Institute of Oceanology, Chinese Academy of Sciences
Article Information
- MIAO Fengping, ZUO Jincheng, LIU Xianghong, JI Naiyun
- Algicidal activities of secondary metabolites of marine macroalgal-derived endophytic fungi
- Journal of Oceanology and Limnology, 37(1): 112-121
- http://dx.doi.org/10.1007/s00343-019-7393-9
Article History
- Received Dec. 22, 2017
- accepted in principle Jan. 22, 2018
- accepted for publication Mar. 16, 2018
2 College of Life Science, Ludong University, Yantai 264025, China
Red tide caused by the outbreak of some planktonic algae and protozoans under certain conditions could significantly change the structure of marine organisms (Song et al., 2003), damage marine fishery, marine aquaculture, and coastal tourism, and even lead to human poisoning and death (Zhou and Zhu, 2006). Besides, red tide could result in heavy losses to the local ecological environment and economic development. Therefore, the use of red tide algae inhibitors has been one of the important contingency measures because of their rapid effects on inhibiting the growth of red tide microalgae (Murray-Gulde et al., 2002). Allelochemicals have attracted significant attention owing to their species-specific effect and ecological safety (Jin and Dong, 2003).
In certain habitat, macroalgal-derived endophytic fungi could produce multifarious secondary metabolites, which are considerably different from the substances produced by other organisms (Ji and Wang, 2016). Rateb and Ebel (2011) showed that the number of new compounds obtained from algaendophytic fungi accounted for 21% of all the new compounds from marine fungi. These compounds, including sesquiterpenes, diterpenoids, steroids, polyketides, and alkaloids, have significant antibacterial, antioxidant, anti-plasmodium, and anticancer activities. However, majority of the studies on secondary metabolites of algal-derived endophytic fungi have focused on medicinal activities rather than algicidal activities (Ji and Wang, 2016). Moreover, most of the algicidal bioassays have examined Chlorella fusca (Osterhage et al., 2000; Krohn et al., 2005), the common marine microalgae, but the red tide algae have rarely been considered (Chen et al., 1996). Based on the available reports (Ji and Wang, 2016), it may be hypothesized that some of the secondary metabolites of endophytic fungi isolated from marine macroalgae could inhibit red tide algae. Herein, the algicidal activities of secondary metabolites isolated and identified from the marine macroalgal-derived endophytic fungi are described against red tide algae Alexandrium tamarense, Prorocentrum donghaiense, Heterosigma akashiwo, and Chattonella marina.
2 MATERIAL AND METHOD 2.1 MaterialAlexandrium tamarense and P. donghaiense were kindly provided by Xiamen University, China, H. akashiwo was kindly provided by Harbin Institute of Technology, Weihai, China, and C. marina was isolated from Yantai seaside by Dr. YANG Cuiyun, Yantai Institute Coastal Zone Research, CAS (Chinese Academy of Sciences). The algae were cultured in a growth chamber containing f/2 medium at 20℃ under 14 h:10 h light-dark (LD) cycle with a light density of 2 000 lx.
The fungal strains, preserved in Yantai Institute Coastal Zone Research, CAS, were isolated from the fresh tissue of surface-sterilized marine macroalgae collected from the seaside of China. All the strains were cultured in potato dextrose broth (PDB) at 28℃ in a growth chamber.
2.2 Preparation of crude extract of secondary metabolitesThe initial fungal cultures were maintained on potato dextrose agar (PDA) plate. Subsequently, pieces of mycelia were cut into small segments and aseptically inoculated into 1 000-mL Erlenmeyer flasks containing 300 mL of PDB, and static fermentation was performed for 30 days at room temperature (25℃). After 30 days, the entire culture (300 mL) was filtered through cheesecloth to separate the mycelia. The culture broth (300 mL) was extracted three times with EtOAc to afford extract Ⅰ after removal of the solvent by evaporation (40℃) at reduced pressure. The dried and powdered mycelia were extracted three times with a mixture of CHCl3 and MeOH (1:1, v/v) and concentrated by evaporation at reduced pressure to yield the gum. Then, the gum was partitioned between H2O and EtOAc. The EtOAc soluble part was collected and evaporated at reduced pressure to afford extract Ⅱ. As the thin-layer chromatography (TLC) profiles of the two extracts were nearly identical, they were combined to obtain the crude extract.
2.3 Algicidal activities of crude extracts and pure compoundsRapid bioassay (Schrader et al., 1997) was used to evaluate the algicidal activities of the crude extracts of secondary metabolites from the algal-derived endophytic fungi. In brief, the stock solution of the crude extract was dissolved in 100% DMSO (technical-grade) and added (2 μL per well) to each well of the microplate with 198 μL microalgal culture containing with medium in a well, respectively. The final volume of each wells were 200 μL. The plates were placed in a growth chamber at 20℃ and 14 h: 10 h LD cycle. After 24 h, the live algal cell number was counted using blood cell counting chamber. Three replications were used for each treatment and the control (2 μL DMSO per well). The 24-h inhibition rates (IRs) were calculated as follows:
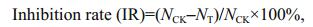
where NCK is the live algal cell number of the control group; NT, live algal cell number of the treatment group.
The crude extracts with high 24-h IRs were considered to have high algicidal activities. The 24-h IRs of the pure compounds isolated from these crude extracts (Liu, 2012; Liu et al., 2012, 2013, 2014; Miao et al., 2012; Sun et al., 2013) were determined according to the above-mentioned procedure, and the 24-h inhibition concentration (24 h EC50) of the compounds with high IR was measured based on the method developed by Schrader et al. (1997).
2.4 Inhibition mechanisms of compounds against H. akashiwo growthAs H. akashiwo generally presented higher growth rate and cell density than the other three algae examined, the effects of compounds with highest algicidal activities on H. akashiwo were examined to explore the underlying mechanisms of growth inhibition of red tide algae.
The H. akashiwo cells in logarithmic phase were treated with DMSO (control), 40% 24 h EC50 of compound 1 (0.25 μg/mL), and 80% 24 h EC50 of compound 1 (0.50 μg/mL), respectively. After 24 h, the cell membrane integrity, Chl a content, soluble protein content, and malondialdehyde (MDA) content were investigated, and the activities of superoxide dismutase (SOD) and peroxidase (POD) were tested. All the experiments were performed in triplicate with control.
2.5 Cell membrane integrityCharacterization of microalgae as viable or nonviable at single-cell level is essential to determine the effects of antialgal agents. Impermeability of the cell membrane to nucleic acid dyes such as propidium iodide (PI) is one of the criteria for "viability" (Shapiro, 2008). Fluorescent dyes such as fluorescein diacetate (FDA) can only stain intact cell membrane, whereas macromolecular fluorescent dyes such as PI can infiltrate into the cell only when the cell membrane is damaged. In the present study, the fluorescence intensity of the algal cells in the treatment and control groups stained with FDA and PI, respectively, were assayed using flow cytometry (FCM).
The H. akashiwo cells in each group were stained with 10 μmol/L PI (Sigma, P4170) and 10 mg/L FDA (Sigma, F7378), respectively, and the cell membrane integrity was measured using FCA. Healthy cells took up FDA to convert fluorescein, which presented emission peak at 515–545 nm when excited at 488 nm. In contrast, cells with damaged cell membrane were stained with PI, which exhibited an emission peak at 600–620 nm when excited at 488 nm. The results obtained were expressed as relative fluorescence intensity.
2.6 Chl a contentThe Chl a content in the algal cells was measured by spectrophotometric method as described by Wintermans and De Mots (1965). In brief, the harvested algal cells were resuspended in 95% ethanol solution and stored at 4℃ in dark for 24 h. Then, the mixture was centrifuged at 4 000×g for 10 min at room temperature, and the supernatant was collected and its absorbance was measured at 665 and 649 nm by using a spectrophotometer. The Chl a content was calculated as follows:

A total of 30 mL of the harvested algal cells were centrifuged at 1 500×g for 10 min at room temperature, and the supernatant was discarded. Subsequently, 2 mL of 0.07% physiological saline were added to the cells and mixed well and centrifuged again. This procedure was repeated twice, and the cells were ground in a homogenizer in ice bath to break the cell wall. The cell debris was dissolved in 2 mL of 0.07% physiological saline and stored at -80℃. The soluble protein and MDA contents as well as SOD and POD activities were determined using assay kits (Jiancheng Biotech, Nanjing, China) according to the manufacturer's instructions.
2.8 Statistical analysisOne-way ANOVA and Duncan's test with P=0.05 were performed using SPSS software to examine the effects of different concentrations of the identified compounds on soluble protein content, MDA content, SOD activity, and POD activity in H. akashiwo cells.
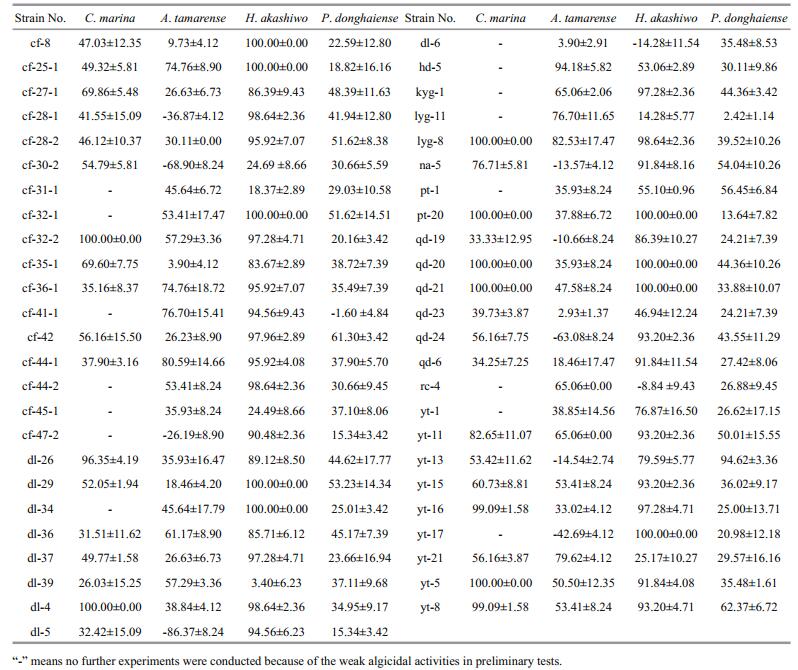
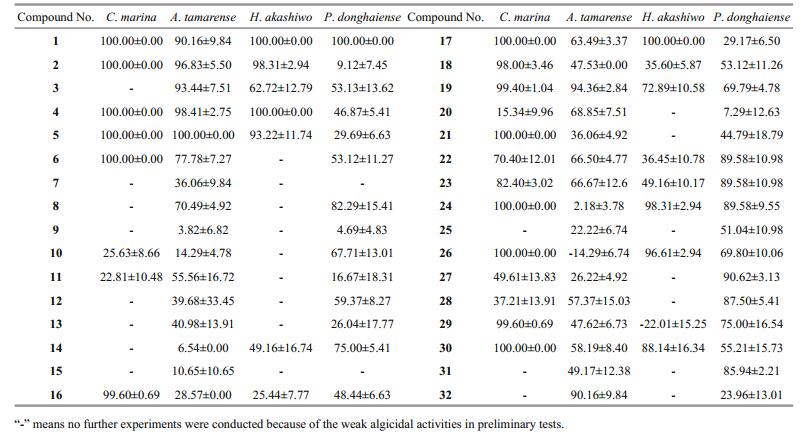
3 RESULT 3.1 Algicidal activities of crude extractsExcept for some crude extracts that promoted algal growth, most of the crude extracts obtained showed varied algicidal activities (Table 1). None of the H. akashiwo cells could survive treatment with 50 μg/mL crude extracts from strains cf-8, cf-25-1, cf-32-1, dl-29, dl-34, pt-20, qd-20, qd-21, and yt-17. Similarly, treatment with 2.5 μg/mL crude extracts from strains cf-32-2, dl-4, lyg-8, pt-20, qd-20, qd-21, and yt-5 resulted in the death of all C. marina cells. Furthermore, 400 μg/mL crude extracts from strains cf-36-1, cf-25-1, lyg-11, cf-41-1, yt-21, cf-44-1, lyg- 8, and hd-5 showed strong antialgal activities against A. tamarense with IR values higher than 70%, while 200 μg/mL crude extracts from strains yt-11, cf-32-1, cf-28-2, dl-29, na-5, pt-1, cf-42, yt-8, and yt-13 presented strong antialgal activities against P. donghaiense with IR values higher than 50%.
|
Based on their algicidal activities, TLC profile, and growth profile of the secondary metabolite crude extracts, fungal strains pt-20, pt-1, cf-42, and dl-29 were selected and cultured on a large scale. A total of 32 different compounds (Fig. 1), comprising seven alkaloids, six anthraquinones, nine terpenes, eight steroids, and two other compounds, were isolated from the secondary metabolites crude extracts (Miao et al., 2012; Liu, 2012; Liu et al., 2012, 2013, 2014; Sun et al., 2013).
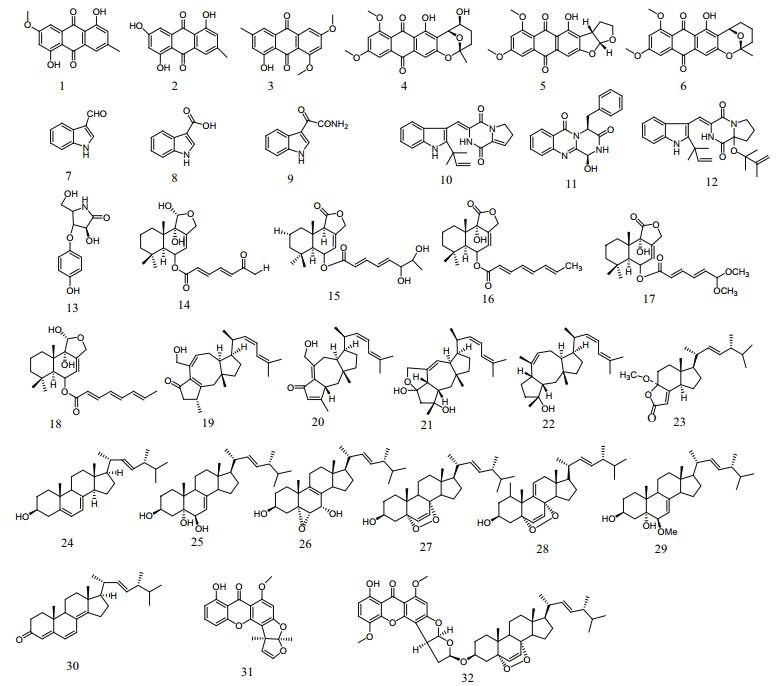
|
| Fig.1 Structures of the compounds isolated from the secondary metabolites crude extracts of fungal strains dl-29, pt-1, pt- 20, and cf-42 Compounds 1-6, 7-13, 14-22, 23-30, and 31-32 are anthraquinones, alkaloids, terpenoids, steroids, and others, respectively. |
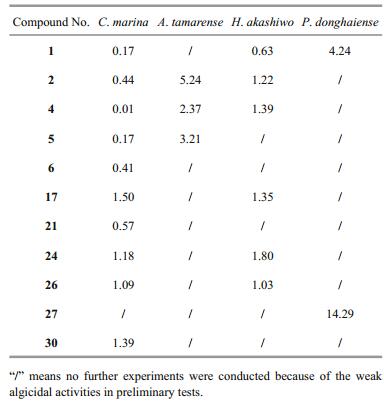
Among them, 11 compounds were considered to have strong algicidal activities (Tables 2 and 3). None of the C. marina cells could survive treatment with compounds 1, 2, 4, 5, 6, 17, 21, 24, 26, and 30 at a final concentration of 5 μg/mL (Table 2), and their 24 h EC50 values ranged from 0.01 to 1.50 μg/mL (Table 3). Compounds 2, 4, and 5, at a final concentration of 40 μg/mL, showed strong algicidal activities against A. tamarense with IR values higher than 95% (Table 2), and their 24 h EC50values were in the range of 2.37–5.24 μg/mL (Table 3). Similarly, at a final concentrations of 2.5 μg/mL, compounds 1, 2, 4, 17, 24, and 26 showed intense algicidal activities against H. akashiwo with IR values higher than 95% (Table 2), and their 24 h EC50values ranged from 0.63 to 1.80 μg/mL (Table 3). The IRs of compounds 1 and 27 against P. donghaiense were both higher than 90% at a concentration of 20 μg/mL (Table 2), and their 24 h EC50 values were 4.24 and 14.29 μg/mL, respectively (Table 3).
|
A total of 11 compounds with lower EC50 values were screened out, including five anthraquinones (compounds 1, 2, 4, 5, 6), two terpenoids (compounds 17, 21), and four steroids (compounds 24, 26, 27, 30). It can be observed from Table 3 that compound 1 (1, 5-dihydroxy-3-methoxy-7-methylanthraquinone) had the highest algicidal activity against C. marina, H. akashiwo, and P. donghaiense, and had higher algicidal activity against A. tamarense.
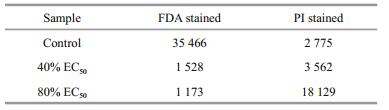
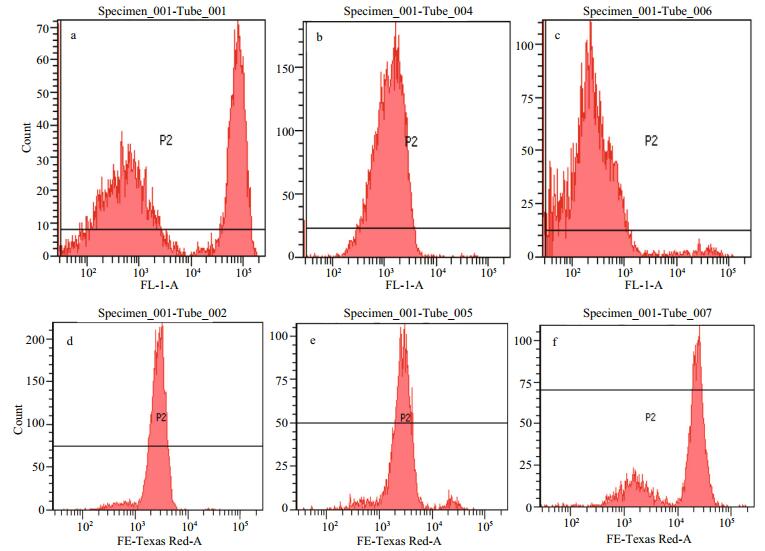
3.3 Inhibitory mechanisms of compound 1 against H. akashiwo growth 3.3.1 Cell membrane integrityAs shown in Fig. 2 and Table 4, the integrity of the algal cell membrane was destroyed by compound 1. The relative fluorescence intensity was 35 466 for the control group stained with FDA, whereas the values decreased to 1 528 and 1 173 for the 40% 24 h EC50 and 80% 24 h EC50 treatment group, which were only 4.31% and 3.31% of that of the control group, respectively. In contrast, the relative fluorescence intensity for the control group stained with PI was 2 775, whereas the values increased to 3 562 and 18 129 for the 40% 24 h EC50 and 80% 24 h EC50 treatment group, respectively. These results clearly showed that the integrity of the algal cell membrane was severely destroyed by compound 1 even at the concentration of 40% 24 h EC50.
|
| Fig.2 FCM spectra of H. akashiwo cells a. control stained with FDA; b. cells treated with compound 1 at 40% 24 h EC50 and stained with FDA; c. cells treated with compound 1 at 80% 24 h EC50 and stained with FDA; d. control stained with PI; e. cells treated with compound 1 at 40% 24 h EC50 and stained with PI; f. cells treated with compound 1 at 80% 24 h EC50 and stained with PI. |
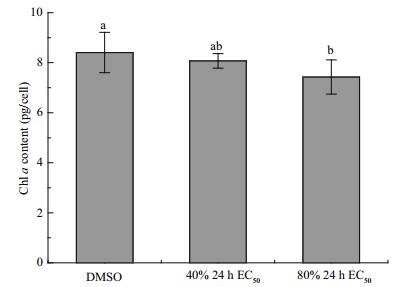
The Chl a content in the algal cells significantly decreased with the increasing concentration of compound 1 (Fig. 3). Treatment with compound 1 at a concentration of 80% 24 h EC50 resulted in a partial reduction in the Chl a content, which was 11.65% lower than that noted in the control (P < 0.05). These results indicated that the active compounds in the secondary metabolites of algal-derived endophytic fungi could reduce the Chl a content in the algal cells.
|
| Fig.3 Chl a content in H. akashiwo cells treated with DMSO (control), 40% 24 h EC50 of compound 1, and 80% 24 h EC50 of compound 1 |
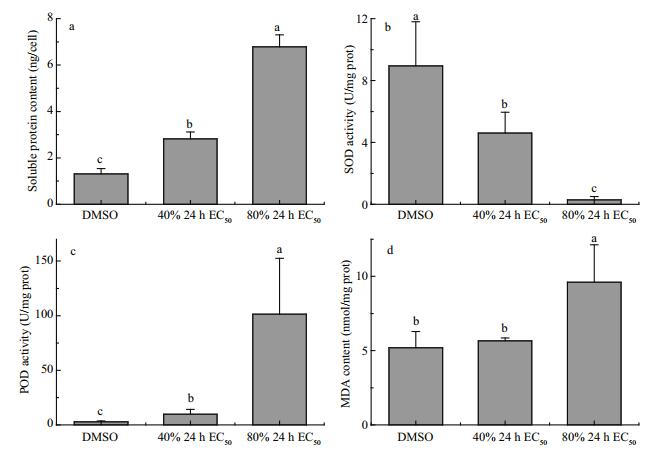
Figure 4a illustrates that both 40% and 80% 24 h EC50 of compound 1 significantly increased the soluble protein contents in H. akashiwo cells (P < 0.05). When compared with the control, the SOD activity in H. akashiwo cells was significantly reduced (P < 0.05) by 48.6% and 96.8% following treatment with 40% and 80% 24 h EC50 of compound 1, respectively (Fig. 4b). In contrast, the POD activity was significantly increased (P < 0.05) by 2.5 and 35.2 times in H. akashiwo cells following treatment with 40% and 80% 24 h EC50 of compound 1, respectively (Fig. 4c). Figure 4d shows that the MDA content in H. akashiwo cells increased with the increasing concentration of compound 1, and that the MDA content in H. akashiwo cells treated with 80% 24 h EC50 of compound 1 was significantly higher than that in the control (P < 0.05).
|
| Fig.4 Soluble protein content (a), SOD activity (b), POD activity (c), and MDA content (d) in H. akashiwo cells treated with DMSO (control), 40% 24 h EC50 of compound 1, and 80% 24 h EC50 of compound 1 |
When compared with other fungi, endophytic fungi isolated from marine macroalgae are considered to be one of the main sources of natural active substances (Cui et al., 2010; Wang et al., 2011; Sun et al., 2013). Many kinds of pure compounds with high bioactivities, such as alkaloids, anthraquinones, and steroids, have been separated from the secondary metabolites of Aspergillus (Miao et al., 2012; Liu, 2012; Liu et al., 2013). In the present study, the four fungal strains with strong algicidal activities against red tide algae belonged to the genus Aspergillus: A. wentii (pt-1), A. ustus (cf-42), and A. versicolor (dl-29, pt-20). A total of 11 compounds with strong algicidal activities were isolated from the secondary metabolites of these fungal strains (Tables 2 and 3), and were found to be composed of five anthraquinones (compounds 1, 2, 4, 5, 6), four steroids (compounds 24, 26, 27, 30), and two terpenoids (compounds17, 21). The anthraquinone compounds appeared to have broad-spectrum algicidal activities (Table 3). In recent years, novel anthraquinone compounds with strong antibacterial, antioxidant, and medicinal activities have been continuously isolated, suggesting that algal-derived endophytic fungi might be one of the important sources of active compounds against red tide algae, and that more attention should be paid to Aspergillus and anthraquinones. It can be observed from Table 2 that compounds 16, 18, 21 showed strong inhibitory activity against C. marina, but had weak effects on the other algae examined. Furthermore, compounds 26, 29 presented strong inhibitory activity against certain algae, while promoted growth of some algal species. These findings indicated that the algicidal activities of compounds might be species-specific because of varied cell structure and cell size (Jin and Dong, 2003; Cantrell et al., 2005).
The exposure to allelochemicals can induce oxidative stress in microalgae cells, thereby affecting microalgal growth and metabolism (Miazek et al., 2014). Chl a is the main photosynthetic pigment of H. akashiwo, and the active compound could weaken photosynthesis and block growth of this alga by destroying Chl a (Fig. 3). Soluble protein is an osmotic regulatory substance in cells, which can decrease the damages to the cells caused by stress. Therefore, the soluble protein content is an important indicator of cell membrane injury during metabolism. SOD and POD are two protective enzymes that widely exist in living organisms. Under stress conditions, SOD and POD could remove excessive reactive oxygen species to protect the cells from damage. MDA is the main product of membrane lipid peroxidation and an important indicator of membrane system damage. The MDA content in a cell could reflect the degree of membrane lipid peroxidation (Qian et al., 2009), membrane damage, and amount of reactive oxygen species in the cell (Zhang et al., 2011). In the present study, an increase in the soluble protein content, POD activity, and MDA content in H. akashiwo cells treated with compound 1 (Fig. 4a, c, d) indicated that the cells had been subjected to stress. Furthermore, a decrease in the SOD activity in H. akashiwo cells treated with compound 1 (Fig. 4b) showed that the cells were affected by severe stress. This result is in agreement with that reported by Qian et al. (2009). Therefore, it can be concluded that the active compounds could restrain the growth of red tide algae by inhibiting photosynthesis (reducing Chl a content), destroying cell membrane, and damaging the antioxidant system.
Interestingly, although the crude extract of strain pt-1 did not show strong algicidal activities (Table 1), compound 1, which was separated from this crude extract, showed the strongest algicidal activities (Table 3). A possible reason for this observation might be the effect of other compounds in the crude extract, which may have weakened the algicidal activities of compound 1, or the low content of compound 1 in the crude extract. Therefore, a moderate criterion for screening of the fungal strains could help in detecting more compounds with strong algicidal activities from the secondary metabolites of algal-derived endophytic fungi.
The solubility of the compounds in different solvent is different. DMSO, known as the "universal solvent" in areas of pharmaceutical sciences and cell biology, could dissolve both polar and nonpolar compounds. Under the same condition, the solubility of these compounds in DMSO is greater than that in water. Therefore, the concentrations of these compounds might not reach the concentrations in the experiment in natural water bodies. However, concentrations of red tide algae under normal conditions are much lower than those in this experiment. And generally, red tide algae at low concentrations would be more susceptible to allelochemicals. Therefore, low concentrations of the compounds might inhibit the growth of the red tide algae in natural water bodies. Furthermore, application of allelochemicals dissolved into DMSO might be used as an emergency treatment measure when red tides broke out, for the low toxicity of this solvent.
5 CONCLUSIONThe following conclusions were drawn from this study:
(1) A total of 32 pure compounds were assayed to possess different degrees of algicidal activities.
(2) Eleven of these compounds including five anthraquinones showed high 24-h IRs for the four red tide algae, with 24 h EC50 values ranging from 0.01 to 14.29 μg/mL. The anthraquinone compounds appeared to have broad-spectrum algicidal activities.
(3) Active compound could decrease the Chl a and SOD contents and increase soluble protein, MDA and POD contents of H. akashiwo.
(4) The anti-algal compound might inhibit the growth of red tide algae by weakening photosynthesis (reducing Chl a content), destroying cell membrane and damaging the antioxidant system.
Cantrell C L, Schrader K K, Mamonov L K, Sitpaeva G T, Kustova T S, Dunbar C, Wedge D E. 2005. Isolation and identification of antifungal and antialgal alkaloids from Haplophyllum sieversii. Journal of Agricultural and Food Chemistry, 53(20): 7 741-7 748.
DOI:10.1021/jf051478v |
Chen C, Imamura N, Nishijima M, Adachi K, Sakai M, Sano H. 1996. Halymecins, new antimicroalgal substances produced by fungi isolated from marine algae. The Journal of Antibiotics, 49(10): 998-1 005.
DOI:10.7164/antibiotics.49.998 |
Cui C M, Li X M, Li C S, Proksch P, Wang B G. 2010. Cytoglobosins A-G, cytochalasans from a marine-derived endophytic fungus, Chaetomium globosum QEN-14. Journal of Natural Products, 73(4): 729-733.
DOI:10.1021/np900569t |
Ji N Y, Wang B G. 2016. Mycochemistry of marine algicolous fungi. Fungal Diversity, 80(1): 301-342.
DOI:10.1007/s13225-016-0358-9 |
Jin Q, Dong S L. 2003. Comparative studies on the allelopathic effects of two different strains of Ulva pertusa on Heterosigma akashiwo and Alexandrium tamarense. Journal of Experimental Marine Biology And Ecology, 293(1): 41-55.
DOI:10.1016/S0022-0981(03)00214-4 |
Krohn K, Dai J Q, Flörke U, Aust H J, Dräger S, Schulz B. 2005. Botryane metabolites from the fungus Geniculosporium sp. isolated from the marine red alga Polysiphonia. Journal of Natural Products, 68(3): 400-405.
|
Liu X H, Miao F P, Li X D, Yin X L, Ji N Y. 2012. A new sesquiterpene from an endophytic Aspergillus versicolor strain. Natural Product Communication, 7(7): 819-820.
|
Liu X H, Miao F P, Liang X R, Ji N Y. 2014. Ergosteroid derivatives from an algicolous strain of Aspergillus ustus. Natural Product Research, 28(15): 1 182-1 186.
DOI:10.1080/14786419.2014.923996 |
Liu X H, Miao F P, Qiao M F, Cichewicz R H, Ji N Y. 2013. Terretonin, ophiobolin, and drimane terpenes with absolute configurations from an algicolous Aspergillus ustus. RSC Advances, 3(2): 588-595.
DOI:10.1039/C2RA22701K |
Liu X H. 2012. Secondary metabolites from three Codium fragile-endophytic fungi and their bioactivities. Shandong Agricultural University, Taian, China. p.34-37. (in Chinese with English abstract)
|
Miao F P, Li X D, Liu X H, Cichewicz R H, Ji N Y. 2012. Secondary metabolites from an algicolous Aspergillus versicolor strain. Marine Drugs, 10(1): 131-139.
|
Miazek K, Remacle C, Richel A, Goffin D. 2014. Effect of lignocellulose related compounds on microalgae growth and product biosynthesis: a review. Energies, 7(7): 4 446-4 481.
DOI:10.3390/en7074446 |
Murray-Gulde C L, Heatley J E, Schwartzman A L, Rodgers J H Jr. 2002. Algicidal effectiveness of clearigate, cutrineplus, and copper sulfate and margins of safety associated with their use. Archives of Environmental Contamination and Toxicology, 43(1): 19-27.
DOI:10.1007/s00244-002-1135-1 |
Osterhage C, Kaminsky R, König G M, Wright A D. 2000. Ascosalipyrrolidinone A, an antimicrobial alkaloid, from the obligate marine fungus Ascochyta salicorniae. Journal of Organic Chemistry, 65(20): 6 412-6 417.
DOI:10.1021/jo000307g |
Qian H F, Xu X Y, Chen W, Jiang H, Jin Y X, Liu W P, Fu Z W. 2009. Allelochemical stress causes oxidative damage and inhibition of photosynthesis in Chlorella vulgaris. Chemosphere, 75(3): 368-375.
DOI:10.1016/j.chemosphere.2008.12.040 |
Rateb M E, Ebel R. 2011. Secondary metabolites of fungi from marine habitats. Natural Product Reports, 28(2): 290-344.
DOI:10.1039/c0np00061b |
Schrader K K, De Regt M Q, Tucker C S, Duke S O. 1997. A rapid bioassay for selective algicides. Weed Technology, 11(4): 767-774.
DOI:10.1017/S0890037X00043414 |
Shapiro H M. 2008. Flow cytometry of bacterial membrane potential and permeability. In: Champney W S ed. New Antibiotic Targets. Humana Press, Totowa, New Jersey. p.175-186.
|
Song X X, Yu Z M, Gao Y H. 2003. Removal of different species of red tide organisms with an effective claycomplex system. Chinese Journal of Applied Ecology, 14(7): 1 165-1 168.
(in Chinese with English abstract) |
Sun K L, Wang Y, Fu P, Liu P P, Zhu W M. 2013a. Studies on the secondary metabolites of Eurotium herbariorum HT-2 symbiotic with Enteromorpha prolifera. Chinese Journal of Marine Drugs, 32(1): 37-45.
(in Chinese with English abstract) |
Sun R R, Miao F P, Zhang J, Wang G, Yin X L, Ji N Y. 2013b. Three new xanthone derivatives from an algicolous isolate of Aspergillus wentii. Magnetic Resonance in Chemistry, 51(1): 65-68.
DOI:10.1002/mrc.v51.1 |
Wang F, Li H J, Lan W J. 2011. The metabolites of the marine fungus Penicillium sp. Associated with soft coral Sarcophyton tortuosum. Acta Scientiarum Naturalium Universitatis Sunyatseni, 50(6): 72-77.
(in Chinese with English abstract) |
Wintermans J F G M, De Mots A. 1965. Spectrophotometric characteristics of chlorophylls a and b and their pheophytins in ethanol. Biochimica et Biophysica Acta (BBA) - Biophysics including Photosynthesis, 109(2): 448-453.
DOI:10.1016/0926-6585(65)90170-6 |
Zhang S L, Zhang B, Dai W, Zhang X M. 2011. Oxidative damage and antioxidant responses in Microcystis aeruginosa exposed to the allelochemical berberine isolated from golden thread. Journal of Plant Physiology, 168(7): 639-643.
DOI:10.1016/j.jplph.2010.10.005 |
Zhou M J, Zhu M Y. 2006. Progress of the project "Ecology and oceanography of harmful algal blooms in China". Advances in Earth Science, 21(7): 673-679.
(in Chinese with English abstract) |
 2019, Vol. 37
2019, Vol. 37