application of E. prolifera as feed ingredient or supplement for feeding fish (Asino et al., 2011; Yang et al., 2016). As most marine macroalgae, E. prolifera contains relatively high fibre and this may limit its effective utilization by fish (Buddington, 1987); E. prolifera in the form of ultrafine powder might help fish digestion. As dry power, it is also much more convenience for storage and transport, as well as for feed manufacture as it is easier to mix with other feed ingredients. To the best of our knowledge, no information is currently available on E. prolifera being proceeded as ultrafine powder form and used as feed additive for feeding fish; the present study was hence carried out to investigate whether ultrafine powder of E. prolifera could be used as effective feed supplement for the red tilapia.
2 MATERIAL AND METHODAll animals used in this study have been approved by the Animal Ethical Committee of Jimei University and experiments were carried out in accordance with the approved guidelines of the university.
2.1 Production of E. prolifera ultrafine powder and the experimental dietsEnteromorpha prolifera was harvested from a manmade canal for dragon boat racing in Xiamen, China during March to April 2012. After rinsing with clean seawater, the harvested E. prolifera was transported to a laboratory of Jimei University and oven-dried at 60℃ for 4 h before being pulverized for 30 s by a low temperature pulverizer at 10℃. The resultant power was then sieved through a mesh screen (74 μm) and only those passed through the screen were collected and stored in plastic bags for late use.
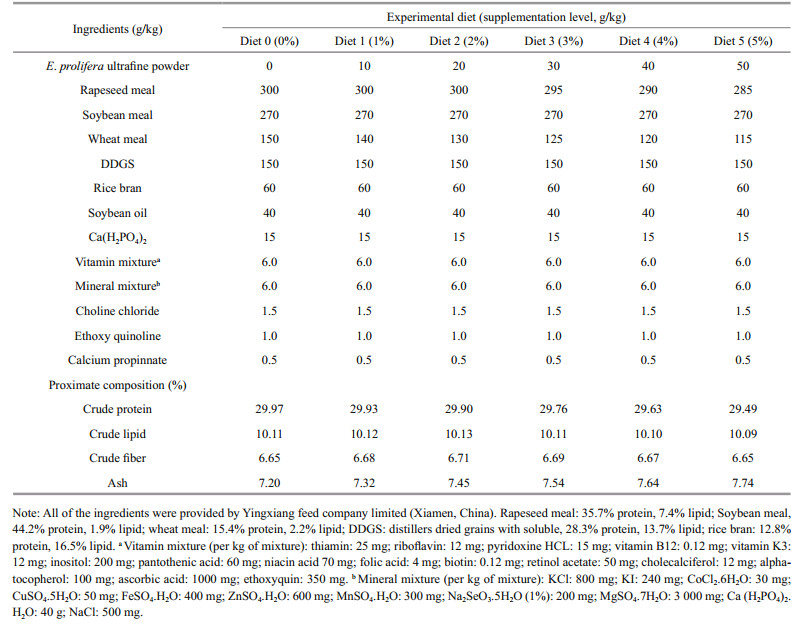
Six experimental diets were formulated to be isonitrogenous and isoenergetic, the ingredients and results of proximate analysis of these diets are shown in Table 1 (the proximate analysis was carried out according to AOAC, 1995). To make the experimental diets, ultrafine powder of E. prolifera was thoroughly mixed with other feed ingredients (purchased from Yingxiang feed Co. Ltd., Xiamen, China) at the designated ratio of 0 g/kg or 0% (Diet 0; control), 10 g/kg or 1% (Diet 1), 20 g/kg or 2% (Diet 2), 30 g/kg or 3% (Diet 3), 40 g/kg or 4% (Diet 4) and 50 g/kg or 5% (Diet 5), respectively. Afterwards, they were squeezed to produce 2.5 mm pellets using a moist pelleting machine (CD4XITX, South China University of Technology, Guangzhou, China). The above products were then dried at room temperature and stored at -25℃ until used.
The feeding trial (2012.7–2012.8) was performed at the fisheries experimental station of Fisheries College, Jimei University, Xiamen, China. The red tilapia fingerlings were fed the control diet (Diet 0) for 2 weeks and after the acclimatization period, health individuals (weight: 4.68±0.48 g; length: 5.16±0.63 cm) were randomly selected and transferred to 18 fiberglass tanks containing 120 L water. Each of the tanks connected to a closed recirculating system (water temperature: 30±4℃, dissolved oxygen: 5.7– 8.0 mg/L, and pH: 6.5–7.9, water quality parameters were measured using HACH/HQ30D portable digital multiparameter analyzer). Each treatment was triplicated and each tank stocked 30 individuals. The fish were fed the correspondent experimentally diets to satiation twice daily at 09:00 and 17:00. After each feeding, uneaten feed was recovered and dried before being weighed to estimate the amount of feed consumed.
The feeding experiment lasted for 7 weeks. At the end of the experiment, all surviving fish were not fed 24 h and batch weighted and counted from each tank. The calculation formulas are as follows:
percentage weight gain: PWG (%)=100×(Wt–W0)/W0;
specific growth rate: SGR=100×(lnWt–lnW0)/t;
feed conversion ratio: FCR=100×FI/(Wt–W0);
condition factor: CF=100×weight/length3;
hepatosomatic index: HI=100×liver mass/body mass;
survival rate: SR (%)=100×Nt/N0,
where W0 and Wt are the initial and the final body weight, respectively; t is the duration of experiment (days); FI is feed intake; N0 and Nt are the initial and the final number of fish in each tank, respectively.
For serum immune parameters analysis, 6 fish from each replicate tank were randomly selected and euthanatized. Blood was extracted from each fish and centrifuged (3 000 r/min for 10 min, 4℃) to collect serum. Serum samples from three fish were pooled and therefore six serum samples from each treatment were obtained and kept at -80℃ until analysis.
For calculating hepatosomatic index (HI) and digestive enzyme analysis, 2 individuals from each tank (six samples from each treatment) were euthanatized and dissected, and then intestine, stomach and liver were weighted and quickly treated by liquid nitrogen flash freezer for digestive enzyme analysis.
2.3 Digestive enzyme analysisFor digestive enzyme analysis, the intestine and stomach were rinsed with chilled distilled water, homogenized and centrifuged (2 500 r/min for 10 min). The resultant supernatants were then quickly collected and kept at -20℃. The pepsin, erepsin, gastric amylase, intestinal amylase, gastric lipase and intestinal lipase activities were analyzed according to the manufacture's instruction of respective test kits (Jiancheng technology Co. Ltd., Nanjing, China).
2.4 Serum nonspecific immune enzymes determinationSerum lysozyme activity was measured by a turbidometric assay using lyophilized Micrococcus lysodeikticus (Jiancheng technology Co. Ltd., Nanjing, Jiangsu, China) (Ellis 1990). A volume of 950 μL M. lysodeikticus at a concentration of 200 mg/mL (w/v) in 0.05 mol/L PBS (pH 6.2) was added to 50 μL of serum sample. The decrease in OD was recorded at 530 nm using a spectrophotometer (UV-2802S, Shimadzu, Kyoto, Japan) after 1 and 6 min at 25℃. A unit of lysozyme activity was defined as the amount of serum causing a reduction in absorbance of 0.001 units per min.
The total superoxide dismutase (T-SOD) activity was determined by an enzymatic assay method using a reagent kit (Jiancheng technology Co. Ltd., Nanjing, Jiangsu, China) as described by Sun et al. (2010). The alkaline phosphatase (AKP) and acid phosphatase (ACP) activities were assayed using AKP and ACP kits (Jiancheng technology Co. Ltd., Nanjing, Jiangsu, China) according to manufacturer's instruction.
2.5 Statistical analysisData are presented as mean±standard deviation (SD). One-way analysis of variance (ANONA) was employed for analysis of the data. Where significant differences (P < 0.05) were detected, the least significant difference (LSD) was used to separate the differences among treatment means. The software used for statistical analysis was SPSS, Version 19.0 (Chicago, IL, USA).
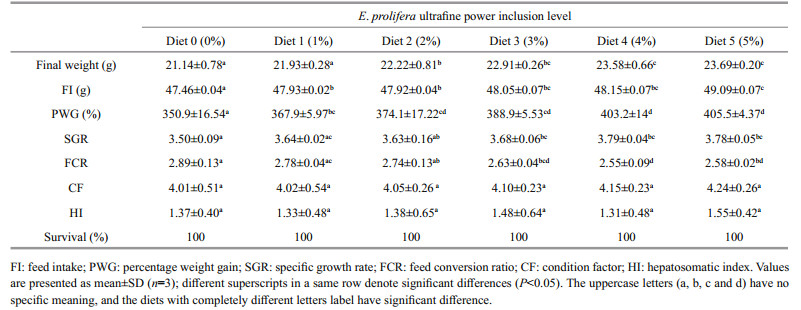
3 RESULT 3.1 Growth performanceThe growth performance of the red tilapia is shown in Table 2. The percentage weight gain (PWG), specific growth rate (SGR), condition factor (CF) and hepatosomatic index (HI) showed an overall trend of increasing with increased inclusion level of E. prolifera powder. In particular, the highest E. prolifera powder inclusion level of 5% (Diet 5) led to the highest increases in PWG (15.4%), SGR (8.0%) and HI (5.7%), respectively when compared to the control. Meanwhile, compared to that of the control, FCR decreased by 11.7% and 10.7% respectively when the fish were fed the diets with E. prolifera inclusion level at 4% (Diet 4) and 5% (Diet 5). Statistics analysis showed that PWG, SGR and FCR of the treatments in which the fish were fed the diets with E. prolifera powder inclusion level between 2% (Diet 2) and 5% (Diet 5) were all significantly better than the control (P < 0.05), however no significant difference in CF, HI and SR were detected for any experimental diets (P > 0.05) (Table 2).
|
The activities of gastrointestinal digestive enzymes are shown in Table 3. The results shown that when the red tilapia were fed the diets with increasing inclusion levels of E. prolifera ultrafine powder, the protease, amylase and lipase activities all showed upward trends, achieving peaks for the feeding treatment with the highest 5% E. prolifera inclusion level (Diet 5). Compared with the control, the pepsin and erepsin increased by 23.1% and 15.7%, respectively, the gastric amylase and the intestinal amylase increased by 33.3% and 53.3%, respectively, and the gastric lipase and the intestinal lipase increased by 16.3% and 28.2%, respectively, when the fish were fed this diet and the differences were statistically significant (P < 0.05).

|
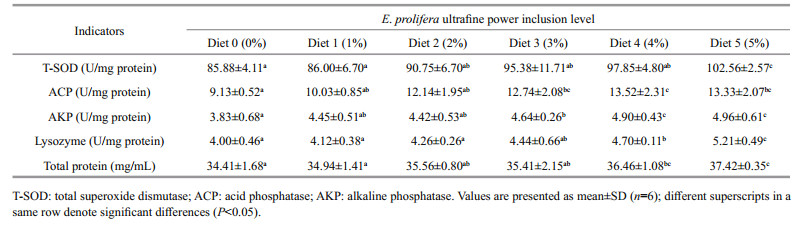
As shown in Table 4, incorporating E. prolifera ultrafine powder in the diets of the red tilapia enhanced serum non-specific immunity and an overall trend of improving with increasing E. prolifera inclusion level was shown. As the result, the T-SOD, ACP and AKP activities of the fish fed both the diets with E. prolifera inclusion level at 4% (Diet 4) and 5% (Diet 5) were significantly higher than the control (P < 0.05). Significantly improved lysozyme activities and serum total protein were also observed in the fish fed the diet with E. prolifera incorporated at 5% (Diet 5) (P < 0.05), which increased by 19.4%, 29.5%, 30.3% and 8.7%, respectively, as compared to the control (Table 4).
|
It is well known that E. prolifera is not only rich in major nutrients, but also contains high levels of various active ingredients—phenols, bioactive peptides, polysaccharide and terpenoids, for example (Chapman and Chapman, 1980; Oohusa, 1993; Zhou et al., 1995; Aguilera-Morales et al., 2005; Harnedy and FitzGerald, 2011). There have been several reports on effects of E. prolifera incorporated as a minor component of practical feeds on the growth of marine fish. For example, Asino et al. (2011) found that adding E. prolifera at 100 (10%) and 150 g/kg (15%) to the diets of juvenile yellow croaker, Pseudosciaena crocea, positive effects on growth performance and feed efficiency were shown. On the other hand, Yousif et al. (2004) showed that the feed efficiency and growth performance of the rabbitfish Siganus canaliculatus was decreased by a dietary 30 g/kg (3%) inclusion level of dehydrated Enteromorpha sp., however, the inclusion of 10 (1%) and 20 g/kg (2%) did not have significantly effect. Likewise, dietary inclusion at 10 (1%), 20 (2%) and 30 g/kg (3%) of Enteromorpha sp. showed no significant effects on growth performance of the bluespot grey mullet Valamugil seheli fry (Yousif, 2012). Besides, Yang et al. (2016) have reported that diets supplemented with 20, 30 and 40 g/kg fermented E. prolifera can similarly improve the growth performance, feed efficiency, digestive enzyme activities and non-specific immune response of red tilapia and the recommended dose of fermented E. prolifera is 37–41 g/kg in the diet of red tilapia.
In the present study, the PWG and SGR showed a clear trend of improvement with increasing supplementation level of E. prolifera ultrafine powder. A possible explanation for this consistent positive result could be that processing of E. prolifera as superfine power in our study enhanced the incorporation of positive components of E. prolifera into the diets, the solubility of such components was likely significantly increased, which led to better digestion and absorption by the fish. Tkacova and Stevulova (1998) reported that superfine grinding technology substantially enhanced dispensability and solubility of the materials.
Other reasons that likely contributed to the improved growth performance observed in this study include the enhanced digestive ability and nonspecific immunity of the red tilapia fed the diets incorporating E. prolifera ultrafine powder. Our results showed that the digestion ability of the red tilapia increased with the increase of inclusion level of E. prolifera ultrafine powder. There are reports that fish digestive enzyme activities are partially correlated to the changes in diet compositions (Glencross, 2009; Booth et al., 2013). Different dietary ingredients with key bioactive chemicals could lead to very different results (Stone et al., 2003). Enteromorpha Prolifera is known to contain many bioactive substances, such as saccharicterpenin, xylooligosaccharide and rich in dietary fiber, which may contribute to the enhanced secretion of digestive enzymes. The different structural units of polysaccharides has been reported to concentrate trace elements for forming functional substances, which could stimulate the gastrointestinal tissue and have biological protective effects on the enzymes from degradation (Kolkovski, 2001). Enteromopha prolifera ultrafine powder may also acted as a feed attractant, not only improved diet ingestion, but also stimulated the digestion of the feeds by the fish.
In aquatic animals, the specific immune mechanisms are diverse and could be immature while the nonspecific immune responses develop earlier and play an important role in the immune defense (Dalmo et al., 1997; Magnadóttir, 2006). It has been confirmed that marine macroalgae as feed additive can promote the stress resistance, immune responses and survival in fish, for instance, Enteromorpha sp., Ulva rigida and Chondrus crispus reportedly improved the respiratory activity of turbot phagocytes (Castro et al., 2004; Wassef et al., 2009, 2013). In this research, all nonspecific immune parameters measured was significantly higher in fish fed the diet containing 50 g/kg of E. prolifera powder (Diet 5) while the T-SOD, ACPandAKPactivities were also significantly higher in fish fed the diet containing 40 g/kg of E. prolifera powder (Diet 4), suggesting enhanced nonspecific immunoregulation in the fish (Saurabh and Sahoo, 2008; Van Muiswinkel and Nakao, 2014). Although the specific mechanisms need further investigation, the polysaccharides from E. prolifera maybe play a vital role in immunostimulation (Cho et al., 2010; Kim et al., 2011). In fact, it has been reported that polysaccharide of E. prolifera was able to significantly increase the immune activity of Chlamys nobilis in an in vitro study (Xu et al., 2005). Castro et al. (2004) reported that polysaccharides from E. prolifera can boost immunity of turbot by enhancing the vitality of neutrophils and macrophages. Besides, polysaccharides from E. prolifera can promote the humoral immunity, cellular immunity and mononuclear phagocytic system of mice Wei et al. (2014). Wei et al. (2015) has also recently reported that polysaccharide from E. prolifera enhanced nonspecific immune responses and protection against Vibrio splendidus infection in sea cucumber.
5 CONCULUSIONBased on the results from this study, at 5% inclusion level, E. prolifera ultrafine powder was shown to significantly improve the growth, immunity and digestive enzyme activities in the red tilapia with most of the measured parameters reached their peaks among different treatments. It is therefore recommended that the dose of E. prolifera ultrafine powder to be incorporated in the diets of the red tilapia should be 5% or 50 g/kg.
6 DATE AVAILABILITY STATEMENTThe datasets and materials supporting the conclusions of this article are included within the article.
7 ACKNOWLEDGMENTWe thank Dr. ZENG Chaoshu of College of Marine & Environmental Sciences, James Cook University, Australia for editing this manuscript.
Aguilera-Morales M, Casas-Valdez M, Carrillo-Domı́Nguez S, González-Acosta B, Pérez-Gil F. 2005. Chemical composition and microbiological assays of marine algae Enteromorpha spp. as a potential food source. J. Food Compos. Anal., 18(1): 79-88.
|
Asino H, Ai Q H, Mai K S. 2011. Evaluation of Enteromorpha prolifera as a feed component in large yellow croaker (Pseudosciaena crocea, Richardson, 1846) diets. Aquac. Res., 42(4): 525-533.
|
Booth M A, Allan G L, Smullen R P. 2013. Digestibility of common feed ingredients by juvenile mulloway Argyrosomus japonicus. Aquaculture, 414-415: 140-148.
|
Bricknell I, Dalmo R A. 2005. The use of immunostimulants in fish larval aquaculture. Fish & Shellfish Immunology, 19(5): 457-472.
|
Buddington R K. 1987. Does the natural diet influence the intestine's ability to regulate glucose absorption?. Journal of Comparative Physiology B, 157(5): 677-688.
DOI:10.1007/BF00700989 |
Castro R, Zarra I, Lamas J. 2004. Water-soluble seaweed extracts modulate the respiratory burst activity of turbot phagocytes. Aquaculture, 229(1-4): 67-68.
|
Chapman V J, Chapman D J. 1980. Seaweeds and Their Uses. Chapman and Hall, London. p.98-148.
|
Chiu A, Li L P, Guo S J, Bai J F, Fedor C, Naylor R L. 2013. Feed and fishmeal use in the production of carp and tilapia in China. Aquaculture, 414-415: 127-134.
|
Cho K, Vaught T G, Ji H, Gu D M, Papasakelariou-Yared C, Horstmann N, Jennings J M, Lee M, Sevilla L M, Kloc M, Reynolds A B, Watt F M, Brennan R G, Kowalczyk A P, McCrea P D. 2010. Xenopus Kazrin interacts with ARVCF-catenin, spectrin and p190B RhoGAP, and modulates RhoA activity and epithelial integrity. J. Cell Sci., 123(23): 4 128-4 144.
|
Dalmo R A, Ingebrigtsen K, Bøgwald J. 1997. Non-specific defence mechanisms in fish, with particular reference to the reticuloendothelial system (RES). J. Fish Dis., 20(4): 241-273.
DOI:10.1046/j.1365-2761.1997.00302.x |
Gatlin Ⅲ D M, Bai S C, Erickson M C. 1992. Effects of dietary vitamin E and synthetic antioxidants on composition and storage quality of channel catfish, Ictalurus punctatus. Aquaculture, 106(3-4): 323-332.
|
Glencross B. 2009. Ingredient evaluation in aquaculture: digestibility, utilisation and other key nutritional parameters. In: Burnell G, Allan G eds. New Technologies in Aquaculture: Improving Production Efficiency, Quality and Environmental Management. Woodhead Publishing Limited and CRC Press LCC, Cambridge, UK. p.387-416. https://www.sciencedirect.com/science/article/pii/B9781845693848500130
|
Harnedy P A, FitzGerald R J. 2011. Bioactive proteins, peptides, and amino acids from macroalgae. J. Phycol., 47(2): 218-232.
|
Hiraoka M, Dan A, Shimada S, Hagihira M, Migita M, Ohno M. 2003. Different life histories of Enteromorpha prolifera (Ulvales, Chlorophyta) from four rivers on Shikoku Island, Japan. Phycologia, 42(3): 275-284.
|
Jian J C, Wu Z H. 2003. Effects of traditional Chinese medicine on nonspecific immunity and disease resistance of large yellow croaker, Pseudosciaena crocea (Richardson). Aquaculture, 218(1-4): 1-9.
DOI:10.1016/S0044-8486(02)00192-8 |
Kim M J, Oh H J, Park J E, Kim G A, Hong S G, Jang G, Kwon M S, Koo B C, Kim T, Kang S K, Ra J C, Ko C, Lee B C. 2011. Generation of transgenic dogs that conditionally express green fluorescent protein. Genesis, 49(6): 472-478.
|
Kolkovski S. 2001. Digestive enzymes in fish larvae and juveniles-implications and applications to formulated diets. Aquaculture, 200(1-2): 181-201.
DOI:10.1016/S0044-8486(01)00700-1 |
Koren G, Bologa M, Pastuszak A. 1994. The way women perceive teratogenic risk: the decision to terminate pregnancy. In: Koren G ed. Maternal-Fetal Toxicology. 2nd edn. Marcel Dekker, Inc., New York. p.727-736.
|
Magnadóttir B. 2006. Innate immunity of fish (overview). Fish & Shellfish Immunology, 20(2): 137-151.
|
Michalak I, Chojnacka K. 2009. Edible macroalga Ulva prolifera as microelemental feed supplement for livestock: the fundamental assumptions of the production method. World Journal of Microbiology and Biotechnology, 25(6): 997-1.
DOI:10.1007/s11274-009-9976-7 |
Oohusa T. 1993. Recent trends in Nori products and markets in Asia. J. Appl. Phycol., 5(2): 155-159.
|
Rawling M D, Merrifield D L, Davies S J. 2009. Preliminary assessment of dietary supplementation of Sangrovit® on red tilapia (Oreochromis niloticus) growth performance and health. Aquaculture, 294(1-2): 118-122.
|
Richter H, Francis G, Becker K. 2002. A reassessment of the maintenance ration of red tilapia. Aquaculture International, 10(1): 1-9.
|
Romana-Eguia M R R, Eguia R V. 1999. Growth of five Asian red tilapia strains in saline environments. Aquaculture, 173(1-4): 161-170.
DOI:10.1016/S0044-8486(98)00484-0 |
Saurabh S, Sahoo P K. 2008. Lysozyme: an important defence molecule of fish innate immune system. Aquac. Res., 39(3): 223-239.
|
Stone D A J, Allan G L, Parkinson S, Frances J. 2003. Replacement of fishmeal in diets for Australian silver perch Bidyanus bidyanus (Mitchell). Ⅱ. Effects of cooking on digestibility of a practical diet containing different starch products. Aquac. Res., 34(3): 195-204.
|
Sun Y Z, Yang H L, Ma R L, Lin W Y. 2010. Probiotic applications of two dominant gut Bacillus strainswith antagonistic activity improved the growth performance and immune responses of grouper Epinephelus coioides. Fish Shellfish Immun., 29: 803-809.
|
Tkacova K, Stevulova N. 1998. Selected problems of the dispersity analysis of milled ultrafine powders. Freiberger Forschungshefte A, A841: 14-25.
|
Van Muiswinkel W B, Nakao M. 2014. A short history of research on immunity to infectious diseases in fish. Dev. Comp. Immunol., 43(2): 130-150.
DOI:10.1016/j.dci.2013.08.016 |
Wassef E A, El-Sayed A F M, Sakr E M. 2013. Pterocladia (Rhodophyta) and Ulva (Chlorophyta) as feed supplements for European seabass, Dicentrarchus labrax L., fry. Journal of Applied Phycology, 25(5): 1 369-1 376.
DOI:10.1007/s10811-013-9995-5 |
Wassef E A, Saleh N E, El-Abd El-Hady H A. 2009. Vegetable oil blend as alternative lipid resources in diets for gilthead seabream, Sparus aurata. Aquaculture International, 17(5): 421-435.
|
Wei J T, Wang S X, Liu G, Dong P, Liu Y F, Liu Y, Di D L. 2014. Polysaccharides from Enteromorpha prolifera enhance the immunity of normal mice. International Journal of Biological Macromolecules, 64: 1-5.
|
Wei S S, Wiens D A, Zha Y, Plank T, Webb S C, Blackman D K, Dunn R A, Conder J A. 2015. Seismic evidence of effects of water on melt transport in the Lau back-arc mantle. Nature, 518(7539): 395-398.
|
Xu D L, Huang X C, Ou C R, Xue C H, Yang W G, Wang H H. 2005. In vitro study on polysaccharides in Enteromorpha with non-specific immunity. Food Science, 26(10): 232-235.
(in Chinese with English abstract) |
Yang H, Li Z B, Chen Q, Li W J, Sun Y Z, Lu J. 2016. Effect of fermented Enteromopha prolifera on the growth performance, digestive enzyme activities and serum nonspecific immunity of red tilapia (Oreochromis mossambicus × Oreochromis niloticus). Aquac. Res., 47(12): 4 024-4 031.
|
Yao D R. 2011. Enteromorpha. Marine Press, Beijing, China. p.1-20, 106-147. (in Chinese)
|
Yousif E. 2012. Synthesis, spectroscopic studies and fungicidal activity of some diorganotin (Ⅳ) with 2-[(phenylcarbonyl) amino] propanoato. Journal of King Saud University - Science, 24(2): 167-170.
DOI:10.1016/j.jksus.2010.12.002 |
Yousif N J, Lifchez S D, Nguyen H H. 2004. Transverse rectus sheath plication in abdominoplasty. Plastic and Reconstructive Surgery, 114(3): 778-784.
|
Zeng C K, Zhang D R, Zhang J F. 1962. Chinese Economic Seaweed. Beijing Science and Technology Press, Beijing, China. p.43-50. (in Chinese)
|
Zhou G C, Bao Z Q, Dixon J E. 1995. Components of a new human protein kinase signal transduction pathway. J. Biol. Chem., 270(21): 12 665-12 669.
DOI:10.1074/jbc.270.21.12665 |
 2018, Vol. 36
2018, Vol. 36