Institute of Oceanology, Chinese Academy of Sciences
Article Information
- ZHU Xiaojuan(朱小娟), LUO Jie(罗杰), ZHOU Chengxu(周成旭), WANG Jianyuan(王建沅), MENG Ran(孟冉), XU Jilin(徐继林), CHEN Juanjuan(陈娟娟), LUO Qijun(骆其君), YAN Xiaojun(严小军)
- Changes of pigments and lipids composition in Haematococcus pluvialis vegetative cell as affected by monochromatic red light compared with white light
- Chinese Journal of Oceanology and Limnology, 36(6): 2257-2267
- http://dx.doi.org/10.1007/s00343-019-7195-0
Article History
- Received Jun. 28, 2017
- accepted in principle Aug. 8, 2017
- accepted for publication Nov. 14, 2017
2 Key Laboratory of Marine Biotechnology of Zhejiang Province, Ningbo University, Ningbo 315211, China;
3 Li Dak Sum Yip Yio Chin Kenneth Li Marine Biopharmaceutical Research Center, Ningbo University, Ningbo 315211, China;
4 Department of Marine Drug and Biological Products, Ningbo Institute of Oceanography, Ningbo 315832, China
Green freshwater microalga Haematococcus pluvialis is the most promising bioresource of the highest-quality astaxanthin, ketocarotenoid with antioxidant activity that has been applied in aquaculture, poultry, nutraceutical and pharmaceutical industries (Lorenz and Cysewski, 2000; Guerin et al., 2003; Li et al., 2011). Color change is prominent in this species and is crucial target in basic research and industry. In the life cycle of H. pluvialis, it includes green motile vegetative cell stage, intermediate stage, such as non-motile green cells or brown cells, and red haematocysts stage when astaxanthin is largely accumulated. During the cell morphological and color change process, cells respond actively to various environmental conditions through complex metabolic process. Because astaxanthin is the ultimate goal in H. pluvialis cultivation, much attention has been paid on studies of astaxanthin accumulation process. Study of the motile vegetative growth phase is much limited. However, vegetative cell growth is the most important part of the whole process and the biomass is critical to a successful culture in industry. Vegetative cell of H. pluvialis is especially susceptible to environmental factor changes, such as light, temperature, nutrients, etc. Once any kind of stress occurred, the cell division stopped or delayed. Through complex metabolic processes, the cells rearrange cellular structure and metabolism. Further stresses lead the cell into formation of aplanospore or haematocysts. This characteristic makes the bottleneck in astaxanthin industry because of the slow growth rate and low cell yields of the vegetative cells (Shah et al., 2016).
Vegetative cell of H. pluvialis is especially light sensitive, both to light intensity and quality. For example, accumulation of astaxanthin can be induced immediately after the start of illumination changes (Katsuda et al., 2004). The effect of light intensity has been extensively studied especially in astaxanthin accumulation process. Light quality also affects both cell growth and astaxanthin accumulation. Previous studies have been trying to find how to apply monochromatic illumination or assemblies to promote the growth rate and yields of vegetative cell (Katsuda et al., 2004; Fu et al., 2013). Light emitting diodes (LEDs) have been recognized as one of the effective light sources for microalgae cultivation and make it a promising protocol to regulate the growth and astaxanthin accumulation by different monochromatic lights or their assemblies (Schulze et al., 2014; Tran et al., 2015). Protocol that applying artificial assembling of different light quality coupled with other conditions to enhance goals of different stages was studied and proposed to use in industry. For example, Saha et al. (2013) tried nine stress conditions combined illumination and nutrition to test biomass and lipids production of a strain of H. pluvialis. Xi et al. (2016) suggested a strategy of wavelength shift, from red light in green cell culture to blue light in astaxanthin accumulation stage, to enhance both products in two-stage process. Wang et al. (2014) suggested a three-stage process which introduces aplanospore instead of vegetative cells to stress conditions. All these optimal final outcomes can be best achieved from maximum vegetative cell biomass. Red light was more effectively used in photosynthesis than white light (Jeon et al., 2006). Tran et al. (2015) suggested applying 1:3 ratio of mixed red-blue light to get the best growth and astaxanthin accumulation. Most of the studies focused on final biomass and astaxanthin products at the end of a cultivation. However, slow growth and low biomass of motile vegetative cells is still the main problem in industry. Monochromatic red light has been proposed being especially beneficial to the maintenance and growth of motile vegetative cells. However, cellular biochemical responses of these vegetative cells as affected by this optimal light quality are not fully understood.
For phototrophic microalga H. pluvialis, changes of photosynthetic pigments are essential in responses to light (Fu et al., 2013). Composition of pigments not only affects cell color, but also determines photosynthesis efficiency, growth rate, maximum cell yields and related metabolism process, such as photosynthetic membrane lipids. Ratios of the pigments are useful indicators to evaluate the cell conditions. For H. pluvialis, in addition to photosynthetic light-harvesting antennae chlorophyll a and b, β-carotene is not only a key accessory pigment in photosynthesis, it is also an important metabolic intermediate in astaxanthin biosynthesis and presents simultaneously in lipid drops with astaxanthin (Lotan and Hirschberg, 1995; Collins et al. 2011; Gao et al., 2013; Chen et al., 2015). For motile vegetative cells, metabolism process of β-carotene is probably related with astaxanthin because biosynthesis of astaxanthin also happened in vegetative cell (Kobayashi et al., 1992).
In photosynthetic process, cell pigment and lipids, both neutral lipids and polar lipids, are correlated with each other and the interactions are complex (Boussiba, 2000; Zhekisheva et al., 2005; Farré et al., 2015). Lipids constitute the basic skeleton of the photosynthetic membrane, whereas pigments, photosynthetic electron transfer-related protein complexes are embedded in a specific order in the photosynthetic membrane. The accumulation of cellular fatty acids is stoichiometricly correlated with the accumulation of astaxanthin, they are feedback- coordinated at the metabolite level (Chen et al., 2015). Neutral lipids, especially triacylglycerol (TAG), are essential for astaxanthin accumulation in H. pluvialis (Zhekisheva et al., 2002). TAG metabolism is part of primary cellular process. It integrates with polar lipids assembly, pigment and energy storage of the cell (Collins et al., 2011).
The aim of this study was to examine what significant responses happened when H. pluvialis motilevegetativecells were exposedtomonochromatic red light, which was proposed as an optimal light condition for this growth phase. Cultures under white light of the same intensity were set as control. Changes of pigment composition and lipids under red light and white light were comparatively studied.
2 MATERIAL AND METHOD 2.1 Haematococcus culture and maintenanceGreen microalga H. pluvialis 797 from the Freshwater Algae Culture Collection at the Institute of Hydrobiology, Chinese Academy of Sciences (Wuhan, China) was used in this study. The culture was grown in BG11 medium and maintained in the Microalgae Collection of Ningbo University, China. The maintenance conditions were 18℃ and 35 μmol photons/(m2·s) (D:L=12 h:12 h) of white fluorescent light.
2.2 Experimental setup:Culture was pre-cultivated in fresh BG11 medium under T3 fluorescent light with density of 50 μmol photons/(m2·s) (D:L=12 h:12 h) at 23±2℃. When it grew to late exponential phase, the culture was inoculated into fresh BG11 medium at initial density of 5×104 cells/mL in 5 000 mL Erlenmeyer flask. The final working volume was 4 000 mL. The flasks were placed under red and white light of the same intensity of 50 μmol photons/(m2·s) (D:L=12 h:12 h) at 20℃. Red light was achieved by assembling a panel of LEDs (650 nm) on the top of culture frame. Culture flasks were illuminated from the top. In order to make sure light intensity were the same, light intensity at the stand points on the frame table was measured using a quantum sensor before the flasks were placed on. Stand points of the same light intensity were marked and the culture flasks were place on the points after the inoculations. Three replicates for each light quality were prepared. The flasks were shaken manually two to three times each day. Flasks under same light quality were shifted randomly among the three stand points each day.
Samples for subsequent analysis of cell number (1 mL), chlorophyll fluorescence (3 mL), neutral lipid (1 mL) and pigments (100 mL) were collected every other day. Cell number was counted using plankton counting chamber (Beijing Purity Instrument Co., LTD, Beijing, China). Chlorophyll fluorescence was measured using pulse-amplitude-modulation fluorometer (Water-PAM WALZ, Germany) according the manufacturer's protocol.
2.3 Pigment analysisFor pigment analysis, 100 mL of the culture was sampled from each flask and centrifuged at 6 000 r/min at 4℃, the pellets were stored in -20℃ in dark until pigment extraction. At the end of the experiment, all the algae pellets were lyophilized. Chlorophyll a (Chl a), chlorophyll b (Chl b), and β-carotene (β-Car) were determined by liquid chromatography-mass spectrometry (LC-MS) according to the method of Wong and Wong (2003). HPLC-MS analysis was carried out in Finnigan Surveror and TSQ Quantum Access system (Thermo Fisher Scientific; USA) equipped with electrospray ionization (ESI) interfaced triple quadrupole mass spectrometer. All the operations, acquiring and analysis of data were processed by Xcalibur (Thermo Fisher Scientific, USA). Chl a was identified with chlorophyll-a standard (Sigma). The ratios of Chl a/Chl b and Chl a/β-Car were calculated on the basis of peak areas in the chromatogram.
2.4 Neutral lipid analysisFor cellular neutral lipid analysis, the method of Chen et al. (2009) was applied with modification. According to pre-experiment, 1 μL Nile Red (0.5 mg/mL, Sigma-Aldorich) was added into 240 μL of the culture on 96-well microtiter plate. Well mixed the cultures by shaking the plate. After the cells were stained for 10 min at 30℃ in dark, Fluorescent Index (FI) was measured by fluorescence spectrophotometer (Varioskan Flash, Thermo) with an excitation wavelength of 530 nm and an emission wavelength of 580 nm. Cultures without being stained were used as blank controls.
2.5 Lipid composition analysisFor lipid composition analysis, 2 000 mL of the cultures were harvested at day 5 by centrifugation at 6 000 r/min at 4℃ after simultaneously being placed on ice for several minutes until centrifugation. All the algal pellets were lyophilized and stored at -20℃ until analysis.
According to Li et al. (2014), the freeze-dried samples were extracted with chloroform: methanol (1:1, v/v) containing 0.05% butylated hydroxytoluene (BHT) and then dried in a rotary evaporator. The residues were dissolved in methanol and used for LC/MS analysis.
UPLC conditions: Reverse-phase analysis was performed on a Waters ACQUITY Ultra Performance LC system (UPLC) using an ACQUITY UPLC BEH C8 analytical column (2.1 mm×100 mm, 1.7 μm). To obtain efficient lipid separation, acetonitrile/isopropyl alcohol/tetrahydrofuran (1:1:1, v/v/v) was used as mobile phase A and water/acetonitrile (1:2, v/v) as mobile phase B; both contained 0.1% formic acid and 0.01% lithium acetate. The initial composition of mobile phase A was 2%, which then reached 80% in 23 min and remained stable for 5 min before returning to the initial 2% in 2 min and equilibrating for 5 min. A 1:4 split of the column effluent was used to achieve a flow rate of approximately 50 μL/min into the ESI source. The injection volume was 5 μL for each analysis.
Mass spectrometry conditions: Mass spectrometry was performed on a Waters Q-TOF Premier mass spectrometer using electron spraying ionization (ESI) in both negative and positive modes. The mass range was from 150 to 1 200 with a scanning duration of 0.3 s. The time-of-flight analyzer was used in V mode, and the lock mass spray for precise mass determination was set by leucine enkephalin.
Data processing: The raw data were obtained by Masslynx 4.1 software (Waters) from UPLC-Q-TOF-MS system and analyzed using the MarkerLynx 4.1 software (Waters). The MarkerLynx matrices, including peak numbers (based on the retention time and m/z), sample names and normalized peak intensity, were exported and analyzed by principal component analysis (PCA), the projection to latent structures with discriminant analysis (PLS-DA), and the orthogonal projection to latent structures with discriminant analysis (OPLS-DA) using SIMCA-P+ V12.0 software.
Lipid identification: Procedures for the identification of lipids (including photosynthetic glycerolipids and other phospholipids) were as described in previous studies (Xu et al., 2010; Yan et al., 2011, 2012)
Briefly, in positive mode, the characteristic ion [C9H16O6Li]+ and [C15H26O11Li]+ at m/z 227.09, 405.14, was for MGDG and DGDG detection respectively. In negative mode, the sulfoquinovosyl head group of [C6H9O7S]- at m/z 225.01 was used as the characteristic fragment ion of SQDG, and characteristic ions at m/z 227.03 [C6H12O7P]-, m/z 171.01 [C3H8O6P]- and m/z 153.00 [C3H6O5P]- were used for PG identification (Li et al., 2015). The rest of lipids were identified according to Su et al. (2013).
2.6 Data analysisAll data are expressed as the mean±standard deviation (SD). Data analysis and graphing were performed using Microsoft Excel 2010 (Microsoft Corp., Redmond, WA, USA) and data were subjected to an ANOVA analysis by SPSS software (version13.0, SPSS Inc., Chicago, IL, USA) using Duncan's test at the level of 5%.
3 RESULT 3.1 Changes in growth and cell densityThe whole proliferative process of H. pluvialis cells grew under red and white light is illustrated in Fig. 1. In 7 days since the inoculation, cell proliferation had no difference between red and white light groups (P > 0.05), showing cell division in logarithmic phase was independent of light quality. Growth rate averaged 0.27/d. However, from day 7 on, growth in white light group declined and cell number in white light reached plateau earlier. Finally, cell density of red light group was significantly higher than that of white light group (P < 0.05).
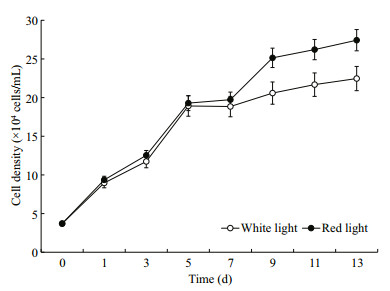
|
| Fig.1 Changes in Haematococcus pluvialis cell density of the white and red light group |
Changes in the chlorophyll fluorescence parameters measured on dark-adapted cells of H. pluvialis grew under red and white light are illustrated in Fig. 2. In 0–7 d, there were no significant differences in the parameters, such as maximal PSII quantum yield (Fv/Fm), electron transport rate (ETR), actual photochemical efficiency of PSII (ΦPSII), non- photochemical quenching (NPQ), and photochemical quenching (qP). In the later period (7–13 d), no significant difference was observed in term of Fv/Fm. However, significant differences existed in other chlorophyll fluorescence parameters, in which white light group had lower values than red light group.
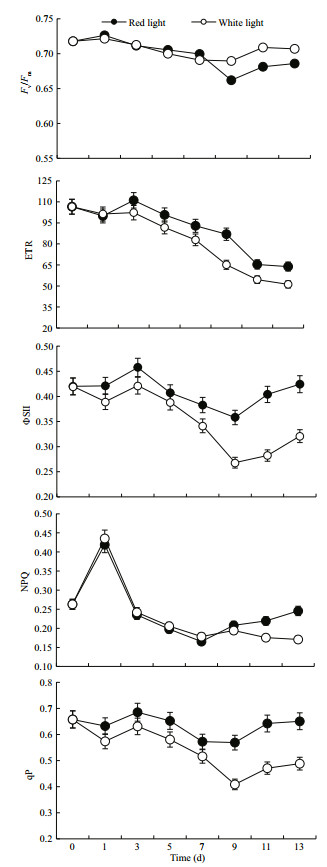
|
| Fig.2 Changes in chlorophyll fluorescence parameters when Haematococcus pluvialis grew under white and red light |
Change of ratios of key photosynthetic pigments, namely Chl a/Chl b (Ⅰ) and Chl a/β-Car (Ⅱ), were analyzed and as showed in Fig. 3. In the early growth phase (1–7 d), Chl a/Chl b ratio increased in both groups, from an initial ratio of 3.08 to the highest ratio of 8.7, and the ratio in red light group was lower than in white light group. After 7 days of cell proliferation, Chl a/Chl b in white light group decreased markedly to a final ratio of 3.1. However, Chl a/Chl b ratio in red light group continued increasing to a maximum of 8.7, where it remained till the end of the experiment (day 13). Chl a/β-Car ratio increased in both groups at the same rate, from an initial ratio of 20 to a final ratio of 39. After 7 d of a similar increase, Chl a/β-Car ratio in the white light group decreased markedly to 21 on day 13, whereas it remained stable at 35 in red light group.
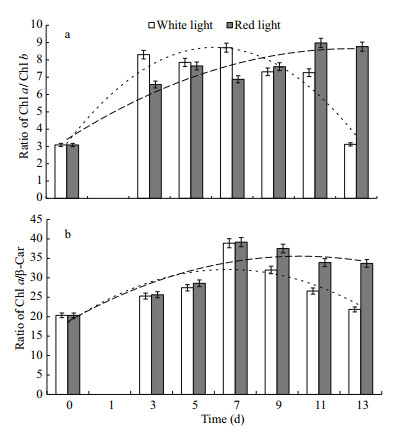
|
| Fig.3 Changes in ratio of key pigments of Haematococcus pluvialis cells during proliferation under different light qualities a: changes in ratio of chlorophyll a to chlorophyll b (Chl a/ Chl b); b. changes in ratio of chlorophyll a to β-carotene (Chl a/β-Car) during proliferation in red and white light. The dotted line indicates the trend of white light group, and the dashed line indicates the trend of red light group. |
Variation of neutral lipid during the cell proliferation under white and red light was obviously different as showed in Fig. 4. In white light group, content of neutral lipid decreased after 2 day's growth, and significantly declined further till day 5 when it came to the lowest point (P < 0.05). Afterwards, neutral lipid content increased gradually back to the initial level at day 11. In red light group, however, cellular neutral lipid kept unchanged at the same level without significant difference as the initial one during the growth period till day 11 (P > 0.05). At day 13, neutral lipid in both group increased to the higher level than the initial ones.
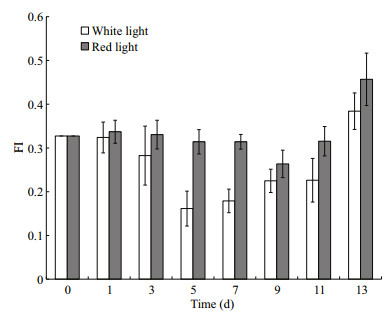
|
| Fig.4 Variation in neutral lipid by method of Nile Red staining, showed as FI (Nile Red fluorescence), in the cell of Haematococcus pluvialis during proliferating under red and white light Error bar: standard deviation. |
In order to detect differences of lipids composition in motile vegetative cells, after growing into exponential phase (5 d) under red and white light, a portion of the algal cells were harvested for lipid composition analysis, and the results were analyzed using MarkerLynx software. A total of 5 831 signal peaks were produced in positive ion mode, and 4 135 signal peaks were generated in negative ion mode. We normalized the signal intensity relative to the total intensity, and a principal component analysis (PCA) was then performed to obtain the plots of the first and second principal components for samples from the red and white light groups in the PI and NI modes (Fig. 5).
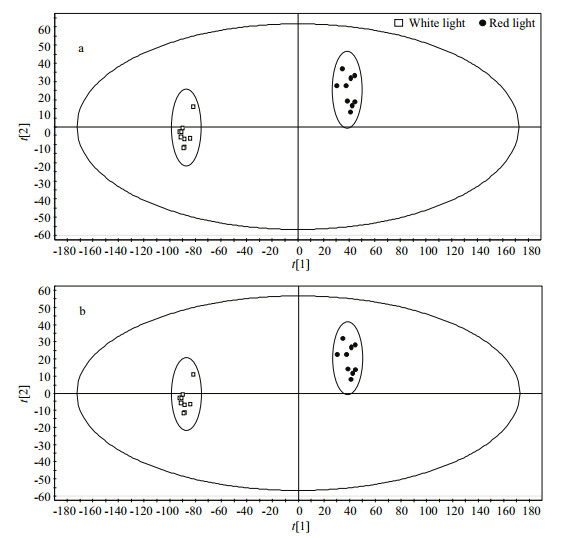
|
| Fig.5 PCA plots of the first and second principal components for the red and white light groups a. ESI+scan; b. ESI-scan. |
The two groups could be separated by both the first and second principal components (Fig. 5). Cross-validation in the PI and NI modes revealed that R2=0.31 and 0.369 (< 0.4), and Q2=-0.301 and -0.335 (< 0.05), respectively. These results indicated that both of the models established in the PI and NI ion modes were valid and that there were significant differences in the lipid composition of the vegetative cells growing under the two different light qualities.
Further analysis showed that, after removing members of the top 20 substances that were ranked in both the PI and NI modes, four lipid markers were obtainedinthe PImode: monogalactosyldiacylglycerol (MGDG), digalactosyldiacylglycerol (DGDG), triglycerides (TAG) and lyso-MGDG; four lipid markers were obtained in the NI mode: sulfoquinovosyldiacylglycerol (SQDG), phosphatidylglycerol (PG), 1, 2-diacylglyceryl-O-4'- (N, N, N-trimethyl) homoserine (DGTS) and Lyso-DGTS. Relative increases or decreases of these lipid markers in red light group versus white light group are shown in Fig. 6. Compared to white light group, lipids that decreased (in percentage) in abundance in red light groups were MGDG (decreased 59.8%), Lyso-MGDG (decreased 98.0%), DGDG (decreased 68.1%), SQDG (decreased 29.2%), Lyso-DGTS (decreased 30.6%), and PG was not detectable in red light group. Lipids that increased in abundance in cells in red light group were DGTS (increased 109%) and TAG (increased 1634%).

|
| Fig.6 The difference in major lipid markers in Haematococcus pluvialis cells between red light group and white light group MGDG: monogalactosyldiacylglycerol; DGDG: digalactosyldiacylglycerol; SQDG: sulfoquinovosyldiacylglycerol; PG: phosphatidylglycerol; TAG: triglycerides; DGTS: diacylglyceryltrimethylhomoserine; Lyso-DGTS: Lyso-diacylglyceryltrimethylhomoserine; Lyso-MGDG: Lyso-monogalactosyldiacylglycerol. Bar error: standard deviation. |
Vegetative cells of H. pluvialis are especially sensitive to various changes of environmental conditions. The cells have high environmental acclimation efficiency. The acclimation of vegetative cells is accomplished with regulation of light harvesting system, non-photochemical quenching and eliminating ROS systems (Foyer and Shigeoka 2011; Demmig-Adams et al., 2012; Han et al., 2012). However, these abilities are limited. Once the cell experiencing high intensity stress, it stops division and changes into anti-stress metabolic state. Slow growth rate and low cell concentration are the major problems at this stage in industry.
The present study especially focused on motile vegetative cells. The growth of the motile vegetative cell was suppressed in white light group earlier than that in red light group, which caused the final biomass difference. The turning point was evident from changes of chlorophyll fluorescence parameters. After 7 days' growth, ΦPSII and qP, which reflects the proportion of opened PSII reaction center, of red light group were higher than white light group (P < 0.05). It showed that the cells in red light group maintained higher photosynthetic activity compared with white light group (Bilger and Schreiber, 1986; Genty et al., 1989). In the later period of growth (9 day), the lower NPQ in white light group (P < 0.05) revealed that the cells are experiencing stress when compared with red light group (Bilger and Björkman, 1990).
Ratios of the pigments are useful indicators to evaluate the cell conditions. Chl a is central photosynthetic chlorophyll, and Chl b helps increase the range of light in photosynthesis. Carotenoids assist photosynthesis and protect cells from free radicals via continuous removal of extra electrons in the meanwhile (Lemoine and Schoefs, 2010). Ratio of Chl a/Chl b is an important parameter for green microalgae in response to different irradiance. Ratio of chlorophyll to carotenoid is always used to evaluate the stress that the cell undertaking. In this study, value of Chl a/Chl b was in the same range as reported by Wang et al. (2014). The highest ratio was approximately 8, much higher than in most microalgae. Changes of increase or decrease of Chl a/Chl b ratio in aquatic algae are species specific in responding to different light intensity (Yamazaki et al., 2005; Beneragama and Goto, 2010; Wang et al., 2014). Light quality also affected the variation in pigment composition during the growth period. In the present study, Chl a/Chl b ratio increased in both red and white light group at exponential growth phase. Chl a/Chl b ratio of vegetative cells growing under white light had higher value than that under red light. However, Chl a/Chl b ratio in red light group increased stably till end of the growth without sharp change, while it decreased significantly in white light group after the culture grew into stationary phase. This difference is in accordance with that of Chl a/β-Car ratio which decreased in white light group when the culture grew into stationary phase. In the study of Saha et al. (2013), contents of the pigments such as Chl a and carotenoids had no significant differences during green vegetative growth phase under nine different illumination conditions. Significant changes occurred only when the cultures grew into astaxanthin accumulation phase when carotenoids increased significantly and Chl a decreased in the mean while. The extents of the changes were different in nine illumination conditions. In the PSII reaction center, β-carotene helps to deactivate singlet oxygen (Telfer, 2002). For H. pluvialis, β-carotene is also precursor of astaxanthin, it can be metabolized into astaxanthin through complex biochemical process in H. pluvialis (Chen et al., 2015). In the present study, decrease of Chl a/β-Car ratio indicated that, when compared with red light group, cells of white light group experienced relatively higher stress. The variation of major pigment ratio during the vegetative growth may be used as a monitoring index to adjust protocol of irradiance to maximize vegetative cell growth and final biomass.
Accumulation of neutral lipids normally is considered storage lipid when cell experiencing stress. But it is not always this case. For example, lipid content in diatom Phaeodactylum tricornutum increased when phosphorus limited while it decreased in green algae Nannochloris atomus and Tetraselmis sp. (Reitan et al., 1994). In the present study, neutral lipid varied during the vegetative cell growth and the variation extent and pattern was different in red and white light group. Especially in white light group during exponential growth, it decreased significantly showing that cell was consuming neutral lipids during this process. These variations were inversely correlated with the variation of major pigment ratios (Fig. 7). In red light group, the variations came later compared with white light group and the values showed no significant difference.
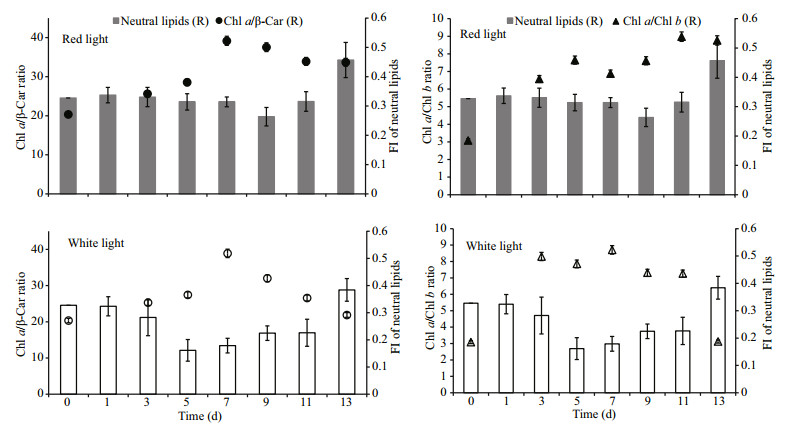
|
| Fig.7 Synchronous variation of neutral lipid and photosynthetic pigment composition of H. pluvialis cultured under red and white light Column: relative content of neutral lipid; dot: ratio of Chl a/β-Car; triangle: ratio of Chl a/Chl b. |
During exponential growth phase under white light, TAG content was lower than in red light group. This phenomenon was in accordance with the variation of cellular neutral lipid during the growth: neutral lipid in white light group decreased while that in red light group kept the initial level without significant change. The response of neutral lipid to these two light qualities may be indicative of a kind of metabolic acclimation. In white light group, decrease of TAG or neutral lipid was accompanied with increase of main photosynthetic membrane lipids, such as MGDG. TAG can be metabolized into MGDG by supplying acyl groups for the biosynthesis of MGDG (Khozin-Goldberg et al., 2000). The concordant changes of neutral lipid and major polar lipids in the present study may due to this metabolic process. TAG primarily participates in synthesizing cartenoids or astaxanthin according many studies (Zhekisheva et al., 2002; Lemoine and Schoefs, 2010). In most cases it refers to conditions when H. pluvialis cell was exposed to high light or nitrogen stresses. Under high irradiance, both TAG and DGTS increased (Gwak et al., 2014). However, the inhibition of secondary carotenogenesis does not lead to the cessation of TAG accumulation (Zhekisheva et al., 2005).
Synthesis of cartenoids and lipids are coordinated with each other in H. pluvialis when it is under stresses (Boussiba, 2000; Zhekisheva et al., 2002; Wang et al., 2003). Photosynthetic membrane lipids were observed changing significantly between green cells and haematocysts. Astaxanthin accumulation cells of H. pluvialis are metabolically active than the motile vegetative cells (Gwak et al., 2014). No previous studies worked on the lipid changes in H. pluvialis motile vegetative cells when affected by light quality. Even though there were no significant differences between red or white light groups in terms of cell number, the differences of lipids were evident. The abundances of typical photosynthetic membrane lipids (MGDG, DGDG, SQDG and PG) were lower in red light than that in white light group. These plastidic membrane lipids are the major components of chloroplast thylakoid membranes, and they play major roles in the structure and function of those membranes (Kobayashi et al., 2007; Mizusawa et al., 2008). Glycolipids such as MGDG, DGDG and SQDG form a majority of thylakoid membrane and also provide a phospholipid bilayer matrix for photosynthetic complexes as the main constituents (Kobayashi, 2016). PG is the only major phospholipid found in thylakoid membranes, plays important roles in photosynthesis and is the important structure in PSII complex (Wada and Murata, 2007). However, considering the facts that there was no significant difference in term of photosynthetic fluorescence parameters in the exponential growth period in red and white light group, the changes of these photosynthetic membrane lipids showed no impacts on photosynthetic activity. In fact, study of Aronsson et al. (2008) showed that when the reduction of MGDG was 40%, there was no impact on PSII activity. Compared with the white light group, about 60% decrease of MGDG in red light group did not affected photosynthetic activity.
Diacylglyceryltrimethylhomoserine (DGTS) is the membrane glycerolipids outside the chloroplast. Triglycerides (TAG) are the main energy-reserving lipids in cells. Unlike the other chloroplastidic membrane lipids, DGTS and TAG significantly increase in red light groups when compared with that in white light groups (P < 0.05). In other words, considering monochromatic red light as the most optimal light source for motile vegetative cell growth, in polychromatic white light source, amounts of lipids outside chloroplast such as DGTS and storage lipid such as TAG decreased while major chloroplastidic membrane lipids increased. Astaxanthin is synthesized in extraplastidic endoplasmic reticulum (Chen et al., 2015). However, β-carotene, intermediate in astaxanthin synthesis, is synthesized in the chloroplast of H. pluvialis. Most membrane systems take part in biochemical material trafficking. Whether photosynthetic membrane lipids contribute to the transportation of β-carotene from chloroplast to extraplastidic compartments need further demonstrations.
In literatures, different strains of H. pluvialis are highly diverse in growth ability and astaxanthin productivity. The relative proportion of major lipids was different in strain of our study from that in Wang et al. (2014) strain in which MGDG was the most abundant membrane glycerolipid. It is worth studies that are the difference of lipids composition and modulation ability of H. pluvialis related to growth and astaxanthin accumulation potency? Whether those of so called good strains in culture or industry have the best proportion of the critical lipids? Because astaxanthin accumulation cells of H. pluvialis, such as "brown" cells or red cysts, are highly metabolically active and complex (Gwak et al., 2014), growing H. pluvialis under red light may be a good way to get homogeneous motile vegetative cells to study and answer these questions.
5 CONCLUSIONIn summary, motile vegetative cell of H. pluvialis will modulate pigment composition, chloroplastidic membrane lipids as well as extraplastidic lipids in respond to the light quality changes. These acclimation processes have prolonged effects such as prolonged cell proliferation time and higher final cell yields when the culture was under monochromatic red light. For vegetative cells, growing under monochromatic red light other than white light showed less anti-stress characteristics and the cells maintained at biflagellated vegetative cells state for longer time.
6 DATA AVAILABILITY STATEMENTThe authors declare that the data supporting the findings of this study are available within the article.
Aflalo C, Meshulam Y, Zarka A, Boussiba S. 2007. On the relative efficiency of two-vs. one-stage production of astaxanthin by the green alga Haematococcus pluvialis. Biotechnology and Bioengineering, 98(1): 300-305.
DOI:10.1002/(ISSN)1097-0290 |
Aronsson H, Schöttler M A, Kelly A A, Sundqvist C, Dörmann P, Karim S, Jarvis P. 2008. Monogalactosyldiacylglycerol deficiency in Arabidopsis affects pigment composition in the prolamellar body and impairs thylakoid membrane energization and photoprotection in leaves. Plant Physiology, 148(1): 580-592.
DOI:10.1104/pp.108.123372 |
Beneragama C K, Goto K. 2010. Chlorophyll a:b Ratio Increases Under Low-light in 'shade-tolerant' Euglena gracilis. Tropical Agricultural Research, 22(1): 12-25.
|
Bilger W, Björkman O. 1990. Role of the xanthophyll cycle in photoprotection elucidated by measurements of lightinduced absorbance changes, fluorescence and photosynthesis in leaves of Hedera canariensis. Photosynthesis Research, 25(3): 173-185.
DOI:10.1007/BF00033159 |
Bilger W, Schreiber U. 1986. Energy-dependent quenching of dark-level chlorophyll fluorescence in intact leaves. Photosynthesis Research, 10(3): 303-308.
DOI:10.1007/BF00118295 |
Boussiba S. 2000. Carotenogenesis in the green alga Haematococcus pluvialis:cellular physiology and stress response. Physiologia Plantarum, 108(2): 111-117.
DOI:10.1034/j.1399-3054.2000.108002111.x |
Chen G Q, Wang B B, Han D X, Sommerfeld M, Lu Y H, Chen F, Hu Q. 2015. Molecular mechanisms of the coordination between astaxanthin and fatty acid biosynthesis in Haematococcus pluvialis (Chlorophyceae). The Plant Journal, 81(1): 95-107.
DOI:10.1111/tpj.12713 |
Chen W, Zhang C W, Song L R, Sommerfeld M, Hu Q. 2009. A high throughput Nile red method for quantitative measurement of neutral lipids in microalgae. Journal of Microbiological Methods, 77(1): 41-47.
DOI:10.1016/j.mimet.2009.01.001 |
Collins A M, Jones H D T, Han D X, Hu Q, Beechem T E, Timlin J A. 2011. Carotenoid distribution in living cells of Haematococcus pluvialis (Chlorophyceae). PLoS One, 6(9): e24302.
DOI:10.1371/journal.pone.0024302 |
Demmig-Adams B, Cohu C M, Muller O, Adams Ⅲ W W. 2012. Modulation of photosynthetic energy conversion efficiency in nature:from seconds to seasons. Photosynthesis Research, 113(1-3): 75-88.
DOI:10.1007/s11120-012-9761-6 |
Fábregas J, Domínguez A, Regueiro M, Maseda A, Otero A. 2000. Optimization of culture medium for the continuous cultivation of the microalga Haematococcus pluvialis. Applied Microbiology and Biotechnology, 53(5): 530-535.
DOI:10.1007/s002530051652 |
Fábregas J, Otero A, Maseda A, Domínguez A. 2001. Twostage cultures for the production of Astaxanthin from Haematococcus pluvialis. Journal of Biotechnology, 89(1): 65-71.
|
Farré G, Twyman R M, Christou P, Capell T, Zhu C F. 2015. Knowledge-driven approaches for engineering complex metabolic pathways in plants. Current Opinion in Biotechnology, 32: 54-60.
DOI:10.1016/j.copbio.2014.11.004 |
Foyer C H, Shigeoka S. 2011. Understanding oxidative stress and antioxidant functions to enhance photosynthesis. Plant Physiology, 155(1): 93-100.
DOI:10.1104/pp.110.166181 |
Fu W Q, Guðmundsson Ó, Paglia G, Herjólfsson G, Andrésson Ó S, Palsson B Ø, Brynjólfsson S. 2013. Enhancement of carotenoid biosynthesis in the green microalga Dunaliella salina with light-emitting diodes and adaptive laboratory evolution. Applied Microbiology and Biotechnology, 97(6): 2 395-2 403.
DOI:10.1007/s00253-012-4502-5 |
Gao Z Q, Meng C X, Gao H Z, Zhang X W, Xu D, Su Y F, Wang Y Y, Zhao Y R, Ye N H. 2013. Analysis of mRNA expression profiles of carotenogenesis and astaxanthin production of Haematococcus pluvialis under exogenous 2, 4-epibrassinolide (EBR). Biological Research, 46(2): 201-206.
DOI:10.4067/S0716-97602013000200012 |
Genty B, Briantais J M, Baker N R. 1989. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochimica et Biophysica Acta (BBA)-General Subjects, 990(1): 87-92.
DOI:10.1016/S0304-4165(89)80016-9 |
Guerin M, Huntley M E, Olaizola M. 2003. Haematococcus astaxanthin:applications for human health and nutrition. Trends in Biotechnology, 21(5): 210-216.
DOI:10.1016/S0167-7799(03)00078-7 |
Gwak Y, Hwang Y S, Wang B B, Kim M, Jeong J, Lee C G, Hu Q, Han D X, Jin E S. 2014. Comparative analyses of lipidomes and transcriptomes reveal a concerted action of multiple defensive systems against photooxidative stress in Haematococcus pluvialis. Journal of Experimental Botany, 65(15): 4 317-4 334.
DOI:10.1093/jxb/eru206 |
Han D X, Wang J F, Sommerfeld M, Hu Q. 2012. Susceptibility and protective mechanisms of motile and non-motile cells of Haematococcus pluvialis (Chlorophyceae) to photooxidative stress. Journal of Phycology, 48(3): 693-705.
DOI:10.1111/j.1529-8817.2012.01147.x |
Jeon Y C, Cho C W, Yun Y S. 2006. Oxygen evolution rate of photosynthetic microalga Haematococcus pluvialis depending on light intensity and quality. Studies in Surface Science and Catalysis, 159: 157-160.
DOI:10.1016/S0167-2991(06)81557-0 |
Katsuda T, Lababpour A, Shimahara K, Katoh S. 2004. Astaxanthin production by Haematococcus pluvialis under illumination with LEDs. Enzyme and Microbial Technology, 35(1): 81-86.
DOI:10.1016/j.enzmictec.2004.03.016 |
Khozin-Goldberg I, Yu H Z, Adlerstein D, Didi-Cohen S, Heimer Y M, Cohen Z. 2000. Triacylglycerols of the red microalga Porphyridium cruentum can contribute to the biosynthesis of eukaryotic galactolipids. Lipids, 35(8): 881-889.
DOI:10.1007/S11745-000-0597-8 |
Kobayashi K, Kondo M, Fukuda H, Nishimura M, Ohta H. 2007. Galactolipid synthesis in chloroplast inner envelope is essential for proper thylakoid biogenesis, photosynthesis, and embryogenesis. Proceedings of the National Academy of Sciences of the United States of America, 104(43): 17 216-17 221.
DOI:10.1073/pnas.0704680104 |
Kobayashi K. 2016. Role of membrane glycerolipids in photosynthesis, thylakoid biogenesis and chloroplast development. Journal of Plant Research, 129(4): 565-580.
DOI:10.1007/s10265-016-0827-y |
Kobayashi M, Kakizono T, Nishio N, Nagai S. 1992. Effects of light intensity, light quality, and illumination cycle on astaxanthin formation in a green alga, Haematococcus pluvialis. Journal of Fermentation and Bioengineering, 74(1): 61-63.
DOI:10.1016/0922-338X(92)90271-U |
Lemoine Y, Schoefs B. 2010. Secondary ketocarotenoid astaxanthin biosynthesis in algae:a multifunctional response to stress. Photosynthesis Research, 106(1-2): 155-177.
DOI:10.1007/s11120-010-9583-3 |
Li J, Zhu D L, Niu J F, Shen S D, Wang G C. 2011. An economic assessment of astaxanthin production by large scale cultivation of Haematococcus pluvialis. Biotechnology Advances, 29(6): 568-574.
DOI:10.1016/j.biotechadv.2011.04.001 |
Li S, Xu J L, Chen J, Chen J J, Zhou C X, Yan X J. 2014. Structural elucidation of co-eluted triglycerides in the marine diatom model organism Thalassiosira pseudonana by ultra-performance liquid chromatography/quadrupole time-of-flight mass spectrometry. Rapid Communications in Mass Spectrometry, 28(3): 245-255.
DOI:10.1002/rcm.6784 |
Li S, Xu J L, Jiang Y, Zhou C X, Yu X J, Zhou Y Y, Chen J J, Yan X J. 2015. Lipidomic analysis can distinguish between two morphologically similar strains of Nannochloropsis oceanica. Journal of Phycology, 51(2): 264-276.
DOI:10.1111/jpy.12271 |
Lorenz R L, Cysewski G R. 2000. Commercial potential for Haematococcus microalgae as a natural source of astaxanthin. Trends in Biotechnology, 18(4): 160-167.
DOI:10.1016/S0167-7799(00)01433-5 |
Lotan T, Hirschberg J. 1995. Cloning and expression in Escherichia coli of the gene encoding β-C-4-oxygenase, that converts β-carotene to the ketocarotenoid canthaxanthin in Haematococcus pluvialis. FEBS Letters, 364(2): 125-128.
DOI:10.1016/0014-5793(95)00368-J |
Mizusawa N, Sakurai I, Kubota H, Wada H. 2008. Role of phosphatidylglycerol in oxygen-evolving complex of photosystem Ⅱ. In: Allen J F, Gantt E, Golbeck J H, Osmond B eds. Photosynthesis. Energy from the Sun.Springer, Berlin. p.463-466. http://www.springerlink.com/content/fulltext.pdf?id=doi:10.1007/978-1-4020-6709-9_104
|
Reitan K I, Rainuzzo J R, Olsen Y. 1994. Effect of nutrient limitation on fatty acid and lipid content of marine microalgae. Journal of Phycology, 30(6): 972-979.
DOI:10.1111/j.0022-3646.1994.00972.x |
Saha S K, McHugh E, Hayes J, Moane S, Walsh D, Murray P. 2013. Effect of various stress-regulatory factors on biomass and lipid production in microalga Haematococcus pluvialis. Bioresource Technology, 128: 118-124.
DOI:10.1016/j.biortech.2012.10.049 |
Schulze P S C, Barreira L A, Pereira H G C, Perales J A, Varela J CS. 2014. Light emitting diodes (LEDs) applied to microalgal production. Trends in Biotechnology, 32(8): 422-430.
DOI:10.1016/j.tibtech.2014.06.001 |
Shah M M R, Liang Y M, Cheng J J, Daroch M. 2016. Astaxanthin-producing green microalga Haematococcus pluvialis:from single cell to high value commercial products. Frontiers in Plant Science, 7: 531.
|
Su X L, Xu J L, Yan X J, Zhao P, Chen J J, Zhou C X, Zhao F, Li S. 2013. Lipidomic changes during different growth stages of Nitzschia closterium f. minutissima.Metabolomics, 9(2): 300-310.
DOI:10.1007/s11306-012-0445-1 |
Telfer A. 2002. What is β-carotene doing in the photosystem Ⅱ reaction centre?. Philosophical Transactions:Biological Sciences, 357(1426): 1 431-1 440.
DOI:10.1098/rstb.2002.1139 |
Tran H L, Lee K H, Hong C H. 2015. Effects of LED irradiation on the growth and astaxanthin production of Haematococcus lacustris. Biosciences Biotechnology Research Asia, 12(2): 1 167-1 173.
DOI:10.13005/bbra/1769 |
Wada H, Murata N. 2007. The essential role of phosphatidylglycerol in photosynthesis. Photosynthesis Research, 92(2): 205-215.
DOI:10.1007/s11120-007-9203-z |
Wang B B, Zhang Z, Hu Q, Sommerfeld M, Lu Y H, Han D X. 2014. Cellular capacities for high-light acclimation and changing lipid profiles across life cycle stages of the green alga Haematococcus pluvialis. PLoS One, 9(9): e106679.
DOI:10.1371/journal.pone.0106679 |
Wang B, Zarka A, Trebst A, Boussiba S. 2003. Astaxanthin accumulation in Haematococcus pluvialis (Chlorophyceae)as an active photoprotective process under high irradiance. Journal of Phycology, 39(6): 1 116-1 124.
DOI:10.1111/j.0022-3646.2003.03-043.x |
Wong C K, Wong C K. 2003. HPLC pigment analysis of marine phytoplankton during a red tide occurrence in Tolo Harbour, Hong Kong. Chemosphere, 52(9): 1 633-1 640.
DOI:10.1016/S0045-6535(03)00503-4 |
Xi T Q, Kim D G, Roh S W, Choi J S, Choi Y E. 2016. Enhancement of astaxanthin production using Haematococcus pluvialis with novel LED wavelength shift strategy. Applied Microbiology and Biotechnology, 100(14): 6 231-6 238.
DOI:10.1007/s00253-016-7301-6 |
Xu J L, Chen D Y, Yan X J, Chen J J, Zhou C X. 2010. Global characterization of the photosynthetic glycerolipids from a marine diatom Stephanodiscus sp. by ultra performance liquid chromatography coupled with electrospray ionization-quadrupole-time of flight mass spectrometry. Analytica Chimica Acta, 663(1): 60-68.
DOI:10.1016/j.aca.2010.01.026 |
Yamazaki J A, Suzuki T, Maruta E, Kamimura Y. 2005. The stoichiometry and antenna size of the two photosystems in marine green algae, Bryopsis maxima and Ulva pertusa, in relation to the light environment of their natural habitat. Journal of Experimental Botany, 56(416): 1 517-1 523.
DOI:10.1093/jxb/eri147 |
Yan X J, Chen D Y, Xu J L, Zhou C X. 2011. Profiles of photosynthetic glycerolipids in three strains of Skeletonema determined by UPLC-Q-TOF-MS. Journal of Applied Phycology, 23(2): 271-282.
DOI:10.1007/s10811-010-9553-3 |
Yan X J, Xu J L, Chen J J, Chen D Y, Xu S L, Luo Q J, Wang Y J. 2012. Lipidomics focusing on serum polar lipids reveals species dependent stress resistance of fish under tropical storm. Metabolomics, 8(2): 299-309.
DOI:10.1007/s11306-011-0307-2 |
Zhekisheva M, Boussiba S, Khozin-Goldberg I, Zarka A, Cohen Z. 2002. Accumulation of oleic acid in Haematococcus pluvialis (Chlorophyceae) under nitrogen starvation or high light is correlated with that of astaxanthin esters. Journal of Phycology, 38(2): 325-331.
DOI:10.1046/j.1529-8817.2002.01107.x |
Zhekisheva M, Zarka A, Khozin-Goldberg I, Cohen Z, Boussiba S. 2005. Inhibition of Astaxanthin synthesis under high irradiance does not abolish triacylglycerol accumulation in the green alga Haematococcus pluvialis(Chlorophyceae). Journal of Phycology, 41(4): 819-826.
DOI:10.1111/jpy.2005.41.issue-4 |
 2018, Vol. 36
2018, Vol. 36


