Institute of Oceanology, Chinese Academy of Sciences
Article Information
- GAO Yaping, JIANG Zengjie, DU Meirong, FANG Jinghui, JIANG Weiwei, FANG Jianguang
- Photosynthetic and metabolic responses of eelgrass Zostera marina L. to short-term high-temperature exposure
- Journal of Oceanology and Limnology, 37(1): 199-209
- http://dx.doi.org/10.1007/s00343-019-7319-6
Article History
- Received Nov. 7, 2017
- accepted in principle Jan. 4, 2018
- accepted for publication Jan. 22, 2018
2 Key Laboratory of Sustainable Development of Marine Fisheries, Ministry of Agriculture, Yellow Sea Fisheries Research; Institute, Chinese Academy of Fishery Sciences, Qingdao 266071, China;
3 University of Chinese Academy of Sciences, Beijing 100049, China;
4 Function Laboratory for Marine Fisheries Science and Food Production Processes, Qingdao National Laboratory for Marine Science and Technology, Qingdao 266071, China
Seagrass, which comprises one of the most abundant and important systems in the coastal ecosystem, has high primary productivity (Rasheed et al., 2008; Kim et al., 2013) and forms the basis of a variety of marine food chains (Connolly et al., 2005; Lepoint et al., 2006). Seagrass beds greatly increase the biodiversity of the surrounding environment (Bertelli and Unsworth, 2014) and contribute to maintenance of the health and stability of the coastal environment (Heck et al., 2003; Thangaradjou et al., 2010). However, seagrass systems are subject to pressure from human activities such as coastal development, the import of land-based pollutants to the ocean, and global climate change (Shin et al., 2002; Ralph et al., 2007; Waycott et al., 2009; Short et al., 2011; Jordà et al., 2012). The global seagrass distribution area has declined since the last century, and this decline seems to be closely related to anthropogenic stress (Short and Wyllie-Echeverria, 1996; Boudouresque et al., 2009). Global climate change, especially the continuous rise of the global ocean temperature, has harmed the health of seagrass to a certain extent (Collier et al., 2011; Waycott et al., 2011), particularly in shallow waters, which are more heavily impacted by solar radiation and air temperature (Pedersen et al., 2016), as well as areas close to the edge of seagrass species' distributional ranges (Micheli et al., 2008; Massa et al., 2009). In recent years, the evidence of the harmful effects of temperature changes in the distribution and growth of seagrass has been increasing. The temperature of the seagrass field at the Great Barrier Reef during low tide has been as high as 43℃. Collier and Waycott (2014) measured the effects of short-term hightemperature exposure on the photosynthesis and growth of four tropical seagrasses and found that it died within 2–3 at 43℃, indicating that frequent thermal events of 40℃ or higher can affect the ecological functions of tropical seagrass. Rasheed and Unsworth (2011) documented the temporal dynamics of intertidal seagrass on the tropical northeastern coast of Australia for 16 years and observed that high temperature is associated with a low biomass of seagrass in the Carpentaria Bay. In recent years, the Mediterranean has rapidly warmed, and field monitoring has demonstrated that interannual variability in Posidonia oceanica shoot mortality was coupled with seawater warming variability (Marbà and Duarte, 2010).
Eelgrass (Zostera marina. L), which is one of the most widely distributed seagrass species in temperate shallow seas, maintains the health and stability of the shallow-sea environment (Hasegawa et al., 2008; Boström et al., 2014). It has an optimum growth temperature of 13.0–24.0℃ (Lee et al., 2007). Contemporary climate change is characterized by a continuous increase in the average temperature and increasing climate variability, such as heat waves, storms and floods (Peterson et al., 2013). A field study conducted by Reusch et al. (2005) revealed that a climate change-related heat wave in central Europe caused water temperatures to exceed 25℃, resulting in the death of more than 50% of eelgrass shoots. When eelgrass is exposed to high water temperature, the increase in respiration is greater than the increase in photosynthesis, resulting in a decreased photosynthesis: respiration ratio (P:R ratio) (Marsh et al., 1986) and an imbalance between carbon uptake and carbon consumption (Zimmerman et al., 1989). This imbalance is also reflected by a decrease in sugar stored in the leaves and rhizomes during periods of high temperature (Burke et al., 1996).
Metabolites are the end products of gene expression. Life activities such as signal transduction and energy transfer are regulated by metabolites (Guy et al., 2008). Metabonomics can qualitatively and quantitatively analyze all low-molecular-weight metabolites of a particular organism or cell in a given physiological period (Dettmer et al., 2007). The development of metabolomics provides a reliable framework for detection of changes in the contents of metabolites under environmental stress. Hightemperature is a common stress for plants that can affect the metabolism of soluble sugars, amino acids, and organic acids (Guy et al., 2008; Yamakawa and Hakata, 2010; Ribeiro et al., 2014). For example, exposure to 26℃ for 3–5 weeks was found to change the contents of sucrose, fructose, and myo-inositol in Z. marina and Z. noltii (Gu et al., 2012). Moreover, the accumulation of some metabolites is closely related to the high-temperature resistance of the plants. For instance, Ashraf et al. (1994) found that soluble sugars and proline largely accumulated in high-temperature-resistant cotton (Gossypium hirsutum), which is conducive to the improvement of high-temperature resistance of plants. Xu et al. (2013) investigated the effects of high-temperature stress on the accumulation of metabolites in the leaves and roots of two Agrostis species, A. scabra (heatresistant) and A. stolonifera (heat-sensitive), by gas chromatography-mass spectroscopy (GC-MS) and found accumulation of organic acids and carbohydrates in the leaves and accumulation of amino acids, organic acids, and carbohydrates in the roots.
High temperature can affect the growth and survival of seagrass. Long-term non-lethal hightemperature exposure also affects seagrass metabolism and gene expression. However, the response of the metabolism of seagrass to short-term exposure to a lethal temperature has not yet been reported. Therefore, the photosynthesis and metabolomic response of eelgrass to short-term high-temperature exposure was investigated in this study with the goal of providing insight into the likely effects of extreme heat events on temperate seagrass.
2 MATERIAL AND METHODEelgrass was collected from an eelgrass bed (122°34'04″E, 37°02'32″N) in Sanggou Bay during the low tide period of June 2016. At this time, the temperature of the surface water was approximately 16℃, and the eelgrass was in the rapid growth stage. Eelgrass shoots without obvious damage were selected and 10-cm diameter PVC core tubes were used to extract intact plugs of sediment and associated selected shoots. The plugs were then transported to the laboratory on ice. Upon arrival in the laboratory, the epiphytes on the eelgrass shoots were removed, and the plugs were put in a 16-L glass cylinder that was placed in a light incubator. Each glass cylinder contained 3–4 plugs with 9–12 shoots. The eelgrass was cultured at 16℃ under a 12 h:12 h light: dark cycle (L:D), and the canopy received a light intensity of approximately 135 μmol quanta/(m2·s).
The water of the eelgrass culture was changed daily using sea water with a salinity of 30 after filtration through a 0.45-μm microporous membrane. After adaption for 2 weeks, eelgrass samples were randomly divided into two groups, with six glass cylinders in each group. One group served as a control, with a culture temperature of 16℃, while the experimental group was cultured in sea water of 32℃ in an incubator (±0.2℃). A temperature of 32℃ was confirmed to severely inhibit the growth of eelgrass in 7 days in our previous research, while the metabolic response was unknown (Gao et al., 2017).
2.1 Physiological responses and data analysisThe eelgrass was treated at 32℃ or 16℃ for 48 h, after which the effective photosynthetic efficiency was measured, which was represented by the effective quantum yield ΔF/F'm (achieved under experimental light conditions, interpreted as photosynthetic efficiency) and maximum yield Fv/Fm (achieved in the dark, interpreted as photo damage) using a chlorophyll spectrometric fluorescence measurement device (Diving PAM, Walz, Germany). To perform the measurement, two terminal shoots in each glass cylinder were randomly selected, and the leaf chip was set at 5–8 cm above the edge of the leaf sheath in the second youngest leaf of each branch to detect the ΔF/F'm under light (135 μmol quanta/(m2·s)) and the Fv/Fm after dark adaptation for 15 min.
After the chlorophyll fluorescence parameters were measured, three shoots were randomly selected from each glass cylinder. The rhizomes and roots were cleaned (removing the substrate and bundling on small stones) and then placed in a 2 000-mL gas-tight, transparent chamber. Chambers containing no seagrass and only seawater were used as controls. The consumption of dissolved oxygen after a certain incubation time (90–120 min) in the dark was measured using a portable probe (Orion 3 Star, Thermo Scientific). The dark respirations of the 16℃ and 32℃ treatment groups were calculated using the dissolved oxygen consumption under dark conditions. At the end of the experiment, the eelgrass in each group was weighed.
Differences in the photosynthetic and respiration rates between the 16℃ and 32℃ treatment groups were analyzed by a Student's t-test at a significance of P < 0.05.
2.2 Metabolic response 2.2.1 Extraction of metabolites from eelgrass shootsAfter 48 h of culture, six replicates of 2–3 g of eelgrass were stored at -80℃ until metabolites were extracted. Metabolite profiling was conducted using GC-time of flight (TOF)-MS (Erban et al., 2007). Briefly, 200 mg of fresh plant sample was extracted using 0.8 mL of extraction liquid (methanol: H2O=3:1 v/v). Ten microliters of adonitol (2 mg/mL in dH2O) were added as an internal standard. The material was then homogenized for 4 min at 40 Hz using a ball mill grinder, after which it was subjected to ultrasound treatment for 5 min (incubated in ice water). The homogenization and ultrasound treatment were repeated, after which samples were centrifuged at 12 000 r/min and 4℃ for 15 min. Next, 200 μL of supernatant was transferred to a GC-vial and vacuum concentrated without heat. Blanks were also prepared using extraction solution, and then the samples were pooled using mixed aliquots from each shoot sample. Forty microliters of methoxyamine hydrochloride (20 mg/mL in pyridine) were added, after which the sample was incubated for 30 min at 80℃. The samples were then trimethylsilylated by adding 60 μL of N-methylN-trifluoroacetamide with 1% trimethylchlorosilane, after which they were incubated for 1 h at 70℃. Finally, samples were cooled to room temperature before GC-MS analysis.
2.2.2 GC-MS analysisGC-TOF-MS analysis was performed using an Agilent 7890 gas chromatograph system (Agilent Technologies, USA) coupled with a Pegasus HT time-of-flight mass spectrometer that was equipped with a DB-5MS capillary column (30 m×250 μm× 0.25 μm) (Agilent J & W Scientific, Folsom, CA, USA). A 1-μL aliquot of the analyte was injected in splitless mode. The carrier gas was helium, the front inlet purge flow rate was 3 mL/min, and the gas flow rate through the column was 1 mL/min. The initial temperature was 50℃, which was held for 1 min, then increased at a rate of 10℃/min until it reached 300℃, where it was kept for 14 min. The injection, transfer line, and ion source temperatures were 280℃, 270℃, and 220℃, respectively. The energy was set at -70 eV in electron impact mode. In fullscan mode, the mass spectra were recorded with a scanning range of 50–550 m/z.
2.2.3 Data analysis, metabolic identification, and pathway analysisChroma TOF4.3X software from LECO Corporation and the LECO-Fiehn Rtx5 database were used to extract raw peaks, process data baselines, identify peaks, and integrate peak areas. Peaks were identified using the retention time index (RI), and the RI tolerance was 5000.
The interquartile range denoising method was used to process metabolites after the peaks were detected. The internal standard normalization method was employed for this analysis. Next, the threedimensional data containing the peak number, sample name, and normalized peak area were analyzed using the SIMCA14.1 software (MKS Data Analytics Solutions, Umea, Sweden) to perform principal component analysis (PCA) and orthogonal projections to latent structures-discriminant analysis (OPLSDA). Principal component analysis is an unsupervised data analysis method that can visualize the inherent clusters between groups, while OPLS-DA is used to identify the differences between groups and help screen metabolites responsible for the classification by eliminating the systematic variants that are not related to discrimination. To better understand the model, the score map, the load map, and the variable importance in the projection were used in combination. A 7-fold cross-validation was employed to estimate the robustness and the predictive ability of the model produced by OPLS-DA. The model quality was described by the parameters R2Y and Q2, and the statistical significance of R2Y and Q2 were estimated by response permutation testing. However, in our experiments, only six replicates in each class were used to build the model; therefore, the row order in the dataset was permutated 10 times (Triba et al., 2015) and the values of the quality parameters were calculated each time by 7-fold cross-validation procedures. The data were also analyzed using Student's t-tests to identify metabolites that were significantly changed between the control and the high-temperature treatment (P < 0.05). The VIP (Variable Importance in the Projection) value (threshold > 1) of the first principal component of the OPLS-DA model and the P-value of the t-test (P < 0.05) were used to identify differentially expressed metabolites.
Metabolites were identified by comparing the mass-to-charge ratio and the abundance of each compound to a standard mass chromatogram in databases from the National Institute of Standards and Technology (NIST) and the Wiley registry of mass spectral data. Peaks with a similarity index of more than 700 were tentatively identified as metabolites, while those with an index of less than 700 were considered unknown metabolites. In addition, commercial databases, including the Kyoto Encyclopedia of Genes and Genomes and NIST, were utilized to search metabolic pathways. The MetaboAnalyst 3.0 Pathway Analysis module was used to combine results from the pathway enrichment analysis with the pathway topology analysis and to identify the most relevant pathways under the experimental conditions tested. All metabolites identified and discussed in this article were identified as Metabolomics Initiative (MSI) Level 2, except for two substances that were level 4.
3 RESULT 3.1 Photochemical efficiency ΔF/F'm, maximum yield Fv/Fm and dark respirationThe high-temperature stress affected the photochemical efficiency (ΔF/F'm). When compared with the control, the ΔF/F'm of eelgrass was significantly decreased to 0.35 (Fig. 1a) after hightemperature treatment for 48 h, which was about 23.9% lower than the control. Although no significant decrease in the maximum yield Fv/Fm was detected (Fig. 1b), high-temperature affected the dark respiration rate. When compared with the control at 16℃, high-temperature treatment for 48 h increased the net respiration rate to 2.66 mg O2/(g FW·h) (Fig. 1c), which was 58.3% higher than the control.
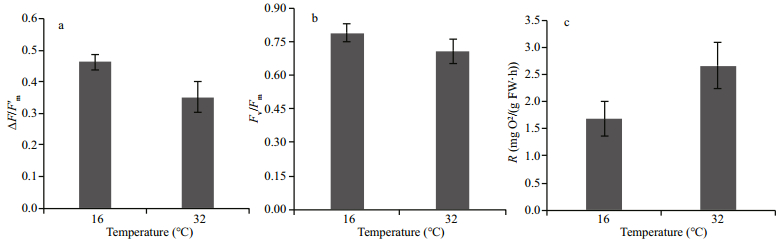
|
| Fig.1 ΔF/F'm (a), Fv/Fm (b) and dark respiration (c) of eelgrass after 48 h at different temperatures Error bars represent the standard deviation (SD). |
The volcanic plot, which is illustrated in Fig. 2, provides a rapid display of metabolic changes based on the fold changes and t-tests of groups of 16℃ and 32℃. A general decrease in metabolites was observed in response to high temperature.
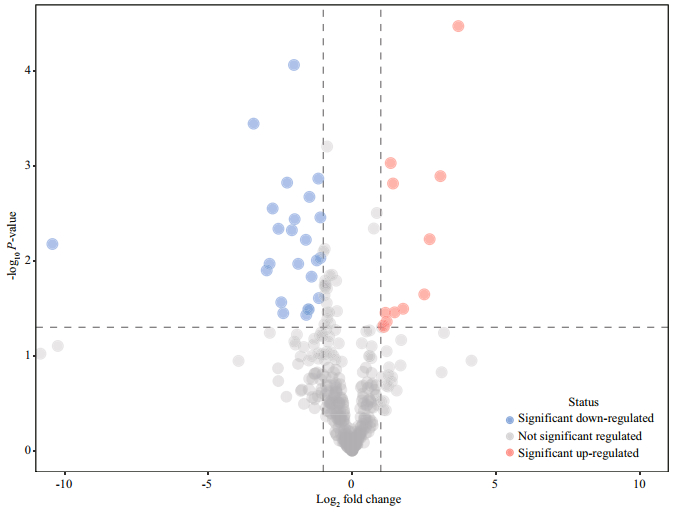
|
| Fig.2 Volcano plot of eelgrass in response to high temperature The dashed lines represent a P-value of 0.05 (y-axis) and a fold change of 2 (x-axis). |
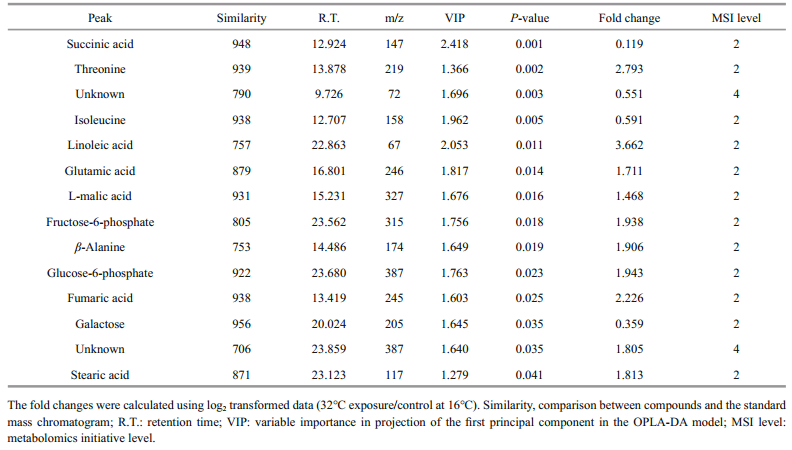
For multivariate analysis of the effects of temperature on the metabolome of Z. marina, exploratory unsupervised PCA analysis was conducted to identify the natural relationships between the two groups. No obvious clustering was found between the two groups in the PCA; therefore, further analysis was conducted by OPLS-DA using temperature as a classifier. The samples were better separated on the score map of OPLA-DA (Fig. 3) and the parameters obtained by 7-fold cross-validation were R2Y=0.995 and Q2=0.657, PCV-ANOVA=0.07. After 10 row permutations of the dataset with 7-fold of cross-validation each time (Triba et al., 2015), 10 Q2 values were obtained and determined to be stable, with a mean of Q2=0.729 (SD=0.03) and PCV-ANOVA= 0.035 (SD=0.012). The model's good predictability was further validated by a permutation test with R2Y=0.982 and Q2=0.024 2. Based on the VIP value from the OPLA-DA mode and substances' similarity, 14 differential metabolites with similarity > 700 and VIP > 1 were identified as significantly changed metabolites, of which 12 were known and two were unknown (Table 1). These significantly changed metabolites in eelgrass shoots were involved in sugar metabolism, the citric acid cycle and metabolism of amino acids and were summarized in a simplified metabolic map (Fig. 4).
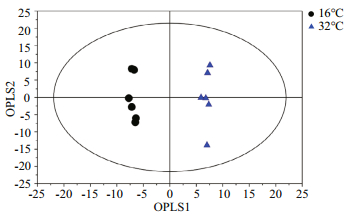
|
| Fig.3 Score plot of OPLS-DA model applied to the control and eelgrass shoots exposed to 32℃ for 48 h The quality factors for the model were R2Y=0.995, Q2=0.729 and PCV-ANOVA=0.035. |
|
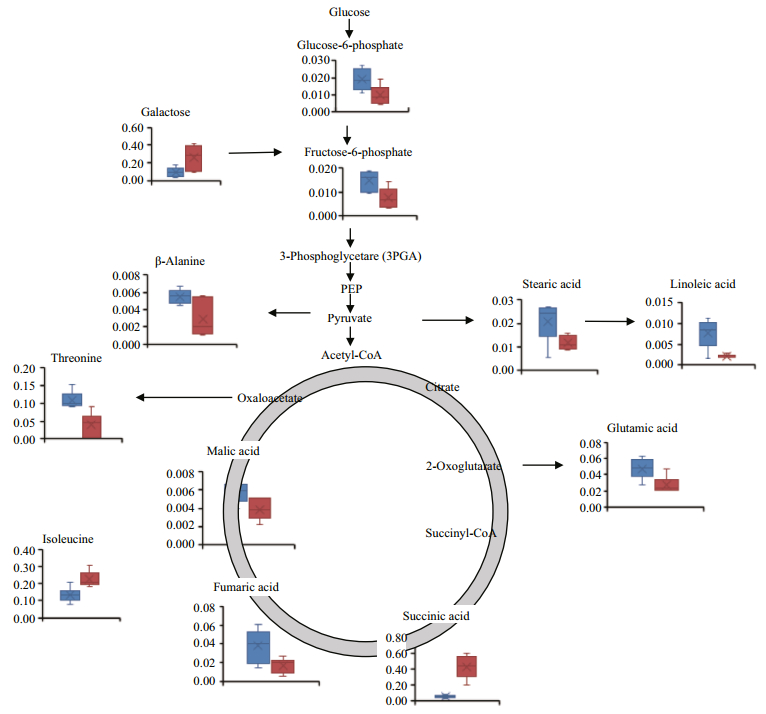
|
| Fig.4 Mapping of relative concentrations of metabolites that were found to change in response to high temperature in the known pathways of metabolism of sugar, the citric acid cycle and metabolism of amino acids Eelgrass was cultured at 16℃ (blue columns) or at 32℃ (red columns). Data shown are the means ± standard errors of six biological replicates. |
Metabolic pathway analysis of the 12 screened differential metabolites was conducted using MetaboAnalyst 3.0, and the metabolic pathways that changed in response to high-temperature treatment (impact > 0.1 and raw P < 0.05) were linoleic acid metabolism and the citrate acid cycle (TCA cycle) (Fig. 5).
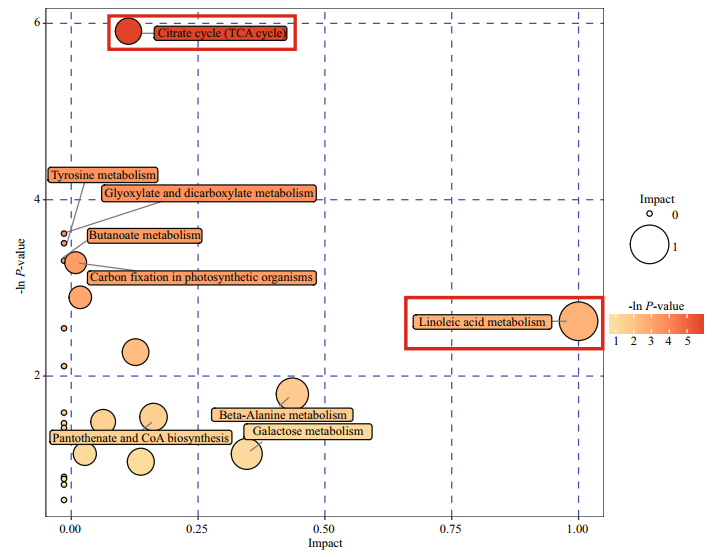
|
| Fig.5 Pathway analysis graphic summary The high temperature response metabolic pathways with raw P < 0.05 and impact score > 0.1 are indicated by red columns. |
The response of eelgrass to short-term hightemperature exposure was reflected in decreased photosynthesis, characterized by ΔF/F'm (Fig. 1a), and enhanced respiration. Under high-temperature, the respiration of eelgrass increased (Fig. 1c); therefore, the energy consumption should have accelerated. Fructose-6-phosphate and glucose-6-phosphate were found to decrease in response to high-temperature, which may have reflected the upregulated downstream reactions in the glycolytic pathway. This has also been found in the responses of metabolites of castor seedlings to high temperatures (Ribeiro et al., 2014). Carbohydrates play an important role in plant growth and coping stress. Most plants accumulate soluble sugar at high temperatures to stabilize their energy supply and membrane function, as well as to regulate gene expression and signal transduction (Kaplan et al., 2004; Wahid et al., 2007; Guy et al., 2008). The accumulation of galactose in eelgrass at high temperature is the same response as that of the leaves of Coffea arabica L. to high temperature (dos Santos et al., 2011). Galactose and other sugars can be used as substrates for a variety of intermediate metabolites to participate in oxidative cross-linking at high temperatures (Seifert and Roberts, 2007). However, unlike in the present study, the major changes in the metabolic products of eelgrass caused by high temperature in the previous study by Gu et al. (2012) were in the levels of sucrose and fructose. This difference was likely because the temperature in our experiments was 32℃, which is higher than the temperature of 26℃ in the long-term heat stress experiments conducted by Gu et al.
An important response of eelgrass to high temperatures is the changes in the intermediate metabolites of the TCA cycle (Fig. 5). The accumulation of succinic acid and the decreases in malic acid and fumaric acid may reflect the insufficient mitochondrial activities of eelgrass when faced with greater energy demand. High temperature can inhibit photosynthesis and enhance the respiration of eelgrass, gradually causing an imbalance in energy supply. In our previous study, severe growth stagnation was found after seven days of high-temperature exposure. Therefore, it is possible that hightemperature treatment for 48 h led to an insufficient energy supply based on the general decrease in metabolites.
Notably, isoleucine in eelgrass increased in response to high temperatures, which has also been observed in other plants exposed to high temperatures. Kaplan et al. (2004) reported an increase in isoleucine concentration in Arabidopsis thaliana in response to heat shock, and the accumulation of isoleucine was also found in spring wheat and castor seedlings under high-temperature stress (Behl et al., 1991; Ribeiro et al., 2014). Because of its unsubstituted aliphatic side chains and branched alkyl groups, isoleucine is one of the most hydrophobic of the 20 standard amino acids. This amino acid is usually located at the core of the protein, where it plays an important role in determining the structure of the globular protein as well as the interactions between membrane proteins and phospholipid bilayers in the transmembrane domain. In plants, isoleucine often conducts anabolism with threonine and methionine as the precursors (Joshi et al., 2010). Under high-temperature conditions, the concentration of threonine decreased and the concentration of isoleucine increased, indicating that isoleucine synthesis was enhanced. It has been proposed that accumulated free branched amino acids, including isoleucine, can be used as substrates for the synthesis of stress-induced proteins and as signaling molecules in the regulation of gene expression (Nambara et al., 1998). Thus, these free branched amino acids accumulate in plants in response to abiotic stresses and coordinate with other reactions to deal with adverse environmental conditions (Simon-sarkadi et al., 2006). The accumulation of isoleucine in eelgrass should be closely related to other molecular changes in response to hightemperature stress. However, this study focused on metabolites, and the information obtained is relatively limited.
Another notable change in the metabolites of eelgrass was the reductions in stearic acid and linoleic acid. The destruction of plant membrane integrity by high-temperature stress is an important cause of impaired cell function (Paulsen, 1994; Marcum, 1998). High temperatures often cause degeneration of proteins in the lipid bilayer of the biomembrane or increases in unsaturated fatty acids, resulting in more membrane fluidity and increased membrane permeability (Savchenko et al., 2002). Although no biological thermometer is evident in plants, it has been suggested that the change in membrane fluidity is critical to the perception of high temperature and the coordinate adjustment of gene expression (Wahid et al., 2007). Several reports suggest that increased concentrations of saturated fatty acids can favor photosynthesis and growth in Arabidopsis thaliana and rice (Murakami et al., 2000; Sohn and Back, 2007). An investigation of the lipid composition and saturation of the membrane in three cultivars of creeping bentgrass (Agrostis stolonifera) showed that the high-temperature-resistant cultivar had a higher level of saturated fatty acids, and all three cultivars showed decreased triene fatty acids, elevated diene fatty acids, and increased membrane saturation under high-temperature stress (Larkindale and Huang, 2004). Similarly, in genetically modified tomato (Lycopersicon esculentum Mill), the increased degree of fatty acids saturation was correlated with hightemperature resistance (Liu et al., 2010). The decrease of saturated stearic acid and non-saturated linoleic acid in eelgrass indicates that high temperature may have changed the fuludity of the membrane, which implies that the stability of the membrane system was damaged by high temperature. However, the metabolites detected in this study were limited, and the changes in the concentrations of other lipids in the membrane were not determined. Therefore, further comprehensive investigations of the response of the lipid composition of eelgrass to high temperatures are warranted.
5 CONCLUSIONAfter short-term exposure to a lethal temperature of 32℃, eelgrass showed decreased photosynthetic activity and increased respiration, with significant reductions in the products involved in glucose metabolism and the TCA cycle, suggesting insufficient energy supply. Isoleucine can be used as a substrate for the synthesis of stress-induced proteins and act as a signaling molecule for regulating gene expression. Therefore, we propose that the reduction in threonine and the accumulation of isoleucine are closely related to other molecular changes in response to hightemperature stress. The decreases in stearic acid and linoleic acid observed in response to high temperature suggest an increase in membrane fluidity, which may also imply the destruction of membrane function at a lethal temperature.
6 DATA AVAILABILITY STATEMENTAll data generated and/or analyzed during this study are included in this published article.
Ashraf M, Saeed M M, Qureshi M J. 1994. Tolerance to high temperature in cotton (Gossypium hirsutum L.) at initial growth stages. Environmental and Experimental Botany, 34(3): 275-283.
DOI:10.1016/0098-8472(94)90048-5 |
Behl R K, Moawad A M, Achtnich W. 1991. Amino acid and protein profile changes in a spring wheat mutant under prolonged heat stress. Annals of Biology, 7: 63-68.
|
Bertelli C M, Unsworth R K F. 2014. Protecting the hand that feeds us:seagrass (Zostera marina) serves as commercial juvenile fish habitat. Marine Pollution Bulletin, 83(2): 425-429.
DOI:10.1016/j.marpolbul.2013.08.011 |
Boström C, Baden S, Bockelmann A C, Dromph K, Fredriksen S, Gustafsson C, Krause-Jensen D, Möller T, Nielsen S L, Olesen B, Olsen J, Pihl L, Rinde E. 2014. Distribution, structure and function of Nordic eelgrass (Zostera marina)ecosystems:implications for coastal management and conservation. Aquatic Conservation:Marine and Freshwater Ecosystems, 24(3): 410-434.
DOI:10.1002/aqc.2424 |
Boudouresque C F, Bernard G, Pergent G, Shili A, Verlaque M. 2009. Regression of Mediterranean seagrasses caused by natural processes and anthropogenic disturbances and stress:a critical review. Botanica Marina, 52(5): 395-418.
|
Burke M K, Dennison W C, Moore K A. 1996. Non-structural carbohydrate reserves of eelgrass Zostera marina. Marine Ecology Progress Series, 137: 195-201.
DOI:10.3354/meps137195 |
Collier C J, Uthicke S, Waycott M. 2011. Thermal tolerance of two seagrass species at contrasting light levels:implications for future distribution in the Great Barrier Reef. Limnology and Oceanography, 56(6): 2200-2210.
DOI:10.4319/lo.2011.56.6.2200 |
Collier C J, Waycott M. 2014. Temperature extremes reduce seagrass growth and induce mortality. Marine Pollution Bulletin, 83(2): 483-490.
DOI:10.1016/j.marpolbul.2014.03.050 |
Connolly R M, Hindell J S, Gorman D. 2005. Seagrass and epiphytic algae support nutrition of a fisheries species, Sillago schomburgkii, in adjacent intertidal habitats. Marine Ecology Progress Series, 286: 69-79.
DOI:10.3354/meps286069 |
Dettmer K, Aronov P A, Hammock B D. 2007. Mass spectrometry-based metabolomics. Mass Spectrometry Reviews, 26(1): 51-78.
|
dos Santos T B, Budzinski I G F, Marur C J, Petkowicz C L O, Pereira L F P, Vieira L G E. 2011. Expression of three galactinol synthase isoforms in Coffea arabica L. and accumulation of raffinose and stachyose in response to abiotic stresses. Plant Physiology and Biochemistry, 49(4): 441-448.
DOI:10.1016/j.plaphy.2011.01.023 |
Erban A, Schauer N, Fernie A R, Kopka J. 2007. Nonsupervised construction and application of mass spectral and retention time index libraries from time-of-flight gas chromatography-mass spectrometry metabolite profiles.In: Weckwerth W ed. Metabolomics: Methods and Protocols. Humana Press, Clifton County, NJ, USA. 358: p.19-38.
|
Gao Y, Fang J, Du M, Fang J, Jiang W, Jiang Z. 2017. Response of the eelgrass (Zostera marina L.) to the combined effects of high temperatures and the herbicide, atrazine. Aquatic Botany, 142: 41-47.
DOI:10.1016/j.aquabot.2017.06.005 |
Gu J, Weber K, Klemp E, Winters G, Franssen S U, Wienpahl I, Huylmans A K, Zecher K, Reusch T B H, BornbergBauer E, Weber A P M. 2012. Identifying core features of adaptive metabolic mechanisms for chronic heat stress attenuation contributing to systems robustness. Integrative Biology, 4(5): 480-493.
DOI:10.1039/c2ib00109h |
Guy C, Kaplan F, Kopka J, Selbig J, Hincha D K. 2008. Metabolomics of temperature stress. Physiologia Plantarum, 132(2): 220-235.
|
Hasegawa N, Hori M, Mukai H. 2008. Seasonal changes in eelgrass functions:current velocity reduction, prevention of sediment resuspension, and control of sediment-water column nutrient flux in relation to eelgrass dynamics. Hydrobiologia, 596(1): 387-399.
DOI:10.1007/s10750-007-9111-4 |
Heck Jr K L, Hays G, Orth R J. 2003. Critical evaluation of the nursery role hypothesis for seagrass meadows. Marine Ecology Progress Series, 253: 123-136.
DOI:10.3354/meps253123 |
Jordà G, Marbà N, Duarte C M. 2012. Mediterranean seagrass vulnerable to regional climate warming. Nature Climate Change, 2(11): 821-824.
DOI:10.1038/nclimate1533 |
Joshi V, Joung J G, Fei Z J, Jander G. 2010. Interdependence of threonine, methionine and isoleucine metabolism in plants:accumulation and transcriptional regulation under abiotic stress. Amino Acids, 39(4): 933-947.
DOI:10.1007/s00726-010-0505-7 |
Kaplan F, Kopka J, Haskell D W, Zhao W, Schiller K C, Gatzke N, Sung D Y, Guy C L. 2004. Exploring the temperaturestress metabolome of Arabidopsis. Plant Physiology, 136(4): 4159-4168.
DOI:10.1104/pp.104.052142 |
Kim J B, Lee W C, Lee K S, Park J I. 2013. Growth dynamics of eelgrass, Zostera marina, in the intertidal zone of Seomjin Estuary, Korea. Ocean Science Journal, 48(3): 239-250.
DOI:10.1007/s12601-013-0021-2 |
Larkindale J, Huang B R. 2004. Changes of lipid composition and saturation level in leaves and roots for heat-stressed and heat-acclimated creeping bentgrass (Agrostis stolonifera). Environmental and Experimental Botany, 51(1): 57-67.
DOI:10.1016/S0098-8472(03)00060-1 |
Lee K S, Park S R, Kim Y K. 2007. Effects of irradiance, temperature, and nutrients on growth dynamics of seagrasses:a review. Journal of Experimental Marine Biology and Ecology, 350(1-2): 144-175.
DOI:10.1016/j.jembe.2007.06.016 |
Lepoint G, Cox A S, Dauby P, Poulicek M, Gobert S. 2006. Food sources of two detritivore amphipods associated with the seagrass Posidonia oceanica leaf litter. Marine Biology Research, 2(5): 355-365.
DOI:10.1080/17451000600962797 |
Liu X, Yang J H, Li B, Yang X M, Meng Q W. 2010. Antisense expression of tomato chloroplast omega-3 fatty acid desaturase gene (LeFAD7) enhances the tomato hightemperature tolerance through reductions of trienoic fatty acids and alterations of physiological parameters. Photosynthetica, 48(1): 59-66.
DOI:10.1007/s11099-010-0009-4 |
Marbà N, Duarte C M. 2010. Mediterranean warming triggers seagrass (Posidonia oceanica) shoot mortality. Global Change Biology, 16(8): 2366-2375.
|
Marcum K B. 1998. Cell membrane thermostability and whole-plant heat tolerance of Kentucky bluegrass. Crop Science, 38(5): 1214-1218.
DOI:10.2135/cropsci1998.0011183X003800050017x |
Marsh Jr J A, Dennison W C, Alberte R S. 1986. Effects of temperature on photosynthesis and respiration in eelgrass(Zostera marina L.). Journal of Experimental Marine Biology and Ecology, 101(3): 257-267.
DOI:10.1016/0022-0981(86)90267-4 |
Massa S I, Arnaud-Haond S, Pearson G A, Serrão E A. 2009. Temperature tolerance and survival of intertidal populations of the seagrass Zostera noltii (Hornemann) in Southern Europe (Ria Formosa, Portugal). Hydrobiologia, 619(1): 195-201.
DOI:10.1007/s10750-008-9609-4 |
Micheli F, Bishop M J, Peterson C H, Rivera J. 2008. Alteration of seagrass species composition and function over two decades. Ecological Monographs, 78(2): 225-244.
DOI:10.1890/06-1605.1 |
Murakami Y, Tsuyama M, Kobayashi Y, Kodama H, Iba K. 2000. Trienoic fatty acids and plant tolerance of high temperature. Science, 287(5452): 476-479.
DOI:10.1126/science.287.5452.476 |
Nambara E, Kawaide H, Kamiya Y, Naito S. 1998. Characterization of an Arabidopsis thaliana mutant that has a defect in ABA accumulation:ABA-dependent and ABA-independent accumulation of free amino acids during dehydration. Plant and Cell Physiology, 39(8): 853-858.
DOI:10.1093/oxfordjournals.pcp.a029444 |
Paulsen G M. 1994. High temperature responses of crop plants.In: Boote K J, Bennett J M, Sinclair T R, Paulsen G M eds. Physiology and Determination of Crop Yield. ASA, CSSA, SSSA, Madison, WI. p.365-389.
|
Pedersen O, Colmer T D, Borum J, Zavala-Perez A, Kendrick G A. 2016. Heat stress of two tropical seagrass species during low tides-impact on underwater net photosynthesis, dark respiration and diel in situ internal aeration. New Phytologist, 210(4): 1207-1218.
DOI:10.1111/nph.13900 |
Peterson T C, Heim Jr R R, Hirsch R, Kaiser D P, Brooks H, Diffenbaugh N S, Dole R M, Giovannettone J P, Guirguis K, Karl T R, Katz R W, Kunkel K, Lettenmaier D, McCabe G J, Paciorek C J, Ryberg K R, Schubert S, Silva V B S, Stewart B C, Vecchia A V, Villarini G, Vose R S, Walsh J, Wehner M, Wolock D, Wolter K, Woodhouse C A, Wuebbles D. 2013. Monitoring and understanding changes in heat waves, cold waves, floods, and droughts in the United States:state of knowledge. Bulletin of the American Meteorological Society, 94(6): 821-834.
DOI:10.1175/BAMS-D-12-00066.1 |
Ralph P J, Tomasko D, Moore K, Seddon S, Macinnis-Ng C M O. 2007. Human impacts on seagrasses: eutrophication, sedimentation, and contamination. In: Larkum A W D, Orth R J, Duarte C M eds. SEAGRASSES: Biology, Ecology and Conservation. Springer, Dordrecht, Netherlands. p.567-593.
|
Rasheed M A, Dew K R, McKenzie L J, Coles R G, Kerville S P, Campbell S J. 2008. Productivity, carbon assimilation and intra-annual change in tropical reef platform seagrass communities of the Torres Strait, north-eastern Australia. Continental Shelf Research, 28(16): 2292-2303.
DOI:10.1016/j.csr.2008.03.026 |
Rasheed M A, Unsworth R K F. 2011. Long-term climateassociated dynamics of a tropical seagrass meadow:implications for the future. Marine Ecology Progress Series, 422: 93-103.
DOI:10.3354/meps08925 |
Reusch T B H, Ehlers A, Hämmerli A, Worm B. 2005. Ecosystem recovery after climatic extremes enhanced by genotypic diversity. Proceedings of the National Academy of Sciences of the United States of America, 102(8): 2826-2831.
DOI:10.1073/pnas.0500008102 |
Ribeiro P R, Fernandez L G, De Castro R D, Ligterink W, Hilhorst H W. 2014. Physiological and biochemical responses of Ricinus communis seedlings to different temperatures:a metabolomics approach. BMC Plant Biology, 14: 223.
DOI:10.1186/s12870-014-0223-5 |
Savchenko G E, Klyuchareva E A, Abramchik L M, Serdyuchenko E V. 2002. Effect of periodic heat shock on the inner membrane system of etioplasts. Russian Journal of Plant Physiology, 49(3): 349-359.
DOI:10.1023/A:1015592902659 |
Seifert G J, Roberts K. 2007. The biology of arabinogalactan proteins. Annual Review of Plant Biology, 58: 137-161.
DOI:10.1146/annurev.arplant.58.032806.103801 |
Shin H, Cho K H, Oh Y S. 2002. Zostera geojeensis, a new species of seagrass from Korea. Algae, 17(2): 71-74.
DOI:10.4490/ALGAE.2002.17.2.071 |
Short F T, Polidoro B, Livingstone S R, Carpenter K E, Bandeira S, Bujang J S, Calumpong H P, Carruthers T J B, Coles R G, Dennison W C, Erftemeijer P L A, Fortes M D, Freeman A S, Jagtap T G, Kamal A H M, Kendrick G A, Kenworthy W J, La Nafie Y A, Nasution I M, Orth R J, Prathep A, Sanciangco J C, van Tussenbroek B, Vergara S G, Waycott M, Zieman J C. 2011. Extinction risk assessment of the world's seagrass species. Biological Conservation, 144(7): 1961-1971.
DOI:10.1016/j.biocon.2011.04.010 |
Short F T, Wyllie-Echeverria S. 1996. Natural and humaninduced disturbance of seagrasses. Environmental Conservation, 23(1): 17-27.
DOI:10.1017/S0376892900038212 |
Simon-Sarkadi L, Kocsy G, Várhegyi Á, Galiba G, De Ronde J A. 2006. Stress-induced changes in the free amino acid composition in transgenic soybean plants having increased proline content. Biologia Plantarum, 50(4): 793-796.
DOI:10.1007/s10535-006-0134-x |
Sohn S O, Back K. 2007. Transgenic rice tolerant to high temperature with elevated contents of dienoic fatty acids. Biologia Plantarum, 51(2): 340-342.
DOI:10.1007/s10535-007-0067-z |
Thangaradjou T, Nobi E P, Dilipan E, Sivakumar K, Susila S. 2010. Heavy metal enrichment in seagrasses of Andaman Islands and its implication to the health of the coastal ecosystem. Indian Journal of Marine Sciences, 39(1): 85-91.
|
Triba M N, Le Moyec L, Amathieu R, Goossens C, Bouchemal N, Nahon P, Rutledge D N, Savarin P. 2015. PLS/OPLS models in metabolomics:the impact of permutation of dataset rows on the K-fold cross-validation quality parameters. Molecular BioSystems, 11(1): 13-19.
DOI:10.1039/C4MB00414K |
Wahid A, Gelani S, Ashraf M, Foolad M R. 2007. Heat tolerance in plants:an overview. Environmental and Experimental Botany, 61(3): 199-223.
DOI:10.1016/j.envexpbot.2007.05.011 |
Waycott M, Duarte C M, Carruthers T J B, Orth R J, Dennison W C, Olyarnik S, Calladine A, Fourqurean J W, Heck Jr K L, Hughes A R, Kendrick G A, Kenworthy W J, Short F T, Williams S L, Paine R T. 2009. Accelerating loss of seagrasses across the globe threatens coastal ecosystems. Proceedings of the National Academy of Sciences of the United States of America, 106(30): 12377-12381.
DOI:10.1073/pnas.0905620106 |
Waycott M, McKenzie L, Mellors J E, Ellison J C, Sheaves M T, Collier C, Schwarz A M, Webb A, Johnson J E, Payri C E. 2011. Vulnerability of mangroves, seagrasses and intertidal flats in the tropical Pacific to climate change. In: Bell J D, Johnson J E, Hobday A J eds. Vulnerability of Tropical Pacific Fisheries and Aquaculture to Climate Change. Secretariat of the Pacific Community, Noumea, New Caledonia. p.297-368.
|
Xu Y, Du H M, Huang B R. 2013. Identification of metabolites associated with superior heat tolerance in thermal bentgrass through metabolic profiling. Crop Science, 53(4): 1626-1635.
DOI:10.2135/cropsci2013.01.0045 |
Yamakawa H, Hakata M. 2010. Atlas of rice grain fillingrelated metabolism under high temperature:joint analysis of metabolome and transcriptome demonstrated inhibition of starch accumulation and induction of amino acid accumulation. Plant and Cell Physiology, 51(5): 795-809.
DOI:10.1093/pcp/pcq034 |
Zimmerman R C, Smith R D, Alberte R S. 1989. Thermal acclimation and whole-plant carbon balance in Zostera marina L. (eelgrass). Journal of Experimental Marine Biology and Ecology, 130(2): 93-109.
DOI:10.1016/0022-0981(89)90197-4 |
 2019, Vol. 37
2019, Vol. 37


