Institute of Oceanology, Chinese Academy of Sciences
Article Information
- LIU Yibing(刘一兵), LIU Shiwen(刘诗文), LIU Bingli(刘冰莉), QIN Jianguang(秦建光), XU Tong(许通), LI Xiaoxu(李孝绪)
- Cryopreservation of strip spawned sperm using programmable freezing technique in the blue mussel Mytilus galloprovincialis
- Chinese Journal of Oceanology and Limnology, 36(6): 2351-2357
- http://dx.doi.org/10.1007/s00343-019-7252-8
Article History
- Received Sep. 4, 2017
- accepted in principle Oct. 18, 2017
- accepted for publication Dec. 19, 2017
2 College of Science and Engineering, The Flinders University of South Australia, Adelaide, South Australia 5042, Australia;
3 College of Fisheries and Life Science, Dalian Ocean University, Dalian 116023, China;
4 South Australian Research and Development Institute, West Beach, South Australia 5024, Australia
The blue mussel, Mytilus galloprovincialis is one of the most important aquaculture species in the world (Liu et al., 2016a). In order to maintain its long-term sustainable development and competitive advantages, techniques that could assist all year-round hatchery production or genetic improvement programmes have become a major interest to the industry (Pettersen et al., 2010; Lazo and Pita, 2012). Sperm cryopreservation has been considered as an effective technique that would achieve these goals and has been largely investigated in oysters and abalone (Hassan et al., 2015; Liu et al., 2015a). In M. galloprovincialis, sperm cryopreservation has been investigated by Matteo et al. (2009) and Liu et al. (2016a). In the former, although approximately 65% fertilization rate was achieved with sperm spawned naturally, this method of sperm collection could compromise sperm quality due to potential contamination (such as mucus, seawater and feces), thus resulting in low post-thaw sperm quality (Paniagua-Chavez et al., 1998; Gwo et al., 2002; Dong et al., 2005). In the latter, on the other hand, although 90% fertilization rate has been achieved with sperm collected by strip spawned method, the non-programmable freezing technique is generally less reliable in comparison with the programmable freezing technique (Clulow et al., 2008). For example, in comparison with the nonprogrammable freezing technique, better post-thaw sperm qualities, such as motility and/or plasma membrane integrity, have achieved with programmable freezing technique in dog (Rota et al., 2005), human (Stanic et al., 2000), stallion (Clulow et al., 2008) and honey bee, Api mellifera (Hopkins and Herr, 2010).These advantages usually make the programmable freezing technique as the preferential selection for the application of cryopreservation in breeding programmes and semen cryobank establishments (Stanic et al., 2000; Clulow et al., 2008; Tiersch, 2008). Nevertheless, the programmable freezing technique has not been evaluated to cryopreserve strip spawned sperm in the M. galloprovincialis.
Studies in livestock and aquatic species have demonstrated that the addition of sugar is an effective strategy to improve the quality of post-thaw sperm (Lyons et al., 2005; Purdy, 2006; Cabrita et al., 2010; Liu et al., 2014a, 2015a), and has been applied in rams (Jafaroghli et al., 2011), boars (GómezFernández et al., 2012), black-lip pearl oysters, Pinctada margaritifera (Lyons et al., 2005), farmed greenlip abalone, Haliotis laevigata and blacklip abalone, H. rubra (Liu et al., 2014a, 2015b).
The integrity of sperm components and organelles is of importance for sperm to perform their functions and can be evaluated under fluorescent microscopy using specific fluorescence dyes (Zhang et al., 2012; Liu et al., 2016a). This staining technique has been applied in various species to provide detailed information for sperm quality assessment in cryopreservation studies, such as in farmed greenlip abalone (Liu et al., 2014a) and blue mussel (Liu et al., 2016a).
In the present research, the sperm collected with the strip spawned method were used to investigate the parameters (cooling rate, thawing temperature, endpoint temperature and sperm to oocyte ratio) key to the development of programmable freezing technique in M.galloprovincialis. The supplementation of various types and concentrations of sugar was also evaluated for the purpose of improvement of postthaw sperm quality after cryopreservation.
2 MATERIAL AND MATHOD 2.1 Broodstock and gamete collectionThe mature blue mussels (65-85 mm in length) were supplied by Kinkawooka Mussels in Port Lincoln, South Australia and delivered to the South Australian Research and Development Institute (SARDI), Adelaide with a chilled transportation. When the strip spawned method was applied, randomly selected animals were open and genders were determined by the gonad colour with the male being milky white. The male gonads were removed individually and transferred in a culture dish with prefilled 1 μm filtered seawater (FSW) at the volume of 30 mL. Sperm were scraped from the gonad and left undisturbed for 30 min. The sperm debris were removed with 90 μm and 25 μm sieves. The sperm motility was estimated under a light microscope using subsamples. In each experiment, sperm with motility > 70% was mixed equally from five or more males and stored on ice. The sperm concentration was determined as described by Liu et al. (2016a) for the same species and standardized to 4×108/mL in this study. The sperm were used within 2 h of collection.
Females were induced to spawn separately using the thermal shock method described by Liu et al. (2016a). Oocytes from one mussel were collected on a 35 μm sieve after through a 90-μm sieve to remove the debris. After rinse they were washed into a container. When no fertilized oocytes (with one or two polar bodies or dividing cells) were observed under a light microscope 15 min later, oocytes from three or more females were mixed and stored on ice (0℃). The oocyte density was determined (Liu et al., 2016a) and standardized to 1×105/mL in this study. The fresh oocyte was used within 2 h after spawning.
2.2 Chemical preparation and equipmentDimethyl sulfoxide (DMSO), sucrose, glucose, trehalose and propidium iodide (PI) were all in the AR grade and purchased from Sigma-Aldrich Pty Ltd., St Louis, MO, USA. The live/dead sperm viability kit (L-7011) and the LysoTrack Green DND- 26 (LYSO-G) packages (L-7526) were purchased from Invitrogen Australia. The former was used to evaluate sperm Plasma Membrane Integrity (PMI) whereas the latter to evaluate Acrosome Integrity (AI). The cryoprotective stock solutions and the working solutions for evaluating PMI and AI were prepared as described by Liu et al. (2016a).
A CL863 programmable freeze controller (Cryologic, Mulgrave, Victoria, Australia) was used. The straws (Minitube, Germany) were placed into a cryochamber (model: CC23F) and frozen by liquid nitrogen (LN). The procedures used in this study to regulate temperatures during freezing and establish the required water temperature in the thawing bath were the same as reported in our previous research on greenlip abalone sperm cryopreservation (Liu et al., 2016b).
2.3 Evaluation of sperm qualityIn the present study, the sperm quality was evaluated by fertilization rate or sperm PMI and AI according to the methods described by Liu et al. (2016a) for the same species. SYBR14/PI and LYSO-G/PI were used for sperm PMI and AI assessments, respectively.
2.4 Experiment 2.4.1 Effects of cooling rates on post-thaw sperm fertilization rateDMSO at a final concentration of 6% or 8% and an equilibration period of 10 min has been identified by Liu et al. (2016a) for the non-programmable cryopreservation method in this species and was applied in this and subsequent experiments. In this experiment, the effects of cooling rates at -3, -4, -5, -6, and -7℃/min on post-thaw sperm fertilization rate were evaluated. Pre-cold freshly strip spawned sperm were equilibrated on ice for 10 min in 6% and 8% DMSO (final concentrations). They were then moved into cryo-straws (0.25 mL) and placed into the programmable freezer. The straws were held at 2℃ for 5 min and then frozen at different cooling rates to the endpoint temperature (-30℃) before being stored in LN. After at least 12 h in LN, the straws were thawed in a 60℃ seawater bath until ice melted and recovered in an 18℃ seawater bath. The fertilization rate was used to evaluate post-thaw sperm quality at a sperm to oocyte ratio of 10 000:1. The sperm to oocyte ratio has been determined according to the method described by Liu et al. (2016a). In this and subsequent experiments all treatments were repeated three times using different sperm pools.
2.4.2 Effects of thawing temperatures on post-thaw sperm fertilization rateSperm mixed with 8% DMSO and frozen at -4℃/ min produced the highest post-thaw sperm fertilization rate in the previous experiment and these parameters were applied hereafter. In the present experiment, the following thawing temperatures were evaluated: 20, 30, 40, 50, 60, 70 and 80℃. The other procedures were as described in Section 2.4.1.
2.4.3 Effects of endpoint temperatures on post-thaw sperm fertilization rateSperm thawed at 20℃ produced the highest postthaw sperm fertilization rate and this temperature was used hereafter. In the current experiment, the following endpoint temperatures were assessed: -20, -30, -40, -50, -60, -70 and -80℃. The other procedures were as described in Section 2.4.2.
2.4.4 Assessment of sugar type and concentration on post-thaw sperm fertilization rateThe endpoint temperature at -30℃ produced the highest post-thaw sperm fertilization rates and was used in this and subsequent experiments. In this experiment, 8% DMSO and its combination with glucose, sucrose and trehalose at a concentration of 0.5%, 1%, 2%, and 4% were evaluated (Liu et al., 2016a). The other procedures were the same as in Section 2.4.3.
2.4.5 Effects of different sperm to oocyte ratios on post-thaw sperm fertilization rate, PMI and AIAs no improvement in post-thaw sperm fertilization rate was shown by adding different types and concentrations of sugar in the previous experiment, 8% DMSO was used to assess the effects of different sperm to oocyte ratios (10 000:1, 20 000:1, 30 000:1, 40 000:1 and 50 000:1) in this experiment. The other procedures were the same as in Section 2.4.4, although PMI and AI were applied to assess sperm quality.
2.5 Statistical analysisResults were presented as mean±standard deviation (SD) and data were arcsine transformed to comply with the ANOVA assumption of data normality and homoscedasticity before statistical analyses using SPSS 20 (SPSS, Chicago, IL, USA). A t-test for independent samples was applied to compare the sperm PMI and AI in both cryopreserved and fresh sperm. Two-way analysis of variance (ANOVA) was applied in the Section 2.4.1 and one-way ANOVA was applied in the others. The least-significant difference (LSD) comparison test was applied when the significance was observed at P < 0.05.
3 RESULT 3.1 Effects of cooling rates on post-thaw sperm fertilization rateSignificant interaction (P < 0.05) between cooling rate and DMSO concentration was found. The postthaw sperm fertilization rates were higher at -4 and -5℃/min than other cooling rates in both 6% and 8% DMSO (Fig. 1). No significant difference (P > 0.05) was found between these two cooling rates in 6% DMSO (39.9%±4.7% and 42.8%±4.1% fertilization rates for -4 and -5℃/min, respectively), whereas significant difference was found in 8% DMSO achieving the highest post-thaw sperm fertilization rate of 50% (P < 0.05).

|
| Fig.1 Post-thaw sperm fertilization rate (%) after freezing at different cooling rates in 6% and 8% DMSO (n=3) of M. galloprovincialis Bars with different capital letters within each DMSO concentration differ significantly between various cooling rates (P < 0.05). Bars with different lowercase letters within each cooling rate differ significantly between DMSO concentrations (P < 0.05). |
Post-thaw sperm fertilization rates fluctuated from 51.2%±0.7% to 60.6%±4.7% at different thawing temperatures (Fig. 2). Significant difference (P < 0.05) was found between 20℃ (60.6%±4.7% fertilization rate) and 70℃ (51.2%±0.7% fertilization rate) thawing temperatures, whereas each of these two temperatures had no significant difference with others (P > 0.05).
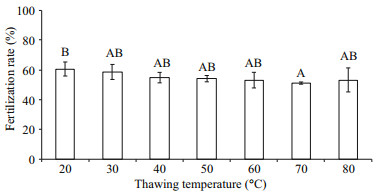
|
| Fig.2 Post-thaw sperm fertilization rates (%) after cryopreservation in 8% DMSO and thawed at different temperatures (n=3) of M. galloprovincialis Bars with different letters show significant difference (P < 0.05). |
The highest post-thaw sperm fertilization rate of 60.2%±4.0% was achieved at the endpoint temperature of -30℃ which was significantly higher than other endpoint temperatures (Fig. 3).
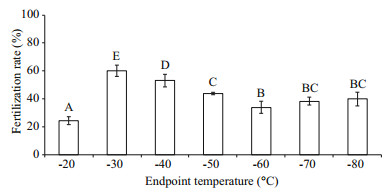
|
| Fig.3 Post-thaw sperm fertilization rates (%) after being frozen to different endpoint temperatures in a programmable controller before being transferred into LN (n=3) of M. galloprovincialis Bars with different letters show significant difference (P < 0.05). |
No significant improvement in post-thaw sperm fertilization rate was found in all sugar types and concentrations evaluated (P > 0.05; Fig. 4), ranging from 22.6%±5.1% to 45.1%±4.9%. Instead the postthaw sperm fertilization rate decreased with the increase in the sugar concentrations.
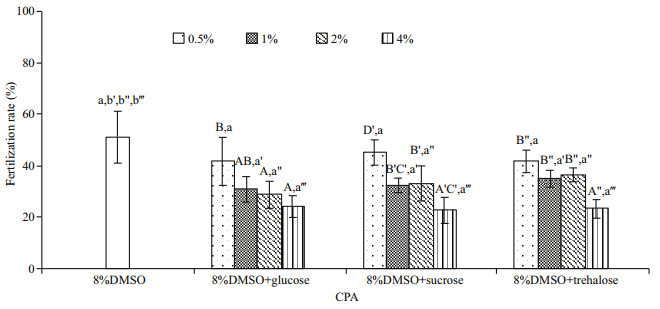
|
| Fig.4 Comparison of post-thaw sperm fertilization rates (%) after being frozen in 8% DMSO and its combination with different sugar types and concentrations (n=3) of M. galloprovincialis Bars with different capital letters within each sugar concentration (+8% DMSO) differ significantly between different sugar types (P < 0.05). Bars with different lowercase letters within each CPA differ significantly between different sugar concentrations (P < 0.05). |
Figure 5 showed that the post-thaw sperm fertilization rate increased with the increase of sperm to oocyte ratio, reaching the highest of 91.4%±6.0% at ratio of 50 000:1. However, no significant difference was found between sperm to oocyte ratio from 30 000:1 to 50 000:1 (P > 0.05). The PMI and AI values in post-thaw sperm were significantly lower than those in fresh sperm (P < 0.01; Table 1).
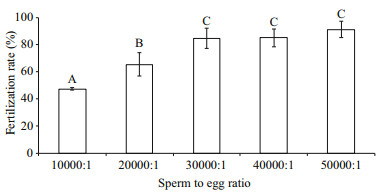
|
| Fig.5 Post-thaw sperm fertilization rates (%) at different sperm to oocyte ratios after cryopreservation in 8% DMSO (n=3) of M. galloprovincialis Bars with different letters differ significantly (P < 0.05). |

|
In this study a programmable freezing technique has been developed for the blue mussel sperm collected with the strip spawned method. The addition of glucose, sucrose or trehalose in the cryoprotectant could not further improve the post-thaw sperm fertilization rate. On the other hand, the fertilization rate of post-thaw sperm increased with the increase in sperm to oocyte ratios.
In cryopreservation, an optimal cooling rate could balance the effects from the formation of intracellular ice and the increase in solute concentrations, leading to maximal maintenance on sperm quality (Liu et al., 2015a). In the present study, a programmable freeze controller was selected as it could strictly regulate the cooling rate. The best post-thaw sperm fertilization rate was produced at -4℃/min. This cooling rate is faster than that optimised in European flat oyster, Ostrea edulis (-3℃/min; Vitiello et al., 2011), but slower than most other species investigated so far, including H. laevigata (-5℃/min; Liu et al., 2016b), disc abalone, H. discus hannai (-50℃/min; Kang et al., 2004), small abalone, H. diversicolor supertexa (-12 or -15℃/min; Gwo et al., 2002), red abalone, H. rufescens (-16℃/min; Salinas-Flores et al., 2005), Pacific oysters, Crassostrea gigas (-6℃/min; Ieropoli et al., 2004), scallops Chlamys farreri (-20℃/min; Li et al., 2000) and macha surf clams, Mesodesma donacium (-18℃/min; Dupré and Guerrero, 2011).
Thawing temperature evaluation is one of the important steps for the development of sperm cryopreservation as optimal thawing temperature could minimise recrystallization and reduce osmotic stress to sperm (Gao and Critser, 2000; Herráez et al., 2012). In the current study, the thawing temperature of 20℃ produced the highest post-thaw sperm fertilization rate, which agrees with the study in Pacific oysters (Adams et al., 2004). However, this temperature is lower than most of the studies in marine mollusk (Liu et al., 2015a) as well as the study in the same species (Liu et al., 2016a). The difference in optimal thawing temperature revealed by Liu et al. (2016a) may be due to the fact that a nonprogrammable freezing technique was used in their investigation.
Theoretically, the optimal endpoint temperature should be species specific while the temperatures lower or higher than the optimal temperature or temperature range will affect the post-thaw sperm survivals (Gwo, 2008). In this study, the highest postthaw sperm fertilization rate was achieved at -30℃ endpoint temperature, which is much higher than the -90℃ reported in the small abalone (Gwo et al., 2002). In the current study, reasons causing significant reduction in post-thaw sperm quality at -20℃ endpoint temperature were not clear. It might be due to inadequate sperm dehydration, therefore causing severe damage by intracellular ice formation in the subsequent freezing steps. This phenomenon has also been observed in farmed H. laevigata (Liu et al., 2016b), marine shrimp, Sicyonia ingentis (Anchordoguy et al., 1988) and African catfish, Clarias gariepinus (Viveiros et al., 2001) where sperm viabilities were also lower at higher endpoint temperatures.
In livestock and fish sperm cryopreservation, sugar is often consisted in cryoprotective medium. The addition of sugar would reduce the formation of intracellular ice, stabilize the membrane during freezing and serve as an energy source (Suquet et al., 2000; Purdy, 2006; Cabrita et al., 2010). In this study, no positive effect on post-thaw sperm fertilization was obtained by the addition of trehalose, sucrose or glucose. These results were in agreement with the findings on Japanese pearl oysters, P. fucata martensii (Kawamoto et al., 2007) and the same species with the non-programmable freezing method (Liu et al., 2016a), where the post-thaw sperm quality could not be improved by the addition of sugar. In contrast, Liu et al.(2014b, 2015b) have found that the addition of glucose significantly improved the post-thaw sperm fertilization rate in farmed greenlip and blacklip abalone.
A higher sperm to oocyte ratio is normally needed in fertilization to compensate cryodamages on sperm after cryopreservation (Liu et al., 2016a). In this study, higher sperm to oocyte ratios (30 000:1 to 50 000:1) were required to produce 85% post-thaw sperm fertilization rates, which are 1 500 to 2 500 times higher than the ratios used in fresh sperm. These ratios were higher than those reported in most published studies in marine mollusk (Liu et al., 2015a, 2016a). The reason causing this higher sperm to oocyte ratio may be partly due to the fact that the sperm PMI and AI were severely damaged during the cryopreservation process (Table 1) and potential high variations in sperm maturity when they were stripspawned.
5 CONCLUSIONThe current study has developed a programmable freezing technique for sperm collected with strip spawned method in the blue mussel, M. galloprovincialis. A post-thaw sperm fertilization rate of > 85% has been achieved when the sperm were cryopreserved in 8% DMSO at the cooling rate of -4℃/min from 2℃ to -30℃ endpoint temperature, thawed in a 20℃ seawater bath and fertilized at the sperm to oocyte ratio of 30 000:1 to 50 000:1. The addition of sugars in 8% DMSO did not improve the post-thaw sperm fertilization rate.
6 DATA AVAILABILITY STATEMENTThe data that support the findings of this study are available from the corresponding author upon reasonable request.
7 ACKNOWLEDGEMENTWe thank Mr Andy Dyle for the provision of blue mussel broodstock and Mr Mark Gluis of SARDI for technical support.
Adams S L, Smith J F, Roberts R D, Janke A R, Kaspar H F, Tervit H R, Pugh P A, Webb S C, King N G. 2004. Cryopreservation of sperm of the Pacific oyster (Crassostrea gigas): development of a practical method for commercial spat production. Aquaculture, 242(1-4): 271-282.
DOI:10.1016/j.aquaculture.2004.08.034 |
Anchordoguy T, Crowe J H, Griffin F J, Clark Jr W H. 1988. Cryopreservation of sperm from the marine shrimp sicyonia ingentis. Cryobiology, 25(3): 238-243.
DOI:10.1016/0011-2240(88)90031-4 |
Cabrita E, Sarasquete C, Martínez-Páramo S, Robles V, Beir o J, Pérez‐Cerezales S, Herráez M P. 2010. Cryopreservation of fish sperm: applications and perspectives. Journal of Applied Ichthyology, 26(5): 623-635.
DOI:10.1111/jai.2010.26.issue-5 |
Clulow J R, Mansfield L J, Morris L H A, Evans G, Maxwell W M C. 2008. A comparison between freezing methods for the cryopreservation of stallion spermatozoa. Animal Reproduction Science, 108(3-4): 298-308.
DOI:10.1016/j.anireprosci.2007.08.014 |
Di Matteo O, Langellotti A L, Masullo P, Sansone G. 2009. Cryopreservation of the Mediterranean mussel (Mytilus galloprovincialis) spermatozoa. Cryobiology, 58(2): 145-150.
DOI:10.1016/j.cryobiol.2008.09.015 |
Dong Q X, Eudeline B, Huang C J, Allen Jr S K, Tiersch T R. 2005. Commercial-scale sperm cryopreservation of diploid and tetraploid pacific oysters, Crassostrea gigas. Cryobiology, 50(1): 1-16.
DOI:10.1016/j.cryobiol.2004.09.003 |
Dupré E, Guerrero A. 2011. Cryopreservation of Macha surf clam spermatozoa. in: Tiersch T R, Green C C eds. Cryopreservation in Aquatic Species. 2nd edn. World Aquaculture Society, Baton Rouge. p.574-580.
|
Gao D Y, Critser J K. 2000. Mechanisms of cryoinjury in living cells. ILAR Journal, 41(4): 187-196.
DOI:10.1093/ilar.41.4.187 |
Gómez-Fernández J, Gómez-Izquierdo E, Tomás C, Mocé E, De Mercado E. 2012. Effect of different monosaccharides and disaccharides on boar sperm quality after cryopreservation. Animal Reproduction Science, 133(1-2): 109-116.
DOI:10.1016/j.anireprosci.2012.06.010 |
Gwo J C, Chen C W, Cheng H Y. 2002. Semen cryopreservation of small abalone (Haliotis diversicolor supertexa). Theriogenology, 58(8): 1 563-1 578.
DOI:10.1016/S0093-691X(02)01055-5 |
Gwo J C. 2008. Cryopreservation of small abalone (Haliotis diversicolor supertexa) semen. in: Cabrita E, Robles V, Herráez P eds. Methods in Reproductive Aquaculture: Marine and freshwater Species. CRC Press, New York. No.6 LIU et al.: Strip spawned sperm cryopreservation in M. galloprovincialis 2357 480p.
|
Hassan M M, Qin J G, Li X X. 2015. Sperm cryopreservation in oysters: a review of its current status and potentials for future application in aquaculture. Aquaculture, 438: 24-32.
DOI:10.1016/j.aquaculture.2014.12.037 |
Herráez P, Cabrita E, Robles V. 2012. Fish gamete and embryo cryopreservation: state of the art. in: Fletcher G L, Rise M L eds. Aquaculture Biotechnology. Wiley-Blackwell, Oxford, UK. p.303-317.
|
Hopkins B K, Herr C. 2010. Factors affecting the successful cryopreservation of honey bee (Apis mellifera) spermatozoa. Apidologie, 41(5): 548-556.
DOI:10.1051/apido/20010006 |
Ieropoli S, Masullo P, Santo M D E, Sansone G. 2004. Effects of extender composition, cooling rate and freezing on the fertilisation viability of spermatozoa of the Pacific oyster (Crassostrea gigas). Cryobiology, 49(3): 250-257.
DOI:10.1016/j.cryobiol.2004.08.005 |
Jafaroghli M, Khalili B, Farshad A, Zamiri M J. 2011. The effect of supplementation of cryopreservation diluents with sugars on the post-thawing fertility of ram semen. Small Ruminant Research, 96(1): 58-63.
DOI:10.1016/j.smallrumres.2010.11.010 |
Kang K H, Kim J M, Kim Y H. 2004. Short-term storage and cryopreservation of abalone (Haliotis discus hannai) sperm. The Korean Journal of Malacology, 20(1): 17-26.
|
Kawamoto T, Narita T, Isowa K, Aoki H, Hayashi M, Komaru A, Ohta H. 2007. Effects of cryopreservation methods on post-thaw motility of spermatozoa from the Japanese pearl oyster, Pinctada fucata martensii. Cryobiology, 54(1): 19-26.
DOI:10.1016/j.cryobiol.2006.10.190 |
Lazo C S, Pita I M. 2012. Effect of temperature on survival, growth and development of Mytilus galloprovincialis larvae. Aquaculture Research, 43(8): 1 127-1 133.
DOI:10.1111/are.2012.43.issue-8 |
Li C, Li J, Xue Q Z. 2000. Cryopreservation of the spermatozoa of Chlamys (Azumapecten) farreri. Marine Fisheries Research, 21(1): 57-62.
(in Chinese with English abstract) |
Liu B L, Liu Y B, Liu S W, Xu T, Liu Q, Li X X. 2016a. Cryopreservation of strip spawned sperm using nonprogrammable freezing technique in the blue mussel Mytilus galloprovincialis. Aquaculture Research, 47(12): 3 888-3 898.
DOI:10.1111/are.2016.47.issue-12 |
Liu Y B, Li X X, Robinson N, Qin J G. 2015a. Sperm cryopreservation in marine mollusk: a review. Aquaculture International, 23(6): 1 505-1 524.
DOI:10.1007/s10499-015-9900-0 |
Liu Y B, Li X X, Xu T, Robinson N, Qin J G. 2014a. Improvement in non-programmable sperm cryopreservation technique in farmed greenlip abalone Haliotis laevigata. Aquaculture, 434: 362-366.
DOI:10.1016/j.aquaculture.2014.08.033 |
Liu Y B, Li X X, Xu T, Robinson N, Qin J G. 2016b. Greenlip abalone (Haliotis laevigata Donovan, 1808) sperm cryopreservation using a programmable freezing technique and testing the addition of amino acid and vitamin. Aquaculture Research, 47(5): 1 499-1 510.
DOI:10.1111/are.12609 |
Liu Y B, Xu T, Robinson N, Qin J G, Li X X. 2014b. Cryopreservation of sperm in farmed Australian greenlip abalone Haliotis laevigata. Cryobiology, 68(2): 185-193.
DOI:10.1016/j.cryobiol.2014.01.002 |
Liu Y B, Xu T, Robinson N, Qin J G, Li X X. 2015b. Cryopreservation of sperm in farmed blacklip abalone (Haliotis rubra Leach, 1814). Aquaculture Research, 46(11): 2 628-2 636.
DOI:10.1111/are.2015.46.issue-11 |
Lyons L, Jerry D R, Southgate P C. 2005. Cryopreservation of black-lip pearl oyster (Pinctada margaritifera, L.) spermatozoa: effects of cryoprotectants on spermatozoa motility. Journal of Shellfish Research, 24(4): 1 187-1 190.
DOI:10.2983/0730-8000(2005)24[1187:COBPOP]2.0.CO;2 |
Paniagua-Chavez C G, Buchanan J T, Tiersch T R. 1998. Effect of extender solutions and dilution on motility and fertilizing ability of eastern oyster sperm. Journal of Shellfish Research, 17: 231-237.
|
Pettersen A K, Turchini G M, Jahangard S, Ingram B A, Sherman C D H. 2010. Effects of different dietary microalgae on survival, growth, settlement and fatty acid composition of blue mussel (Mytilus galloprovincialis) larvae. Aquaculture, 309(1-4): 115-124.
DOI:10.1016/j.aquaculture.2010.09.024 |
Purdy P H. 2006. A review on goat sperm cryopreservation. Small Ruminant Research, 63(3): 215-225.
DOI:10.1016/j.smallrumres.2005.02.015 |
Rota A, Rota A, Martini M, Milani C, Romagnoli S. 2005. Evaluation of dog semen quality after slow (biological freezer) or rapid (nitrogen vapours) freezing. Reproduction Nutrition Development, 45(1): 29-37.
DOI:10.1051/rnd:2005002 |
Salinas-Flores L, Paniagua-Chavez C G, Jenkins J A, Tiersch T R. 2005. Cryopreservation of sperm of red abalone (Haliotis rufescens). Journal of Shellfish Research, 24(2): 415-420.
DOI:10.2983/0730-8000(2005)24[415:COSORA]2.0.CO;2 |
Stanic P, Tandara M, Sonicki Z, Simunic V, Radakovic B, Suchanek E. 2000. Comparison of protective media and freezing techniques for cryopreservation of human semen. European Journal of Obstetrics & Gynecology and Reproductive Biology, 91(1): 65-70.
|
Suquet M, Dreanno C, Fauvel C, Cosson J, Billard R. 2000. Cryopreservation of sperm in marine fish. Aquaculture Research, 31(3): 231-243.
DOI:10.1046/j.1365-2109.2000.00445.x |
Tiersch T R. 2008. Strategies for commercialization of cryopreserved fish semen. Revista Brasileira de Zootecnia, 37 suplemento especial: 15-19.
|
Vitiello V, Carlino P A, Del Prete F, Langellotti A L, Sansone G. 2011. Effects of cooling and freezing on the motility of Ostrea edulis (L., 1758) spermatozoa after thawing. Cryobiology, 63(2): 118-124.
DOI:10.1016/j.cryobiol.2011.07.004 |
Viveiros A T M, Lock E J, Woelders H, Komen J. 2001. Influence of cooling rates and plunging temperatures in an interrupted slow-freezing procedure for semen of the African catfish, Clarias gariepinus. Cryobiology, 43(3): 276-287.
DOI:10.1006/cryo.2001.2362 |
Zhang X M, Li X X, Clarke S, Li X. 2012. The development of Pacific oysters Crassostrea gigas produced using cryopreserved sperm. In: Qin J G ed. Oysters: Physiology, Ecological Distribution and Mortality. Nova Science, New York. p.1-18.
|
 2018, Vol. 36
2018, Vol. 36


