Institute of Oceanology, Chinese Academy of Sciences
Article Information
- LI Yanguo, CUI Dandan, ZHUO Pinli, ZHANG Lin, SUN Xue, XU Nianjun
- A new approach to promote astaxanthin accumulation via Na2WO4 in Haematococcus pluvialis
- Journal of Oceanology and Limnology, 37(1): 176-185
- http://dx.doi.org/10.1007/s00343-018-7317-0
Article History
- Received Nov. 14, 2017
- accepted in principle Feb. 5, 2018
- accepted for publication Apr. 10, 2018
Astaxanthin (3, 3′-dihydroxy-β, β-carotene-4, 4′-dione) is a keto-carotenoid with high antioxidant potency, which showed beneficial effects on human health and preventing diseases, such as diabetes, cardiovascular disease, cancer, and showed the effects of immuno-modulation and anti-aging activity (Yamashita, 2013). The U.S. Food and Drug Administration (FDA) and European Commission has approved the security of natural astaxanthin (Pashkow et al., 2008; Ambati et al., 2014), furthermore, it has been marketed as a new food resource for approximately 10 years without any adverse effects by the Ministry of Health, China. Astaxanthin has an estimated market of USD $2.57 billion by 2025 with irresistible business opportunity (Algae Industry Magazine, 2017).
The unicellular green alga Haematococcus pluvialis shows its highest astaxanthin content under different environmental stresses, including nitrogen deficiency, high light, or exposure to chemical regulators, such as ferrous sulfate and sodium acetate (Boussiba, 2000; Han et al., 2013). The astaxanthin content can be up to 4% of the dry weight (Lee and Ding, 1994; Ranga et al., 2009). Astaxanthin is stored in cytosolic lipid bodies in the form of monoesters or diesters (Ambati et al., 2013), which may enhance the stability, bioavailability and antioxidant properties of astaxanthin (Barbosa et al., 1999). The astaxanthin synthesis pathway in H. pluvialis is as follows. First, isopentenyl pyrophosphate (IPP) is synthesized from pyruvate and glyceraldehyde-3-phosphate by the nonmevalonate DOXP/MEP (Deoxyxylulose-5- phosphate) pathway (Disch et al., 1998). Then, isopentenyl diphosphate isomerase (Ipi), phytoene synthase (Psy) and so on catalyze the reaction from IPP to β-carotene. Finally, carotenoid ketolase (Bkt) and carotenoid hydroxylase catalyze the conversion of β-carotene to astaxanthin (Li, 2007).
Nitrate starvation is an effective method to increase the accumulation of astaxanthin, which can lead to extremely low levels of nitrate reductase (NR) activity and excess production of reactive oxygen species (ROS) in H. pluvialis (Dong et al., 2006; Vidhyavathi et al., 2008). For practical production, transferring large amounts of algae mass is difficult under nitrogen-deficient conditions; thus, a new approach to induce intracellular nitrate starvation in H. pluvialis is needed. Sodium tungstate (Na2WO4) is a NR inhibitor that significantly inhibits NR activity in the microalgae Nannochloropsis oculata and Isochrysis galbana (Shen et al., 2011; Fan, 2012). One possible mechanism for this action is that tungsten (W) can replace the molybdenum (Mo) in NR structure, which is the key element involved in the electron transfer process (Yu et al., 2010). Furthermore, Na2WO4 may induce metal stress in H. pluvialis. Because the effects of Na2WO4 in H. pluvialis have not been reported, we tested the effects and optimum concentration of Na2WO4 on astaxanthin accumulation in H. pluvialis. To elucidate the mechanism underlying astaxanthin accumulation in response to Na2WO4, we evaluated the cellular morphology, NR activity, soluble sugar and protein contents, and chlorophyll fluorescence of H. pluvialis at the optimum Na2WO4 concentration and examined the transcriptional expression of carotenogenic genes by quantitative real-time PCR.
2 MATERIAL AND METHOD 2.1 Algae culture and treatmentHaematococcus pluvialis was obtained from the Algae Collection Lab of Ningbo University and cultured in 250 mL Erlenmeyer flasks with 150 mL of NBU3# medium under a light intensity of 25 μmol/ (m2∙s) on a 12 h:12 h light:dark cycle at 24℃. The NBU3# medium was composed of the following components (in mg/L): KNO3, 100; K2HPO4, 10; MnSO4, 0.25; FeSO4·7H2O, 2.5; Na2EDTA, 20; VB12, 5×10-7; and VB1, 5×10-6 (Wang et al., 2014).
To explore the optimum concentration of Na2WO4, cells in the middle exponential stage were exposed to 1.0, 2.0, 3.0 and 4.0 mmol/L of Na2WO4 separately under continuous levels of high irradiance at 80 μmol/(m2∙s) (Lv et al., 2016). Algae were collected on days 5 and 10 to examine the astaxanthin content and cell density. Samples without Na2WO4 were used as the controls. The cell numbers were counted using a plankton counting chamber.
Haematococcus pluvialis cells in the exponential stage were exposed to an optimum concentration of Na2WO4under continuous irradiance at 80 μmol/(m2∙s). The cells were harvested after treatment for 0 d, 0.5 d, 1 d, 3 d and 5 d, and the samples were used to examine the cellular morphology, NR activity, soluble sugar and protein contents, chlorophyll fluorescence, and carotenogenic gene expression.
2.2 Morphological observations and astaxanthin determinationA 400× optical microscope was used for the morphological observations and photography. The astaxanthin content was extracted and measured according to the methods of Boussiba et al. (1992). Algae cells were harvested and treated with a 5% KOH solution in 30% methanol to degrade the chlorophyll. Then, astaxanthin was extracted with dimethylsulfoxide (DMSO) three times until the precipitate became colorless. The absorbance of the extracts was determined at 490 nm with a spectrophotometer (Metash UV-6100A, China). The blank contained only DMSO.
2.3 Nitrate reductase activity and soluble sugar and protein contents determinationsThe samples were frozen in liquid nitrogen and then ground completely into a fine powder. The powder was transferred and diluted to 2.0 mL. After centrifugation, the supernatant was used to determine the NR activity and the soluble sugar and protein contents. The NR activity was measured with a NR assay kit (Suzhou Comin Biotechnology, Suzhou, China), the soluble sugar was determined using the anthrone-sulfate method, and the soluble protein was determined using the Bradford method (Li, 2000).
2.4 Chlorophyll fluorescence determinationChlorophyll fluorescence was obtained using the Water-PAM (Walz, Germany). The samples were incubated in the dark for 15 min at 24℃. Fv'/Fm' was obtained from the induction curve under culture light intensity. The relative electron transport rate (rETR) was measured by applying a sequence of increasing actinic irradiance; each actinic light incubation lasted for 10 s (White et al., 2011). Ek was obtained from the curve-fitting model according to the method of Eilers and Peeters (1988).
2.5 RNA isolation and quantitative real-time PCRThe samples were frozen in liquid nitrogen and then ground into a fine powder. Total RNA was extracted using the Plant Total RNA kit (OMEGA, USA) according to the user manual. The cDNA used for the quantitative real-time PCR was synthesized from the total RNA using the TransScript All-in-One First-Strand cDNA Synthesis SuperMix for qPCR (TransGen Biotech; China).
The gene-specific primers were designed using the Primer Premier 6 software (Table 1). Then, the PCR products were quantified with a real-time PCR system (Eppendorf, Germany) using SYBR green fluorescence. The PCR amplification profiles were 94℃ for 30 s, followed by 40 cycles of 95℃ for 5 s, 58℃ for 15 s, and 72℃ for 10 s. The 2-ΔΔCt method was used to analyze the quantitative real-time PCR data (Livak and Schmittgen, 2001), and samples collected at 0 d were used as the control group.
All experiments were conducted in triplicate, and the data were analyzed with one-way ANOVA (SPSS 22.0). Duncan's test was used to test differences among the groups from different trials, and p-values less than 0.05 were considered significantly different.
3 RESULT 3.1 Optimum Na2WO4 concentrationThe cell densities of H. pluvialis after treatment with different Na2WO4 concentrations were shown in Fig. 1. The cell density in the control group increased continually, with the maximum of 18.72×104 cells/mL obtained at 10 d. The cell density nearly stabilized after application of 1.0 and 2.0 mmol/L of Na2WO4, decreased significantly with the application of 3.0 and 4.0 mmol/L of Na2WO4 from days 0 to 5 (P < 0.05), and increased again by day 10 in the 1.0, 2.0, 3.0 mmol/L, but there are no significantly different between them (P < 0.05); treatment with 4.0 mmol/L of Na2WO4 resulted in the lowest cells density on the day 5 and 10. The astaxanthin content in the motile cells at day 0 was extremely low at 5.12 pg/cell (0.45 mg/L) but increased significantly after treatment with Na2WO4 (Fig. 2). The value reached a peak at day 10 of 49.41 pg/cell (4.81 mg/L) after application of 3.0 mmol/Lof Na2WO4; this astaxanthin concentration was 1.94-fold higher than the control. In summary, 3.0 mmol/L of Na2WO4was the optimum concentration to induce the accumulation of astaxanthin in H. pluvialis.
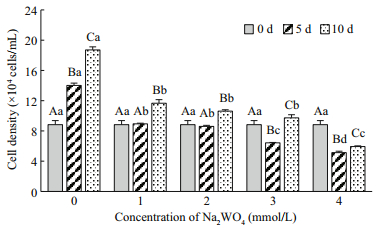
|
| Fig.1 The effects of different concentrations of Na2WO4 on the cell density of H. pluvialis Different uppercase letters among the different treatment times indicate significant differences (P < 0.05). Different lowercase letters among the different Na2WO4 concentrations indicate significant differences (P < 0.05). The same as that in the blow. |
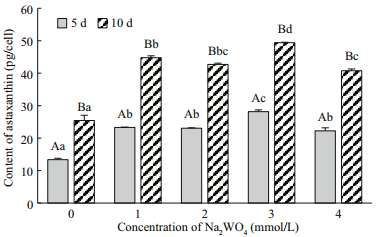
|
| Fig.2 The effects of different concentrations of Na2WO4 on the astaxanthin content of H. pluvialis |
Changes in the cell morphology of H. pluvialis after exposure to Na2WO4 at the optimal concentration are shown in Fig. 3. On the first day, most of these cells were round or oval motile cells with two flagella growing from the back of the protoplasts and extending through the periplasm to the cell wall (Fig. 3a). After treatment with 3 mmol/L of Na2WO4 for 0.5 d, the algae cells progressively changed from motile cells to non-motile cells, accompanied by abscission of the flagella, a more uniform morphology, and disappearance of the periplasmic space (Fig. 3b). At day 3, the algae color changed from green to red, which indicated that astaxanthin had accumulated in the cells. Meanwhile, the cell inclusions became more compact, and the cell wall tended to be thicker (Fig. 3c). After 5 days of treatment, the chloroplasts were pushed to the edges of the cells, most of the area was filled with astaxanthin, and the cell wall was visible and thick (Fig. 3d). We continued application of Na2WO4 to 10 d; microscopic observation showed that the algae cells became totally red and were obviously enlarged, which were typical characteristics of akinetes (Fig. 3e).

|
| Fig.3 The morphology of H. pluvialis treated with 3.0 mmol/L of Na2WO4 (×400 microscope) a. motile cells; b. non-motile cells; c. cells treated for 3 d; d. cells treated for 5 d; e. akinete. |
Haematococcus pluvialis cells in the exponential stage were exposed to 3 mmol/L of Na2WO4 under continuous irradiance at 80 μmol/(m2·s). The NR activity was increased significantly in the control cells at day 0.5 and then tended to decline slowly. The NR activity in the treatment cells decreased significantly at day 0.5 and was much lower than the activity in the control at all experimental time points (P < 0.05) (Fig. 4), which indicated that Na2WO4 significantly inhibited NR activity.

|
| Fig.4 The effects of Na2WO4 on nitrate reductase (NR) activity of H. pluvialis Treatment: 3.0 mmol/L of Na2WO4; control: without Na2WO4. The same as that in the blow |
The soluble sugar content is shown in Fig. 5a. At 0.5 d, the soluble sugar content in both groups was significantly increased (P < 0.05). The control group maintained a stable level through the subsequent timeframe, but the soluble sugar content in the treatment cells increased gradually and reached levels that were significantly higher than the level in the control cells. The maximum soluble sugar content was 2.23×10-5 μg/cell at day 5. The soluble protein content in the both groups increased rapidly and reached maximums of 7.12×10-6 μg/cell and 7.51×10-6 μg/cell at day 1, respectively (Fig. 5b). Then, the soluble protein contents significantly decreased in both groups from 3 to 5 d (P < 0.05), although the content declined more slowly in the treatment cells than in the control.
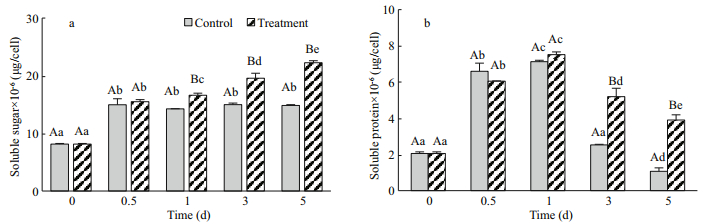
|
| Fig.5 The effects of Na2WO4 on the soluble sugar (a) and protein (b) contents of H. pluvialis |
Fv'/Fm' reflects the actual photosynthetic efficiency of the algae and can be used to characterize the lightdependent photosynthesis reactions. Within the first 3 days, the Fv'/Fm' was lower in the treatment cells than that in the control, and the Fv'/Fm' in the treatment cells then remained stable from days 3–5 (Fig. 6a). The results showed that Na2WO4 decreased the photosynthetic efficiency, although the rapid accumulation of astaxanthin might protect the photosynthetic machinery.
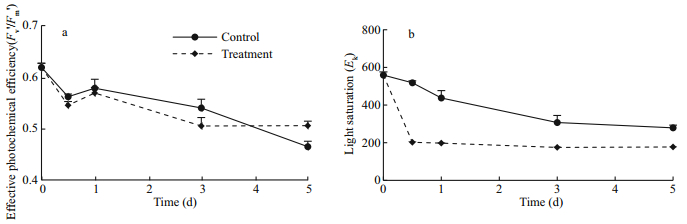
|
| Fig.6 The effects of Na2WO4 on Fv'/Fm' (a) and Ek (b) of H. pluvialis |
Ek represents the minimum saturating irradiance and interrelates with the maximum photosynthetic rate of the algae (Ralph and Gademann, 2005). The initial Ek was 558.87 μmol/(m2·s), and the Ek of both groups declined after treatment with 3 mmol/L of Na2WO4 and continuous irradiance. At 0.5 d, the Ek of the treatment groups decreased rapidly to 36.19% of the initial value and then tended to stabilize (Fig. 6b). The Ek of the treatment groups was always lower than that of the control, and the inducing irradiance (80 μmol/(m2·s)) was still within the Ek range. The results showed that Na2WO4 decreased the Ek of H. pluvialis.
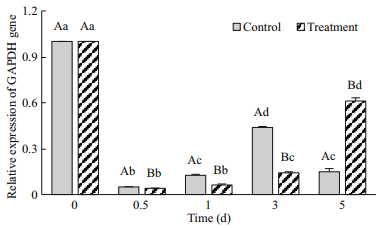
GAPDH is a key enzyme in the Calvin cycle (dark reactions of photosynthesis). GAPDH gene expression was down-regulated in both groups (Fig. 7). The treatment cells expression level was lower than the control at day 0.5 to 3 and then increased significantly at day 5 (P < 0.05), which corresponded to the trend observed for Fv'/Fm'. These results revealed that GAPDH gene expression was affected by Na2WO4.
|
| Fig.7 The effects of Na2WO4 on GAPDH gene expression in H. pluvialis |
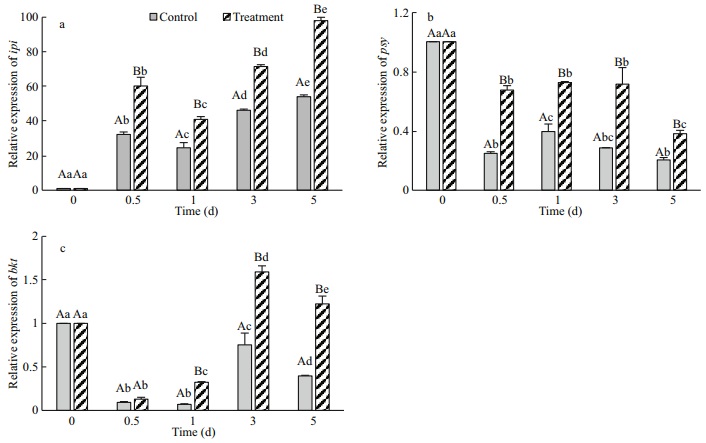
Ipi is the initial and key enzyme involved in carotenoid biosynthesis. This enzyme catalyzes the reversible isomerization reaction between IPP and dimethylpropyl pyrophosphate (DMAPP) (Wouters et al., 2003). As shown in Fig. 8a, ipi expression was significantly lower in the control cells than in the treatment cells (P < 0.05), and the peak transcriptional of 54.01-fold occurred on day 5 due to the effect of light (80 μmol/(m2·s)). In the treatment group, ipi expression increased continually and reached a peak on day 5 of 98.03-fold (P < 0.05), which was 1.82 times higher than the peak of the control. The results showed that high light levels promoted ipi expression and that the addition of Na2WO4 accelerated the effect remarkably.
|
| Fig.8 The effects of Na2WO4 on the expression of carotenogenic genes of H. pluvialis |
Psy is a rate-limiting enzyme in carotenoid biosynthesis (Liang et al., 2006). After induction with high light and Na2WO4, the psy expression levels were down-regulated in both groups (Fig. 8b), but the expression level was significantly higher in the treatment cells than in the control from days 0.5 to 5 (P < 0.05). No significant change was observed in the treatment cells at days 0.5 to 3 (P > 0.05), which was followed by a decrease in the expression level that was possibly due to feedback by astaxanthin. The results indicated that psy expression was downregulated when the algae were exposed to high light conditions and that the addition of Na2WO4 increased the expression of this gene significantly.
Bkt participates in the final step of astaxanthin synthesis (Huang et al., 2006). As shown in Fig. 8c, the bkt expression level was down-regulated in the control cells and was significantly lower than that in the treatment cells (P < 0.05). In the treatment groups, the initial increase and maximum transcriptional expression of bkt occurred on day 3 with a 1.59-fold increase. The results indicated that the addition of Na2WO4 significantly promoted bkt expression, especially after 3 days of treatment.
4 DISCUSSIONNa2WO4 is mainly used for dyes, electroplating, fabric, as a cellulose fire retardant, and as a catalyst but is also known for its anti-diabetic effects. Researchers have identified the pathways through which sodium tungstate improves pancreatic function and beta cell proliferation (Domínguez et al., 2003). Na2WO4 is a typic NR inhibitor for plant experiment, as well we in microalgae. For example, Shen et al. (2011) used it in Nannochloropsis oculata for fatty acid accumulation, and Fan (2012) reported that Na2WO4 could promote the lipid accumulation in Isochrysis galbana. In this study, Na2WO4 was used to induce astaxanthin accumulation in H. pluvialis through inhibition of NR activity. The cellular morphology changed obviously when algae were exposed to Na2WO4. Astaxanthin accumulated rapidly after application of 3.0 mmol/L of Na2WO4, and the color of the cells completely turned to red at 10 d when the astaxanthin content reached 49.41 pg/cell. Boussiba and Vonshak (1991) reported that the astaxanthin content reached 65 pg/cell under 170 μmol/(m2·s), and Kobayashi et al. (1997) obtained completely mature algae cells with an astaxanthin content of 49.7 pg/cell. By comparing our data with data from previous studies, we can confirm that Na2WO4 effectively promotes the accumulation of astaxanthin. But there is no doubt that Na2WO4 may have some toxic effect on algae cells, for the cells density decreased and some algae cells appeared bleach or rupture. On the other hand, Na2WO4 is easy to dissolve in water, while astaxanthin is usually extracted by organic solvent, then the product won't be affected.
NR is the initial and rate-limiting enzyme in nitrate assimilation, and its activity is closely related to algae growth and metabolism. Research has showed that Na2WO4 is a NR inhibitor in higher plants. Liang et al. (2008) reported that the NR activity of Brassica campestris was inhibited by 0.8 mmol/L of Na2WO4 and that higher concentrations of Na2WO4 showed toxic effects. Studies also showed that 1.0 mmol/L Na2WO4 had the best inhibitory effects on the NR activity of Brassica napus roots and that the rate of nitrate assimilation was significantly decreased by this treatment (Yang et al., 2012). NR is extremely sensitive to the nitrate concentration and light intensity (Zhao et al., 2004; Tian et al., 2009). The increase in NR activity in the control cells was probably caused by continuous light exposure. In the treated cells, Na2WO4 showed significant inhibitory effects on the NR activity of H. pluvialis from days 0.5 to 5. Under these conditions, we expect that the absorption of nitrate in the culture medium will be inhibited, thereby blocking amino acid and protein synthesis and inducing a form of nitrogen deficiency. At the same time, we confirmed that astaxanthin accumulation was significantly higher in the treated cells (3 mmol/L Na2WO4) than in the control cells at day 5, indicating that nitrogen deficiency could accelerate astaxanthin accumulation. This conclusion is consistent with the hypothesis that nitrogen deficiency has a greater effect on astaxanthin synthesis by exerting a stronger blocking effect on cell division (Fábregas et al., 1998).
Soluble sugar is a critical substance for maintaining cell osmotic pressure, increasing plant resistance to stress (Wang and Tang, 2014), and forming cell walls and other structures. Previous studies have reported that carbohydrates, such as sucrose, altrose and fructose-6-phosphate, are increased under various stress conditions in H. pluvialis (Su et al., 2014; Lv et al., 2016). In our study, the soluble sugar content of H. pluvialis was significantly increased in both groups at 0.5 d, which might increase the stress resistance of the cells rapidly. The soluble sugar content in the control cells exhibited a stable trend and in the treatment cells increased continuously, which demonstrated that the nitrogen deficiency caused by Na2WO4 promoted an increase in the soluble sugar content, which could improve the stress resistance of the cells. Similarly, other researchers concluded that the soluble sugar content increased with nitrogen deficiency in higher plant roots (Liang et al., 2008). We speculate that soluble sugar not only can be regarded as a resistance compound but can also be used to synthesize astaxanthin, the cell wall (Fig. 1) and other secondary metabolites in H. pluvialis.
Soluble protein has functions such as regulating osmotic pressure and relieving metal stress and thus can also indicate cellular resistance to stress (Xu et al., 2008). Studies have shown that H. pluvialis produces a large amount of protease-resistant, heatstable proteins under high light conditions and nitrogen starvation (Pelah et al., 2004). Our research showed that the soluble protein content in both groups increased significantly at 0–1 d, presumably to protect the cells. Then, the soluble protein content of the control cells decreased more rapidly than the content in the treatment cells, indicating that soluble proteins might play important roles in continual resistance to adverse environmental conditions.
Nitrogen is an important element in chlorophyll. Nitrogen deficiency affects the chlorophyll content and functions in H. pluvialis and suppresses photosynthesis. Previous studies noted that Fv'/Fm' and rETR were significantly decreased by a lack of nitrogen, which suppressed photosynthesis in H. pluvialis (Chen et al., 2012). In our study, Na2WO4 resulted in low NR activity and nitrogen deficiency, which led to a significant decrease in Fv'/Fm' and Ek. Then, Fv'/Fm' and Ek tended to stabilize following the rapid accumulation of astaxanthin, revealing the protective effects of astaxanthin on the photosynthetic system (Li et al., 2008). GAPDH participates in the reduction reaction of 3-phosphoglyceric and the conversion to glyceraldehyde-3-phosphate in the Calvin cycle, which relies on the electron carrier NADPH that is generated by the light-dependent reactions (Raven et al., 2005). Kim et al. (2011) performed a transcriptomic analysis and showed that nitrogen deficiency inhibited the expression of chlorophyll biosynthesis genes and light-harvesting complex (LHC) related genes in H. pluvialis. In our study, GAPDH gene expression was down-regulated consistently with Fv'/Fm'. We speculated that the NADPH yield decreased with the decline of photosynthesis in the chloroplasts, which resulted in down-regulation of GAPDH gene transcription.
Many studies have examined the expression of carotenoid genes under different conditions by transcriptomics and quantitative real-time PCR techniques and have demonstrated that induction of carotenoid gene expression, including ipi, psy and bkt, plays an important role in regulating astaxanthin accumulation under various stress conditions (Gao et al., 2012; Gwak et al., 2014). Our results showed that transcriptional expression of ipi and psy was upregulated at 0–48 h in response to high light stress with fast accumulation of astaxanthin (Li et al., 2010). Gao et al. (2013) showed that 24-epibrassinolide (EBR) enhanced the astaxanthin content and ipi, psy and bkt expression at 24–48 h. Although the stress conditions were different, we found that transcription of ipi, psy and bkt was up-regulated significantly after application of Na2WO4 compared with that in the control, and ipi showed maximal amplification. Our results indicated that Na2WO4 induced carotenoid gene expression and ultimately led to a high quantity of astaxanthin.
5 CONCLUSIONOur study provides the first evidence that Na2WO4 can effectively promote the accumulation of astaxanthin in H. pluvialis by inhibiting nitrate assimilation, which is a new approach to promote the accumulation of astaxanthin. We conjectured that WO42- replaced or affected the molybdenum (Mo) in the NR structure, which inhibited NR activity and caused nitrogen deficiency. Then, chlorophyll, amino acid, and protein synthesis was blocked, and some metabolic pathways might be inhibited or disrupted, especially nitrogen assimilation and photosynthesis. When stress signals are transmitted to the algae nucleus, up-regulation of carotenogenic genes leads to high astaxanthin accumulation levels. Furthermore, the soluble sugars and proteins cooperated with astaxanthin to protect and relieve photosynthesis and other metabolic processes from stress.
6 DATA AVAILABILITY STATEMENTAll data supporting the findings of this study are available within the article.
Algae Industry Magazine. 2017. Advanced technologies driving astaxanthin market. http://www.algaeindustrymagazine.com/report-suggests-astaxanthinmarket-2-57-billion-2025/. Accessed on 2017-08-6.
|
Ambati R R, Phang S M, Ravi S, Aswathanarayana R G. 2014. Astaxanthin:sources, extraction, stability, biological activities and its commercial applications-a review. Marine Drugs, 12(1): 128-152.
DOI:10.3390/md12010128 |
Ambati R R, Sindhuja H N, Dharmesh S M, Sankar K U, Sarada R, Ravishankar G A. 2013. Effective inhibition of skin cancer, tyrosinase, and antioxidative properties by astaxanthin and astaxanthin esters from the green alga Haematococcus pluvialis. Journal of Agricultural and Food Chemistry, 61(16): 3842-3851.
DOI:10.1021/jf304609j |
Barbosa M J, Morais R, Choubert G. 1999. Effect of carotenoid source and dietary lipid content on blood astaxanthin concentration in rainbow trout (Oncorhynchus mykiss). Aquaculture, 176(3-4): 331-341.
DOI:10.1016/S0044-8486(99)00115-5 |
Boussiba S, Fan L, Vonshak A. 1992. Enhancement and determination of astaxanthin accumulation in green alga Haematococcus pluvialis. Methods in Enzymology, 213: 386-391.
DOI:10.1016/0076-6879(92)13140-S |
Boussiba S, Vonshak A. 1991. Astaxanthin accumulation in the green alga Haematococcus pluvialis. Plant and Cell Physiology, 32(7): 1077-1082.
DOI:10.1093/oxfordjournals.pcp.a078171 |
Boussiba S. 2000. Carotenogenesis in the green alga Haematococcus pluvialis:cellular physiology and stress response. Physiologia Plantarum, 108(2): 111-117.
DOI:10.1034/j.1399-3054.2000.108002111.x |
Chen S X, Liang Y, Wang H. 2012. Effects of different nitrogen and phosphorus concentrations on the chlorophyll fluorescence parameters of Haematococcus pluvialis. Freshwater Fisheries, 42(1): 15-19.
(in Chinese with English abstract) |
Disch A, Schwender J, Müller C, Lichtenthaler H K, Rohmer M. 1998. Distribution of the mevalonate and glyceraldehyde phosphate/pyruvate pathways for isoprenoid biosynthesis in unicellular algae and the cyanobacterium Synechocystis PCC 6714. Biochemical Journal, 333(2): 381-388.
DOI:10.1042/bj3330381 |
Domínguez J E, Muñoz M C, Zafra D. 2003. The antidiabetic agent sodium tungstate activates glycogen synthesis through an insulin receptor-independent pathway. Journal of Biological Chemistry, 278(44): 42785-42794.
DOI:10.1074/jbc.M308334200 |
Dong Q L, Zhao X M, Xing X Y, Gong J X, HU J Z. 2006. Biosynthesis of astaxanthin in Haematococcus pluvialis caused by suppression on nitrogen and carbon metabolisms. Chemical Engineering (China), 34(12): 48-49, 57.
(in Chinese with English abstract) |
Eilers P H C, Peeters J C H. 1988. A model for the relationship between light intensity and the rate of photosynthesis in phytoplankton. Ecological Modelling, 42(3-4): 199-215.
DOI:10.1016/0304-3800(88)90057-9 |
Fábregas J, Domínguez A, Álvarez D G, Lamela T, Otero A. 1998. Induction of astaxanthin accumulation by nitrogen and magnesium deficiencies in Haematococcus pluvialis. Biotechnology Letters, 20(6): 623-626.
DOI:10.1023/A:1005322416796 |
Fan L M. 2012. Preliminary Study on the Breeding of HighLipid Content Marine Golden Algae. Ocean University of China, Qingdao, China.
(in Chinese with English abstract)
|
Gao Z Q, Meng C X, Gao H Z, Zhang X W, Xu D, Su Y F, Wang Y Y, Zhao Y R, Ye N H. 2013. Analysis of mRNA expression profiles of carotenogenesis and astaxanthin production of Haematococcus pluvialis under exogenous 2, 4-epibrassinolide (EBR). Biological Research, 46(2): 201-206.
DOI:10.4067/S0716-97602013000200012 |
Gao Z Q, Meng C X, Zhang X W, Xu D, Zhao Y F, Wang Y T, Lv H X, Yang L M, Chen L Q, Ye N H. 2012. Differential expression of carotenogenic genes, associated changes on astaxanthin production and photosynthesis features induced by JA in H. pluvialis. PLoS One, 7(8): e42243.
DOI:10.1371/journal.pone.0042243 |
Gwak Y, Hwang Y S, Wang B B, Kim M, Jeong J, Lee C G, Hu Q, Han D X, Jin E. 2014. Comparative analyses of lipidomes and transcriptomes reveal a concerted action of multiple defensive systems against photooxidative stress in Haematococcus pluvialis. Journal of Experimental Botany, 65(15): 4317-4334.
DOI:10.1093/jxb/eru206 |
Han D X, Li Y T, Hu Q. 2013. Astaxanthin in microalgae:pathways, functions and biotechnological implications. Algae, 28(2): 131-147.
DOI:10.4490/algae.2013.28.2.131 |
Huang J C, Chen F, Sandmann G. 2006. Stress-related differential expression of multiple β-carotene ketolase genes in the unicellular green alga Haematococcus pluvialis. Journal of Biotechnology, 122(2): 176-185.
DOI:10.1016/j.jbiotec.2005.09.002 |
Kim D K, Hong S J, Bae J H, Yim N, Jin E, Lee C G. 2011. Transcriptomic analysis of Haematococcus lacustris during astaxanthin accumulation under high irradiance and nutrient starvation. Biotechnology and Bioprocess Engineering, 16: 698-705.
DOI:10.1007/s12257-011-0081-z |
Kobayashi M, Kakizono T, Nishio N, Nagai S, Kurimura Y, Tsuji Y. 1997. Antioxidant role of astaxanthin in the green alga Haematococcus pluvialis. Applied Microbiology and Biotechnology, 48(3): 351-356.
DOI:10.1007/s002530051061 |
Lee Y K, Ding S Y. 1994. Cell cycle and accumulation of astaxanthin in Haematococcus lacustris (Chlorophyta). Journal of Phycology, 30(3): 445-449.
DOI:10.1111/j.0022-3646.1994.00445.x |
Li H S. 2000. Principles and Techniques of Plant Physiological Biochemical Experiment. Higher Education Press, Beijing, China.
(in Chinese)
|
Li Y T, Sommerfeld M, Chen F, Hu Q. 2008. Consumption of oxygen by astaxanthin biosynthesis:a protective mechanism against oxidative stress in Haematococcus pluvialis (Chlorophyceae). Journal of Plant Physiology, 165(17): 1783-1797.
DOI:10.1016/j.jplph.2007.12.007 |
Li Y T, Sommerfeld M, Chen F, Hu Q. 2010. Effect of photon flux densities on regulation of carotenogenesis and cell viability of Haematococcus pluvialis (Chlorophyceae). Journal of Applied Phycology, 22(3): 253-263.
DOI:10.1007/s10811-009-9453-6 |
Li Y T. 2007. The role of carotenogenesis in the response of the green alga Haematococcus Pluvialis to oxidative stress. The University of Hong Kong, Hong Kong, China.
|
Liang C W, Zhao F Q, Qin S, Tan C P, Wei W, Meng C X. 2006. Molecular cloning and characterization of phytoene synthase gene from a unicellular green alga Haematococcus pluvialis. Progress in Biochemistry and Biophysics, 33(9): 854-860.
|
Liang L, Chen J, Su X J, Xu H, Yuan X H, Chen J F. 2008. Effects of nitrate reductase activity in root on absorption and metabolism of nitrate in Brassica campestris L. ssp.Chinesis Makino. Jiangsu Agricultural Sciences, 36(3): 153-155.
(in Chinese with English abstract) |
Livak K J, Schmittgen T D. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCt method. Methods, 25(4): 402-408.
DOI:10.1006/meth.2001.1262 |
Lv H X, Xia F, Liu M, Cui X G, Wahid F, Jia S R. 2016. Metabolomic profiling of the astaxanthin accumulation process induced by high light in Haematococcus pluvialis. Algal Research, 20: 35-43.
DOI:10.1016/j.algal.2016.09.019 |
Pashkow F J, Watumull D G, Campbell C L. 2008. Astaxanthin:a novel potential treatment for oxidative stress and inflammation in cardiovascular disease. American Journal of Cardiology, 101(10): S58-S68.
DOI:10.1016/j.amjcard.2008.02.010 |
Pelah D, Marton I, Wang W, Shoseyov O, Altman A, Cohen E. 2004. Accumulation and protection activity of proteaseresistant heat-stable proteins in Haematococcus pluvialis during high light and nitrogen starvation. Journal of Applied Phycology, 16(2): 153-156.
DOI:10.1023/B:JAPH.0000044944.53478.b2 |
Ralph P J, Gademann R. 2005. Rapid light curves:a powerful tool to assess photosynthetic activity. Aquatic Botany, 82(3): 222-237.
DOI:10.1016/j.aquabot.2005.02.006 |
Ranga R, Sarada A R, Baskaran V, Ravishankar G A. 2009. Identification of carotenoids from green alga Haematococcus pluvialis by HPLC and LC-MS (APCI)and their antioxidant properties. Journal of Microbiology and Biotechnology, 19(11): 1333-1341.
|
Raven P H, Evert R F, Eichhorn S E. 2005. Biology of Plants. Freeman and Company, New York.
|
Shen X, Jiao Y Y, Zhu B H, Yang P G, Pan K H. 2011. Effect of nitrogen deficiency and sodium tungstate in medium on the fatty acid composition of Nannochloropsis oculata. Transactions of Oceanology and Limnology, (4): 78-82.
(in Chinese with English abstract) |
Su Y X, Wang J X, Shi M L, Niu X F, Yu X H, Gao L J, Zhang X Q, Chen L, Zhang W W. 2014. Metabolomic and network analysis of astaxanthin-producing Haematococcus pluvialis under various stress conditions. Bioresource Technology, 170: 522-529.
DOI:10.1016/j.biortech.2014.08.018 |
Tian H, Duan M Y, Wang L. 2009. Research progress on nitrate reductase functions in plants. Chinese Agricultural Science Bulletin, 25(10): 96-99.
(in Chinese with English abstract) |
Vidhyavathi R, Venkatachalam L, Sarada R, Ravishankar G A. 2008. Regulation of carotenoid biosynthetic genes expression and carotenoid accumulation in the green alga Haematococcus pluvialis under nutrient stress conditions. Journal of Experimental Botany, 59(6): 1409-1418.
DOI:10.1093/jxb/ern048 |
Wang J J, Tang Z H. 2014. The regulation of soluble sugars in the growth and development of plants. Botanical Research, 3(3): 71-76.
(in Chinese with English abstract) DOI:10.12677/BR.2014.33011 |
Wang J Y, Zhou C X, Yan X J, Luo Q J, Jiang Y, Ma B, Tan Y H. 2014. The characteristics of growth and nutrient consumption of Haematococcus pluvialis under red light. Acta Hydrobiologica Sinica, 38(6): 1135-1142.
(in Chinese with English abstract) |
White S, Anandraj A, Bux F. 2011. PAM fluorometry as a tool to assess microalgal nutrient stress and monitor cellular neutral lipids. Bioresource Technology, 102(2): 1675-1682.
DOI:10.1016/j.biortech.2010.09.097 |
Wouters J, Oudjama Y, Ghosh S, Stalon V, Droogmans L, Oldfield E. 2003. Structure and mechanism of action of isopentenylpyrophosphate-dimethylallylpyrophosphate isomerase. Journal of the American Chemical Society, 125(11): 3198-3199.
DOI:10.1021/ja029171p |
Xu L Z, Cai J, Jiang Z M, Peng X B, Su X F, Zhang S X. 2008. Effects of water stress on osmotic adjustment and activity of protect enzymes in the leaves of three sorts of seedlings. Journal of Northwest Forestry University, 23(2): 12-16.
(in Chinese with English abstract) |
Yamashita E. 2013. Astaxanthin as a medical food. Functional Foods in Health and Disease, 3(7): 254-258.
DOI:10.31989/ffhd.v3i7.49 |
Yang R, Qiu W H, Wang Z H, Wang X Y. 2012. Effects of nitrate reductase inhibitor Na2WO4 on nitrate accumulation in oilseed rape. Plant Physiology Journal, 48(1): 51-56.
(in Chinese with English abstract) |
Yu M, Hu C X, Sun X C, Wang Y H. 2010. Influences of Mo on nitrate reductase, glutamine synthetase and nitrogen accumulation and utilization in Mo-efficient and Moinefficient winter wheat cultivars. Agricultural Sciences in China, 9(3): 355-361.
DOI:10.1016/S1671-2927(09)60104-8 |
Zhao T, Chen Y, Xie H, Liang J S. 2004. The research progress of the regulation of nitrate reductase activity and the possible mechanism. Guihaia, 24(4): 367-372.
(in Chinese with English abstract) |
 2019, Vol. 37
2019, Vol. 37



