Institute of Oceanology, Chinese Academy of Sciences
Article Information
- GARCÍA-MUÑOZ Enrique, GUERRERO Francisco, ARECHAGA Garbiñe, PARRA Gema
- Does wetland watershed land use influence amphibian larval development? A relevant effect of agriculture on biota
- Journal of Oceanology and Limnology, 37(1): 160-168
- http://dx.doi.org/10.1007/s00343-019-7378-8
Article History
- Received Dec. 27, 2017
- accepted in principle Feb. 22, 2018
- accepted for publication Apr. 5, 2018
2 Centro de Estudios Avanzados en Ciencias de la Tierra, Campus de las Lagunillas, s/n. E-23071 Jaén, Spain;
3 Departamento de Ciencias de la Salud, Campus de las Lagunillas, s/n. 23071 Jaén, Spain
Intensification of agriculture is widely recognized as one of the most significant human alterations to the global environment (Mann et al., 2009). The responsibility of this activity in the disappearance of wetlands and their pollution has been supported by large body of literature (i.e. Casado and Montes, 1995; Beja and Alcazar, 2003; Parra et al., 2005). Moreover, the improper use and excessive application of agrochemicals generates an impact on the ecosystem structure and function, thereby on its ecological integrity (Troncoso et al., 2000). Agrochemical impacts have consequences at different hierarchical levels: from the individual level, with morphological, physiological and biochemical alterations, to the community level with the loss of diversity, and the impairment of the value and services that healthy ecosystems provide (Montes and Sala, 2007).
Thereby, the agricultural application of fertilizers, pesticides, herbicides and fungicides is receiving increasing attention as a potential cause of amphibian decline, acting singly or in combination with other stressors disturbing their life cycle during the aquatic and terrestrial phases (Relyea and Mills, 2001; Mann et al., 2009; Hua and Relyea, 2014). During the larval period, anuran amphibians are in contact with water and sediments. Many of them act as detrital feeders, so they could be in contact with pollutants due to their feeding habits and by direct absorption through the integument (Ingersoll, 1995). In the study area in the southeast of Spain, the most common agrochemicals used in the intensive olive groves are mainly fertilizers (nitrogen-based compounds) and fungicides (copper sulphate-based compounds), as well as insecticides (Junta de Andalucía 2008; García-Muñoz et al., 2011a). Previous studies have shown that some of these substances (specifically those with nitrogenbased and copper sulphate-based compounds) have lethal and sublethal effects on several amphibian species in the area studied. For instance, concentrations below 0.1 mg/L Cu affected larval growth and the development of Bufo bufo, Epidalea calamita, Discoglossus jeanneae, Pelobates cultripes and Pelophylax perezi negatively. Besides, the behaviour of E. calamita is also affected under a similar copper concentration. On the other hand, the fertilizer ammonium nitrate reduces amphibian survival and larvae total length in B. bufo, E. calamita, Pelodytes ibericus and P. perezi (García-Muñoz et al., 2009, 2010a, 2011a). Changes in larval scape behaviour have been also observed when tadpoles are exposed to ammonium nitrate and to copper sulphate, moreover this sublethal effect has been proposed as a rapid biomarker of wetland pollution (García-Muñoz et al., 2011b). The application of biomarkers (behavioural, histological or biochemical) presents advantages to complement traditional chemical methods for detecting pollution (Handy et al., 2003; Hagger et al., 2006). Among these biomarkers, the lipid peroxidation level highlights the consequences in organisms exposed to pollutants (Cavas and Tarhan, 2003), and is also being used as a biomarker in amphibians (Falfushinska et al., 2008; Gripp et al., 2017). Lipid peroxidation is a process mediated by free radicals (Slater, 1984a, b ), and is considered the best measure of damage due to the increase in reactive oxygen species (ROS) (Halliwell and Gutteridge, 1989).
In order to truly reflect the effects that the contaminants generate at population level, the number of studies addressing the effect of chemical pollution in outdoor mesocosms and whole-sediment experiments is increasing (Cooke, 1973; Day et al., 1995; Relyea, 2006; Mann et al., 2009; Araújo et al., 2010; Burton, 2013; Bókony et al., 2017; Koprivnikar et al., 2017). These types of experiments are very useful when the agrochemicals used within the watershed are difficult to detect since they are subjected to transformation, degradation and dilution processes while reaching the aquatic system (Andreu and Picó, 2004). Although pollutant concentrations in water were below the level of detection, their presence in sediments could generate toxic effects on amphibian tadpoles because they filter and rasp the sediment surface when feeding (algae, dead organic matter…) (Baier et al., 2016). At the end, the wetland sediment accumulates (integrates) all the natural and anthropogenic changes and alterations (de Vicente et al., 2010). Sediments have been recognized as a potential source of the toxicity of a contaminant in aquatic environments and whole-sediment bioassays have been reported to be a powerful tool in studying sediment-related toxicity (Luoma and Carter, 1993).
In this study, an outdoor experiment using two sediments that were different in origin was designed to analyse the effects on amphibian larvae. Our hypothesis is that amphibian larvae can be affected by exposure to sediment that comes from a wetland surrounded by intensive agriculture, where agrochemicals are used regularly. Although wholesediment experiments have not been frequently used with amphibians, the present investigation uses an approach where the environmental sample (polluted sediment) is used as an origin treatment, and the endpoints to be measured are those that have been previously reported in amphibians to be affected by agrochemicals: survival, development, length, and lipid peroxidation level.
2 MATERIAL AND METHOD 2.1 Study speciesBased on previous toxicological studies (García-Muñoz et al., 2010a, 2011b), one of the most sensitive Iberian anuran species was selected, Bufo bufo. This species has disappeared from several wetlands in the southeast of the Iberian Peninsula, and only inhabits moderately or well-preserved ecosystems (García-Muñoz et al., 2010b).
Bufo bufo egg masses (fractions of six different egg clutches in order to ensure natural genetic variability) were collected (by permission of the relevant authorities) from Cañada de las Hazadillas, an aquatic ecosystem located in the southeast of the Iberian Peninsula that does not have a known history of pollution. Egg masses were brought to the laboratory immediately after collection (under temperaturecontrolled conditions). The samples were pooled to gain a genetically varied and representative sample of individuals. Eggs were kept at 20℃ (±0.5℃) on a 12 h light:12 h dark cycle in a temperature-controlled chamber. Individuals were allowed to develop to Gosner stage 25 (Gosner, 1960) in aquariums that were filled with water from the same aquatic ecosystem (pH: 7.2–7.8; alkalinity: 170–250 mg/L; hardness: 129 mg/L; NO3ˉ < 0.1 mg/L). Gosner stage 25 is the usual stage to begin the experiment (Edwards et al., 2006; Brunelli et al., 2009; García-Muñoz et al., 2009).
2.2 Outdoor experimentFour experimental plastic pools (200 cm×200 cm× 50 cm) located in the University of Jaén's experimental garden were used in the present study. They were 0.5 m apart, completely open to the environment and under equal environmental conditions (temperature, humidity and light conditions). In each of them, five centimetres of sediment from specific sites (see below the sediment origin) was added together with 1 200 L of dechlorinated potable water in order to create a water depth of 30 cm. After seven days, 650 tadpoles of B. bufo, randomly selected from different egg masses at Gosner stage 25, were added to each pool. The experiment was carried out for 52 days. The high number of tadpoles was selected in order to recreate the high density in natural ponds, taking into account that B. bufo females lay large clutches with a high number of eggs (Salvador and García-París, 2001). Lamentably and taking into account the elevated number of organisms in each pool, the size of the pools and the space limitation in the experimental garden, the number of experimental replicates was limited to two in each treatment.
2.3 Sediment treatmentsTwo different treatments were used. The first one with sediments from a wetland (Naranjeros wetland; south-eastern Spain; Jaén province; UTM: 30SVG0978) with a watershed that is affected by intensive olive tree agricultural practices (AP). In the second treatment, sediments came from the experimental garden of the University of Jaén with no record of agrochemical uses (NAP). Sediments were brought to the laboratory immediately after collection (only the first five centimetres of wetland sediments were collected) and were added to each pool, creating a plain layer.
Sediment chemical analyses were carried out by inductively coupled plasma mass spectrometry (days zero and 52) to characterize the concentration of copper (Cu2+, μmol/L) and by Continuous Flow Analyzer and spectrometric detection (ISO 13395: 1996; ISO 11732: 1997) for ammonium (NH4+, mg/L) and nitrate (NO3ˉ, mg/L) concentrations. The selected variables are the main components in fertilizers and fungicides usually used in intensive olive farming (Junta de Andalucía 2008; García-Muñoz et al., 2011a).
Water samples were collected on days 7, 14, 37 and 52. The selected variables were the same as those in sediment samples. Copper was analysed with a photometer (Filterphotometer PF11; MachereyNagel) according to a colorimetric technique (detection limit 0.040 mg Cu2+/L). Ammonium was analysed with the indophenol blue method (Rodier, 1989—detection limit 0.010 mg N/L) and nitrate by ultraviolet method (American Public Health Association et al., 1995—detection limit 0.045 mg N/L). Additionally, fluorescence was measured using a field fluorometer (Aquafluor Turner Design Handheld). Chlorophyll-a concentration (Chl a, mg/L) was later calculated using a previously obtained calibration curve determined by fluorometry. Oxygen, temperature, total dissolved solids (TDS) and pH were measured in each pool at the start of the experiment and on days 7, 14, 37 and 52 using a multiparametric probe (YSI-556 MPS; Yellow Springs, OH 45387 USA).
2.4 Larval and metamorph total lengthA subsample of one hundred individuals from each experimental pool was collected at the beginning of the experiment (day zero; start outdoor experiment), deposited in a Petri dish (with 0.5 cm of water), photographed against grid paper and placed again in its experimental pool of origin. The mentioned procedure was repeated 9 times during the whole experimental period. The larval total length (from snout to tail tip) was measured using the ruler function (accurate to 0.01 mm) in the Image-J program. On day 37 the first metamorph individual (Gosner stage 46) was observed. From then on, the number of individuals reaching stage 46 was also recorded each day. Fully metamorphosed individuals were collected and deposited in a dish against grid paper (without water) and were photographed. Metamorph length (snout-vent length; SVL) was measured following the same methodology previously commented.
2.5 Lipid peroxidation and protein contentAt the end of the experimental period, 60 metamorphs (15 metamorphs from each experimental pool) were humanely euthanized in 150 to 200 mg/L e.g. MS-222 (buffered with sodium bicarbonate to achieve pH 7.0) and were frozen at -80℃ for biochemical analysis. Before being analysed, all the individuals were weighed quickly in pre-cooled Eppendorf tubes, using a microbalance (Mettler Toledo microbalance; precision 0.01 mg) and kept in crushed ice until homogenization. The lipid peroxidation was quantified with the concentration of the malondialdehyde (MDA), an end product of lipid peroxidation, following the method proposed by Esterbauer and co-workers (Esterbauer et al., 1991). The standard curve preparation was carried out with 10 mmol/L TMP (1, 1, 3, 3-tetramethoxypropane; Sigma-Aldrich) at 4℃. Protein content was determined colorimetrically using the Bradford method (Bradford, 1976) with Coomassie Brilliant Blue G-250 (Sigma-Aldrich) using bovine serum albumin as a standard.
2.6 Statistical analysisSediment variables were analysed using a t-test to detect differences between the treatments. Water variables were analysed using repeated measures ANOVA to detect intra treatments and intra time differences. The larval total length (log transformed) and metamorph total length (log transformed) were analysed using repeated measures ANOVA. The differences in survival versus mortality in each treatment were tested using Fisher's exact test, P one-tailed. The differences in MDA concentrations (unit of MDA /mg protein log transformed) were tested by a t-test. In order to test normality, a Kolmogorov-Smirnov Z-test was conducted before the statistical analysis. In every case the statistic Z-test showed P values > 0.05. The statistical analyses were performed with SPSS software (SPSS Inc., Chicago, Il).
3 RESULTTable 1 shows temperature, oxygen, TDS, pH and chlorophyll-a mean values in water samples. The repeated measures ANOVA test showed differences throughout the experimental period in the oxygen (F4, 8=54.47; P=0.018), pH (F4, 8=34.15; P=0.028), TDS (F4, 8=64.64; P=0.015) and chlorophyll-a values (F4, 8=17.66 and P < 0.001). However, no statistical differences between the treatments were observed in oxygen (F1, 2=8.43; P=0.101), pH (F1, 2=0.995; P=0.424), and chlorophyll-a values (F1, 2=0.860 and P=0.452). Only TDS values showed differences between the treatments (F1, 2=40.36; P=0.024). No statistical differences were found between the treatments in selected water chemical variables. The repeated measures ANOVA test showed no differences throughout the experimental period and between treatments in ammonium (P=0.423) and nitrate (P=0.461). In all the treatments, ammonium never exceeded the concentration of 1.9 mg NH4+/L, while the maximum nitrate concentration found in the treatments was 6.2 mg NO3ˉ/L. The copper concentration ranged between 0.1 to 0.2 μmol Cu/L, showing statistical differences throughout the experimental period (F4, 8=14.23; P=0.001), although no statistical differences were found between the treatments (F1, 2=3.52; P=0.201).
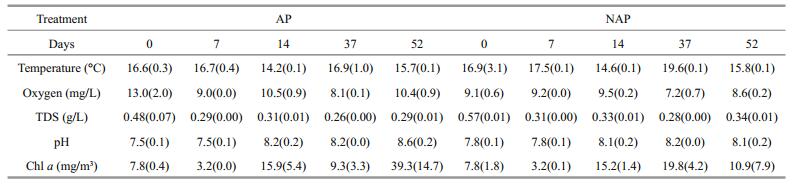
|
No differences in the selected sediment variables were found between the treatments (P > 0.05). Ammonium mean values in AP and NAP treatments were 4.3 (±1.4) and 2.6 (±1.2) mg NH4+/L, respectively. Copper mean values in AP and NAP treatments were 0.3 (±0.1) and 0.2 (±0.01) μmol Cu/L, respectively. The nitrate concentration found in the treatments was lower than 6.1 mg NO3ˉ/L in both cases.
Amphibian survival in the NAP treatment was 79% (±2.82%) while in the AP treatment it was 52.5% (±4.94%). The results of the Fisher's exact test showed differences (P < 0.001) in survival at the end of the experimental period between the treatments. The results showed statistical differences in premetamorphic total length between the treatments just on day 28 (P=0.037). Larvae from the NAP treatment showed bigger sizes when reaching the metamorphosis period than larvae from the AP treatment (Fig. 1). Metamorphs in the NAP treatment showed higher SVL than in the AP treatment, except on day 42 (P=0.300) (Fig. 1). Furthermore, Fig. 2 showed the accumulated number of metamorphs in each treatment, highlighting the lower number of successfully metamorphosed organisms in the AP treatment and a delay in completing metamorphosis. Twenty-six metamorphs were collected on day 37 in the NAP pools and none in the AP pools on the same day. Moreover, a maximum number of metamorphs was observed on day 42 and 52 in NAP and AP treatments respectively.
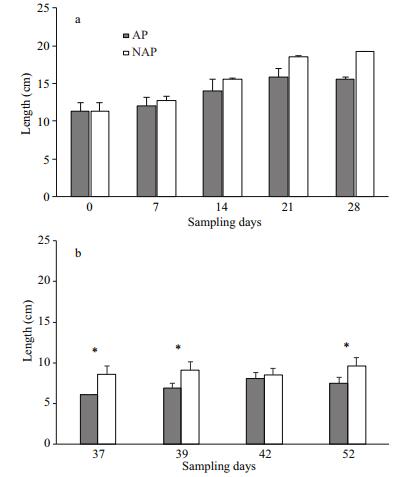
|
| Fig.1 a) Larval total length (TL) mean values (cm) in agricultural practice (AP) and non-agricultural practice (NAP) treatments during 28 days of experimental period; b) metamorph snout to vent length (SVL) mean values (cm) in agricultural practice (AP) and non-agricultural practice (NAP) treatments measured from day 37 to day 52 * indicates significant differences between treatments (P<0.05). |

|
| Fig.2 Accumulated number of metamorphs in agricultural practice (AP) and non-agricultural practice (NAP) treatments during the experimental period |
The lipid peroxidation level was different in each treatment. The MDA concentration was affected by the treatment (t=6.24; P=0.001). Metamorphs from the NAP showed lower MDA concentrations (157.5±55.6 MDA unit/mg protein) than metamorphs from the AP treatment (615±135.8 MDA unit/mg protein).
4 DISCUSSIONThe results obtained in the present study show differences between treatments in survival, length, development and lipid peroxidation level in B. bufo. The differences in the selected endpoints obtained from the present study are relevant because the water column is not the only significant exposure source for amphibians in a natural habitat (Gerdron, 2013). As they are in close contact with the substrate (when foraging during larval development), experiments with sediments need to be conducted. In this sense, the present study had shown four important negative effects at different levels that intensive agricultural practices could generate on natural amphibian populations, although we could not attribute a specific cause to explain the observed effects, apart from the sediment origin.
The first two negative results obtained in this study, a reduction in survival and an increase in the development time of B. bufo, are clearly related to the recruitment of amphibian populations. If fewer tadpoles complete their metamorphosis, a reduction in recruitment to the terrestrial environment is generated and, consequently, a potential reduction in the number of breeding individuals for the future population. In the same way, an increase in development time has an important effect on the total number of individuals that could complete their metamorphosis successfully (Sinsch, 1998). This last aspect is a key factor in Mediterranean landscapes, where the majority of wetlands are temporary (Guerrero et al., 2006) and tadpoles have a short period of time to complete their metamorphosis process. Moreover, in a Mediterranean context, as the development time increases while the spring draws on, there is an increase in wetland water temperature. An increase in this environmental variable alters the development, metabolism and behaviour of individuals (Gripp et al., 2017).
The third negative effect is a reduction in the length of B. bufo. Similar results were found in Bufo americanus larvae exposed to pond sediments with elevated metal levels and chloride water concentrations (Snodgrass et al., 2008). Copper sulphate- and nitrogen-based compounds typically used in the study area generate a reduction in larval length of different amphibian species (García-Muñoz et al., 2009, 2010a). However, other factors responsible for producing variation in the experimental pools (such as the amount and quality of food—not measured in this study—or other type of agrochemicals such as insecticides) could be behind the results obtained. The present study has also shown how a reduction in larval size is related to a smaller size in metamorphs. Similar effects were found in tadpoles of B. americanus (Breden and Kelly, 1982). As a result, this sublethal effect could have drastic consequences because different fitness parameters are directly related to size at metamorphosis (Semlitsch et al., 1988). In this sense it is well known that a reduction in larval size increases susceptibility to predation and reduces the survivorship and fecundity of offspring (Smith, 1987; Boone and Semlitsch, 2002; Altwegg and Reyer, 2003; Boone and James, 2003).
The fourth negative effect found has occurred at a subcellular level. An increase in lipid peroxidation level exposed to chemicals has been described in invertebrates and vertebrates (Radi and Matkovics, 1988; Barata et al., 2005; Gripp et al., 2017). This biochemical biomarker is one of the most appropriate used to relate damage at the individual level with consequences at the population level, due to the fact that subcellular alterations could show their effects at higher hierarchical levels (Peakall, 1992). In this sense, a relationship to higher hierarchical levels could be accomplished through energy expenditure in detoxification processes. In theory, energy investment should be regulated by metabolic expenditure, representing a balance between the cost of survival and activity, and the production of somatic and reproductive tissues (Congdon et al., 2001). Some studies have shown that exposure to pollutants increases metabolic expenditure and it is possible to obtain a relationship with the energetic cost that detoxification implies on individuals embedded in agricultural landscapes (Rowe et al., 1998; Hopkins et al., 1999) and could be correlated with a reduction in growth/development (Beck and Congdon, 2003). Although the interpretation of biomarker information in the field is much more complex than it is in laboratory-controlled conditions, they are meant to be used as early warning indicators and are seen as efficient diagnosis tools (Gerdron, 2013).
The low number of replicates is the main criticism of this study. However, a lower statistical power could lead to erroneous conclusions including false negatives (i.e. concluding that there is no effect when in fact there is one). The lack of statistical power or statistical significance should not conflict with common sense (Barnett and Mathisen, 1997). The statistical autocracy must not make us forget that what really matters is the detection of effects in amphibians during an outdoor experiment that tries to simulate more realistic conditions than a lab experiment. The lack of confirmation of causality with a specific toxic substance could be another possible criticism. From a Toxicity Identification Evaluation (TIE) approach, biological tests (toxicity tests) must be applied in order to detect the chemicals responsible for the toxicity (Camargo et al., 2015). However, the present results do not allow the active substances responsible for the toxicity to be determined. More analytical procedures on the sediment characterization, such as the analysis of sediment grain size distribution, as well as organic material content (OM) or the presence of pesticides, could give more data in order to confirm causality. There is evidence that supports a decrease in tadpole survival and growth with pesticides (Relyea, 2006) or when the proportion of fine sediment increases and the OM content decreases, both reducing the availability of resources for tadpoles (Wood and Richardson, 2009; Courcelles, 2016). In this sense, Mediterranean wetlands surrounded by intensive olive groves showed the presence of pesticides (Robles-Molina et al., 2014), high sediment deposition from the watershed and low OM levels (Gilbert et al., 2015; Vanwalleghem et al., 2011). Moreover, a deep physiological analysis that determines the multiple exposure pathways (integument, diet) also needs to be carried out.
Dealing with a level of uncertainty could be unavoidable when the ecosystem complexity is trying to be reached. Part of this uncertainty could come from experimental procedures and part from the extrapolation from the laboratory to nature (Luoma and Carter, 1993). While the use and development of mesocosms and outdoor experiments allow a better understanding and integration of the effects of chemicals on amphibians (Gerdron 2013), in the present study no causative relationship has been determined, beyond the sediment provenance, in spite of the significant negative effects which were observed. The direct and indirect effects of land use on amphibians are difficult to distinguish, but the indirect effect, including high soil erosion rates and contributions of pollutants from different land use types to wetland sediment, could ultimately determine the wetland habitat quality for amphibians (Snodgrass et al., 2008).
Finally, our results indicate that intensive agricultural practices have indirect relevant consequences on amphibian populations. The effectiveness of the protection of amphibian populations would be achieved, for example, through (ⅰ) an agricultural policy change that favours environmentally-friendly agriculture in wetland watersheds that enhances soil protection and reduces diffuse pollution, and (ⅱ) the creation of buffer zones around wetlands. Both actions will imply a reduction in the agrochemical content of water and sediments of wetlands and also an increase in watershed habitat heterogeneity that could help to increase amphibian diversity in Mediterranean wetlands.
5 CONCLUSIONIn summary, the present study has shown four important effects on amphibian tadpoles, and at different hierarchical levels, generated by sediments in wetland that is surrounded by intensive agriculture: a decrease in survival, a decrease in length, retarded development and an increase in peroxidation level. Regarding this, watershed land uses and agricultural practices can influence the number of successfully metamorphosed amphibians in wetlands and consequently the recruitment. These results allow us to understand the consequences on amphibian populations that inhabit areas affected by intensive agriculture.
6 DATA AVAILABILITY STATEMENTThe authors declare that all data supporting the findings of this study are available within the article.
7 ACKNOWLEDGEMENTOur thanks go to the Consejería de Medio Ambiente (Junta de Andalucía) for permission to use the amphibian surveys. E.G.-M. has a research grant from the FCT postdoctoral programme (SFRH/ BPD/78206/2010). We also like to express our sincere thanks to the reviewers for their valuable comments and suggestions, which have been very helpful in improving the manuscript.
Altwegg R, Reyer H U. 2003. Patterns of natural selection on size at metamorphosis in water frogs. Evolution, 57(4): 872-882.
DOI:10.1111/evo.2003.57.issue-4 |
American Public Health Association. 1995. Standard methods for the examination of water and wastewater. American Public Health Association, Washington, DC. 874p.
|
Andreu V, Picó Y. 2004. Determination of pesticides and their degradation products in soils: current status, gaps and the future. Journal of Limnology, 64: 13-29.
|
Araújo C V M, Tornero V, Lubián L M, Blasco J, van Bergeijk S A, Cañavate P, Cid , Franco D, Prado R, Bartual A, López M G, Ribeiro R, Moreira-Santos M, Torreblanca A, Jurado B, Moreno-Garrido I. 2010. Ring test for wholesediment toxicity assay with-a-benthic marine diatom. Science of the Total Environment, 408(4): 822-828.
DOI:10.1016/j.scitotenv.2009.10.018 |
Baier F, Jedinger M, Gruber E, Zaller J G. 2016. Temperaturedependence of glyphosate-based herbicide's effects on egg and tadpole growth of common toads. Frontiers in Environmental Science, 4: 51.
|
Barata C, Varo I, Navarro J C, Arun S, Porte C. 2005. Antioxidant enzyme activities and lipid peroxidation in the freshwater cladoceran Daphnia magna exposed to redox cycling compounds. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 140(2): 175-186.
|
Barnett M L, Mathisen A M. 1997. Tyranny of the P-value: The conflict between statistical significance and common sense. Journal of Dental Research, 76(1): 534-536.
DOI:10.1177/00220345970760010201 |
Beck C W, Congdon J D. 2003. Energetics of metamorphic climax in the southern toad (Bufo terrestris). Oecologia, 137(3): 344-351.
DOI:10.1007/s00442-003-1374-5 |
Beja P, Alcazar R. 2003. Conservation of Mediterranean temporary ponds under agricultural intensification: an evaluation using amphibians. Biological Conservation, 114(3): 317-326.
DOI:10.1016/S0006-3207(03)00051-X |
Bókony V, Mikó Z, Móricz Á M, Krüzselyi D, Hettyey A. 2017. Chronic exposure to a glyphosate-based herbicide makes toad larvae more toxic. Proceedings of the Royal Society B, 284(1858): 20170493.
DOI:10.1098/rspb.2017.0493 |
Boone M D, James S M. 2003. Interactions of an insecticide, herbicide, and natural stressors in amphibian community mesocosms. Ecological Applications, 13(3): 829-841.
DOI:10.1890/1051-0761(2003)013[0829:IOAIHA]2.0.CO;2 |
Boone M D, Semlitsch R D. 2002. Interactions of an insecticide with competition and pond drying in amphibian communities. Ecological Applications, 12(1): 307-316.
DOI:10.1890/1051-0761(2002)012[0307:IOAIWC]2.0.CO;2 |
Bradford M M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72(1-2): 248-254.
DOI:10.1016/0003-2697(76)90527-3 |
Breden F, Kelly C H. 1982. The effect of conspecific interactions on metamorphosis in Bufo americanus. Ecology, 63(6): 1 682-1 689.
DOI:10.2307/1940110 |
Brunelli E, Bernabò I, Berg C, Lundstedt-Enkel K, Bonacci A, Tripepi S. 2009. Environmentally relevant concentrations of endosulfan impair development, metamorphosis and behaviour in Bufo bufo tadpoles. Aquatic Toxicology, 91(2): 135-142.
DOI:10.1016/j.aquatox.2008.09.006 |
Burton Jr G A. 2013. Sediment ecotoxicity. In: Férard J F, Blaise C eds. Encyclopedia of Aquatic Ecotoxicology. Springer, Dordrecht. p.1 003-1 014.
|
Camargo J B D A, Cruz A C F, Campos B G, Araújo G S, Fonseca T G, Abessa D M S. 2015. Use, development and improvements in the protocol of whole-sediment toxicity identification evaluation using benthic copepods. Marine Pollution Bulletin, 91(2): 511-517.
DOI:10.1016/j.marpolbul.2014.10.015 |
Casado C, Montes C. 1995. Guide of Spanish lakes and wetlands. In: Reyero J M ed. Madrid. 293p.
|
Cavas L, Tarhan L. 2003. Glutathione redox system, GSH-Px activity and lipid peroxidation (LPO)levels in tadpoles of R ridibunda and B. viridis. Cell Biochemistry and Function, 21(1): 75-79.
DOI:10.1002/(ISSN)1099-0844 |
Congdon J D, Dunham A E, Hopkins W A, Rowe C L, Hinton T G. 2001. Resource allocation-based life histories: a conceptual basis for studies of ecological toxicology. Environmental Toxicology and Chemistry, 20(8): 1 698-1 703.
DOI:10.1002/etc.v20:8 |
Cooke A S. 1973. The effects of DDT, when used as a mosquito larvicide, on tadpoles of the frog Rana temporaria. Environmental Pollution, 5(4): 259-273.
DOI:10.1016/0013-9327(73)90003-7 |
Courcelles D M M. 2016. Inorganic fine sediment deposition in rivers with run-of-river hydropower projects and Coastal Tailed Frog (Ascaphus truei) tadpoles in coastal British Columbia. University of British Columbia's Information Repository. 64p.
|
Day K E, Kirby R S, Reynoldson T B. 1995. The effect of manipulations of freshwater sediments on responses of benthic invertebrates in whole‐sediment toxicity tests. Environmental Toxicology and Chemistry, 14(8): 1 333-1 343.
DOI:10.1002/etc.v14:8 |
de Vicente I, Guerrero F, Cruz-Pizarro L. 2010. Chemical composition of wetland sediments as an integrator of trophic state. Aquatic Ecosystem Health and Management, 13(1): 99-103.
DOI:10.1080/14634980903566816 |
Edwards T M, McCoy K A, Barbeau T, McCoy M W, Thro J M, Guillette Jr L J. 2006. Environmental context determines nitrate toxicity in Southern toad (Bufo terrestris) tadpoles. Aquatic Toxicology, 78(1): 50-58.
|
Esterbauer H, Schaur R J, Zollner H. 1991. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radical Biology and Medicine, 11(1): 81-128.
DOI:10.1016/0891-5849(91)90192-6 |
Falfushinska H, Loumbourdis N, Romanchuk L, Stolyar O. 2008. Validation of oxidative stress responses in two populations of frogs from Western Ukraine. Chemosphere, 73(7): 1 096-1 101.
DOI:10.1016/j.chemosphere.2008.07.060 |
García-Muñoz E, Gilbert J D, Parra G, Guerrero F. 2010b. Wetlands classification for amphibian conservation in Mediterranean landscapes. Biodiversity and Conservation, 19(3): 901-911.
DOI:10.1007/s10531-009-9747-7 |
García-Muñoz E, Guerrero F, Parra G. 2009. Effects of copper sulfate on growth, development, and escape behavior in Epidalea calamita embryos and larvae. Archives of Environmental Contamination and Toxicology, 56(3): 557-565.
DOI:10.1007/s00244-008-9201-y |
García-Muñoz E, Guerrero F, Parra G. 2010a. Intraspecific and interspecific tolerance to copper sulphate in five Iberian amphibian species at two developmental stages. Archives of Environmental Contamination and Toxicology, 59(2): 312-321.
DOI:10.1007/s00244-010-9473-x |
García-Muñoz E, Guerrero F, Parra G. 2011a. Effects of previous sublethal pulse to ammonium nitrate on mortality and total length on Epidalea calamita larvae. Chemosphere, 84(5): 671-675.
DOI:10.1016/j.chemosphere.2011.03.031 |
García-Muñoz E, Guerrero F, Parra G. 2011b. Larval escape behavior in anuran amphibians as a wetland rapid pollution biomarker. Marine and Freshwater Behaviour and Physiology, 44(2): 109-123.
DOI:10.1080/10236244.2011.557855 |
Gerdron A. 2013. Amphibian Ecotoxicology. In: Férard J F, Blaise C eds. Encyclopedia of Aquatic Ecotoxicology. Springer, Dordrecht. p.21-37.
|
Gilbert J D, Guerrero F, Jiménez-Melero R, de Vicente I. 2015. Is the bioproduction number a good index of the trophic state in Mediterranean wetlands?. Knowledge and Management of Aquatic Ecosystems, 416: 05.
|
Gosner K L. 1960. A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica, 16(3): 183-190.
|
Gripp H S, Freitas J S, Almeida E A, Bisinoti M C, Moreira A B. 2017. Biochemical effects of fipronil and its metabolites on lipid peroxidation and enzymatic antioxidant defense in tadpoles (Eupemphix nattereri: Leiuperidae). Ecotoxicology and Environmental Safety, 136: 173-179.
DOI:10.1016/j.ecoenv.2016.10.027 |
Guerrero F, Parra G, Jiménez-Gómez F, Salazar C, JiménezMelero R, Galotti A, García-Muñoz E, Lendínez M L, Ortega F. 2006. Ecological studies in Alto Guadalquivir wetlands: a first step towards the application of conservation plans. Limnetica, 25: 95-106.
|
Hagger J A, Jones M B, Leonard D P, Owen R, Galloway T S. 2006. Biomarkers and integrated environmental risk assessment: are there more questions than answers?. Integrated Environmental Assessment and Management, 2(4): 312-329.
DOI:10.1002/ieam.5630020403 |
Halliwell B, Gutteridge J M C. 1989. Free radicals in biology and medicine. Oxford University Press, New York. 543p.
|
Handy R D, Galloway T S, Depledge M H. 2003. A proposal for the use of biomarkers for the assessment of chronic pollution and in regulatory toxicology. Ecotoxicology, 12(1-4): 331-343.
|
Hopkins W A, Rowe C L, Congdon J D. 1999. Elevated trace element concentrations and standard metabolic rate in banded water snakes (Nerodia fasciata) exposed to coal combustion wastes. Environmental Toxicology and Chemistry, 18(6): 1 258-1 263.
DOI:10.1002/etc.v18:6 |
Hua J, Relyea R. 2014. Chemical cocktails in aquatic systems: pesticide effects on the response and recovery of > 20 animal taxa. Environmental Pollution, 189: 18-26.
DOI:10.1016/j.envpol.2014.02.007 |
Ingersoll C G. 1995. Sediment test. In: Rand G M. ed. Fundamentals of Aquatic Toxicology: Effects, Environmental Fate, and Risk Assessment. CRC Press, Florida. p.231-256.
|
Junta de Andalucía. 2008. Reglamento específico de producción integrada de olivar. BOJA 83: 9-38.
|
Koprivnikar J, Riepe T B, Calhoun D M, Johnson P T J. 2017. Whether larval amphibians school does not affect the parasite aggregation rule: testing the effects of host spatial heterogeneity in field and experimental studies. Oikos, 127(1): 99-110.
|
Luoma S N, Carter J L. 1993. Understanding the toxicity of contaminants in sediments: beyond the bioassay-based paradigm. Environmental Toxicology and Chemistry, 12: 793-796.
DOI:10.1002/etc.v12:5 |
Mann R M, Hyne R V, Choung C B, Wilson S P. 2009. Amphibians and agricultural chemicals: Review of the risks in a complex environment. Environmental Pollution, 157(11): 2 903-2 927.
DOI:10.1016/j.envpol.2009.05.015 |
Montes C, Sala O. 2007. La evaluación de los ecosistemas del milenio. Las relaciones entre el funcionamiento de los ecosistemas y el bienestar humano. Ecosistemas, 16(3): 137-147.
|
Parra G, Jiménez-Melero R, Guerrero F. 2005. Agricultural impacts on Mediterranean wetlands: the effect of pesticides on survival and hatching rates in copepods. International Journal of Limnology, 41(3): 161-167.
DOI:10.1051/limn:20054130161 |
Peakall D. 1992. The role of biomarkers in environmental assessment. In: Peakall D ed. Animal Biomarkers as Pollution Indicators. Springer, Dordrecht. p.201-206.
|
Radi A A R, Matkovics B. 1988. Effects of metal ions on the antioxidant enzyme activities, protein contents and lipid peroxidation of carp tissues. Comparative Biochemistry and Physiology Part C: Comparative Pharmacology, 90(1): 69-72.
|
Relyea R A, Mills N. 2001. Predator-induced stress makes the pesticide carbaryl more deadly to gray treefrog tadpoles (Hyla versicolor). Proceedings of the National Academy of Sciences of the United States of America, 98(5): 2 491-2 496.
DOI:10.1073/pnas.031076198 |
Relyea R A. 2006. The effects of pesticides, pH, and predatory stress on amphibians under mesocosm conditions. Ecotoxicology, 15(6): 503-511.
DOI:10.1007/s10646-006-0086-0 |
Robles-Molina J, Gilbert-López B, García-Reyes J F, MolinaDíaz A. 2014. Monitoring of selected priority and emerging contaminants in the Guadalquivir River and other related surface waters in the province of Jaén, South East Spain. Science of the Total Environment, 479-480: 247-257.
DOI:10.1016/j.scitotenv.2014.01.121 |
Rodier J. 1989. Análisis de las aguas. Omega, Barcelona. 1 059p.
|
Rowe C L, Kinney O M, Nagle R D, Congdon J D. 1998. Elevated maintenance costs in an anuran (Rana catesbeiana) exposed to a mixture of trace elements during the embryonic and early larval periods. Physiological and Biochemical Zoology, 71: 27-35.
|
Salvador A, Garcia-Paris M. 2001. Anfibios españoles: identificación, historia natural y distribución. Esfagnos, Madrid. 269p.
|
Semlitsch R D, Scott D E, Pechmann J H K. 1988. Time and size at metamorphosis related to adult fitness in ambystoma talpoideum. Ecology, 69(1): 184-192.
DOI:10.2307/1943173 |
Sinsch U. 1998. Biologie und ökologie der kreuzkröte. Laurenti Verlag, Bochum. 222p.
|
Slater T F. 1984a. Overview of methods used for detecting lipid peroxidation. Methods in Enzymology, 105: 283-293.
DOI:10.1016/S0076-6879(84)05036-9 |
Slater T F. 1984b. Free-radical mechanisms in tissue injury. Biochemical Journal, 222(1): 1-15.
|
Smith D C. 1987. Adult recruitment in chorus frogs: effects of size and date at metamorphosis. Ecology, 68(2): 344-350.
DOI:10.2307/1939265 |
Snodgrass J W, Casey R E, Joseph D, Simon J A. 2008. Microcosm investigations of stormwater pond sediment toxicity to embryonic and larval amphibians: variation in sensitivity among species. Environmental Pollution, 154(2): 291-297.
DOI:10.1016/j.envpol.2007.10.003 |
Troncoso L, Galleguillos R, Larrain A. 2000. Effects of copper on the fitness of the Chilean scallop Argopecten purpuratus (Mollusca: Bivalvia). Hydrobiologia, 420(1): 185-189.
DOI:10.1023/A:1003947407939 |
Vanwalleghem T, Amate J I, de Molina M G, Fernández D S, Gómez J A. 2011. Quantifying the effect of historical soil management on soil erosion rates in Mediterranean olive orchards. Agriculture, Ecosystem and Environment, 142(3-4): 341-351.
DOI:10.1016/j.agee.2011.06.003 |
Wood S L R, Richardson J S. 2009. Impact of sediment and nutrient inputs on growth and survival of tadpoles of the western toad. Freshwater Biology, 54(5): 1 120-1 134.
DOI:10.1111/fwb.2009.54.issue-5 |
 2019, Vol. 37
2019, Vol. 37


