Institute of Oceanology, Chinese Academy of Sciences
Article Information
- YIN Qunjian, ZHANG Weijia, LI Xuegong, ZHOU Lihong, QI Xiaoqing, ZHANG Chan, WU Long-Fei
- Contribution of trimethylamine N-oxide on the growth and pressure tolerance of deep-sea bacteria
- Journal of Oceanology and Limnology, 37(1): 210-222
- http://dx.doi.org/10.1007/s00343-019-7377-9
Article History
- Received Dec. 14, 2017
- accepted in principle Mar. 8, 2018
- accepted for publication Apr. 19, 2018
2 University of Chinese Academy of Sciences, Beijing 100049, China;
3 International Associated Laboratory of Evolution and Development of Magnetotactic Multicellular Organisms, CNRS-Marseille/CAS-Beijing-Qingdao-Sanya;
4 CAS Key Laboratory for Experimental Study under Deep-sea Extreme Conditions, Institute of Deep-sea Science and Engineering, Chinese Academy of Sciences, Sanya 572000, China;
5 AMU, LCB UMR 7283, CNRS-Marseille, 143402, France
The deep-sea with depth over 1 000 m accounts for over 75% of the ocean. Our current knowledge of the microorganisms inhabiting the deep-sea environment is generally obtained by means of high-throughput sequencing, due to the difficulties in bacterial cultivation and analysis under HHP conditions. Metagenomic analysis demonstrated that Gammaproteobacteria and Alphaproteobacteria are the most abundant taxa in diverse deep-sea environments, and Deltaproteobacteria, Acidobacteria and Actinobacteria are often detected as well (DeLong et al., 2006; Sogin et al., 2006; Lauro and Bartlett, 2008; Salazar et al., 2016; Tarn et al., 2016). Stratified deep-sea bacterial community composition has been observed in different sites. Chemolithotrophic microbes are more abundant in the upper abyssal water while heterotrophic, methane and hydrogen utilizing, and sulfur-cycling microbes are enriched in the hadal water (Nunoura et al., 2015; Tarn et al., 2016). However, the molecular basis of deep-sea adaptation remains unresolved since few of the deepsea bacteria have been successively cultivated in laboratory conditions. Piezophiles are microbes that favor growth at pressure conditions higher than atmospheric pressure (0.1 MPa). The majority of piezophiles isolated and cultivated to date are affiliated to the genera of Shewanella, Photobacterium, Colwellia, Moritella and Psychromonas of Gammaproteobacteria (Fang et al., 2010). The studies confined to limited species of Shewanella and Photobacterium demonstrated that bacterial adaptation to the deep-sea environment is a multifactor event including alterations in the composition and structure of macromolecules, regulation of gene transcription and metabolic pathway (Tamegai et al., 2012; Ohke et al., 2013).
TMAO is an important component of organic nitrogen in the marine ecological environment. Its concentration in surface water reaches 76.9 nmol/dm3, higher than all the other methylamines (Gibb and Hatton, 2004; Ge et al., 2011). TMAO can be produced through oxidation of trimethylamine (TMA) by a variety of marine bacteria, phytoplankton, invertebrates and fishes (Barrett and Kwan, 1985; Seibel and Walsh, 2002; McCrindle et al., 2005). Due to the difficulties in sampling techniques and TMAO detection approaches, the concentration of TMAO in deep-sea environment remains unknown. However, highly accumulated TMAO (up to 386±18 mmol/kg) has been observed in the muscle tissue of deep-sea animals, where it functions as an osmolyte and protects the proteins from multiple stresses including low temperature, high concentration of urea and HHP (Yancey et al., 1982, 2001, 2014; Gillett et al., 1997; Saad-Nehme et al., 2001; Zou et al., 2002; He et al., 2009; Petrov et al., 2012). It is speculated that TMAO released from deep-sea animals may create a microenvironment with instant high concentration of TMAO and provides bacteria nearby precious nutrient for growth (Zhang et al., 2016).
Marine microorganisms metabolize TMAO through two different pathways. SAR11 clade and marine Roseobacter clade (MRC) bacteria could utilize TMAO as a carbon and nitrogen source (Lidbury et al., 2014, 2015). TMAO is transported into the cytoplasm through a TMAO-specific ABC transporter system and converted into DMA (dimethylamine) by TMAO demethylase. DMA is further catalyzed into MMA (monomethylamine) and ammonia, and participates into diverse metabolism pathways (Zhu et al., 2014). In addition, diverse species of marine bacteria, including Alteromonas, Campylobacter, Flavobacterium, Photobacterium, Pseudomonas and Vibrio, and most species of Enterobacteriaceae are known to use TMAO as electron acceptor of respiration under anaerobic conditions (Barrett and Kwan, 1985; Dos Santos et al., 1998; Dunn and Stabb, 2008). TMAO reductase that is responsible for TMAO anaerobic respiration has also been detected in numerous deep-sea bacterial strains. Moderately piezophiles P. profundum SS9 and P. phospherum ANT-2200 encode 3 and 4 sets of TMAO reductase (TorA), respectively. Previously, we observed that one of the TMAO reductase isozyme TorA1 is induced under HHP condition in ANT-2200 (Zhang et al., 2016). In addition, we discovered, for the first time, that TMAO improves the pressure tolerance of a piezo-sensitive strain Vibrio fluvialis QY27, and an HHP inducible TMAO reductase is involved in this process (Yin et al., 2018). Yet, whether the utilization of TMAO and the TMAO-promoted pressure tolerance is a common trait of deep-sea bacteria remains unknown.
To answer these questions, we analyzed the ability of TMAO utilization and the pressure tolerance with or without the presence of TMAO of over 200 strains isolated from the South China Sea and the Mariana Trench. Our results demonstrated that there was no apparent correlation between the depth where the bacteria inhabit and their pressure tolerance, regarding to these samples. Strains from the genera of Alteromonas, Halomonas, Marinobacter, Photobacterium and Vibrio showed capacity of TMAO utilization, but none of the isolated Acinebacter, Bacillus, Brevundimonas, Muricauda, Novosphingobium, Rheinheimera, Sphingobium and Stenotrophomonas did. Furthermore, we noticed that TMAO has greater impact on the growth of deep-sea isolates of Vibrio neocaledonicus than shallow-water isolates. For most isolates, the utilization of TMAO does not change their pressure tolerance. Therefore, the TMAO-improved pressure tolerance we recently reported might be a species-specific trait of V. fluvialis.
2 MATERIAL AND METHOD 2.1 Sample collectionSeawater samples of the South China Sea were collected at different depths with Niskin bottles mounted on the rosette sampler. Seawater samples of the Mariana Trench were collected by pressureretaining sampler (Top Industrie, France) mounted on the rosette sampler or Niskin bottles carried by seabed lander. Sediment samples were collected by box sampler or push-core carried by seabed lander. Deepsea amphipods were captured using baited traps carried by seabed lander. The information of sampling sites is listed in table 1.
The samples collected from the South China Sea and the Mariana Trench were first incubated under high pressure vessels (Feiyu Technology Development Co. Ltd., China) for the enrichment of pressure tolerant bacteria. To specify, seawater samples were inoculated into cultural medium YPG (Martini et al., 2013), 2216E (Simon-Colin et al., 2008) and R2A (Smith et al., 2004) with inoculums of 1/10. Sediment samples were processed by mixing 10 mL medium with 1 g sediment. Regarding to the deep-sea amphipods, an individual with length of approximately 1.5 cm was incubated with 3 mL cultural medium. After incubation under high pressure condition under ambient temperature for 10 days, a series dilution was prepared for each of the samples and spread on solid medium for the purification of single colonies. Purified strains were cultured in the corresponding medium at room temperature (approximately 23– 25℃) for molecular identification and strain conservation.
2.3 Phylogenetic analyses based on 16s rRNA gene sequencesThe 16S rRNA sequences were amplified with 27F and 1492R primers, and BLAST against the EzBioCloud Database (https://www.ezbiocloud.net/identify) (Yoon et al., 2017). Sequence identity of 97% and 95% are used as the thresholds for classification of species and genus, respectively (Tindall et al., 2010). The phylogenetic tree was established with the sequences of 16S rRNA, using neighbor-joining (N-J) method in the software MEGA 5, in which 1 000 times repeated bootstrap analysis was used to test the credibility of the phylogenetic tree.
2.4 Cultivation of deep-sea bacterial isolatesFor evaluation of growth at different conditions, bacterial isolates were cultivated in 1 mL liquid medium overnight before inoculated into fresh medium with inoculums of 1:100. The cultures were then transferred into a disposable syringe and blocked with sterilized stopper. The syringes were placed in a high-pressure vessel and pressure was applied with a water pump (Top Industrie, France). After cultivation for 24 h, absorption at 600 nm was measured with a spectrophotometer (Agilent Technologies, the United States). TMAO was supplemented to a final concentration of 1% (w/v) unless otherwise mentioned. All the culture experiments were carried out at room temperature (23–25℃).
For the measurement of the growth curve, cells were cultivated under the condition of 0.1, 10, 20, 30, 40 and 50 MPa, respectively. The absorption at 600 nm was measured every 5 h to obtain the growth curve. The maximum specific growth rate μ was calculated from the logarithm of the growth curve, μ=3.322×Lg(x2/x1)/(t2–t1). The x1 and x2 are the numbers of cells at the time of t1 and t2, respectively.
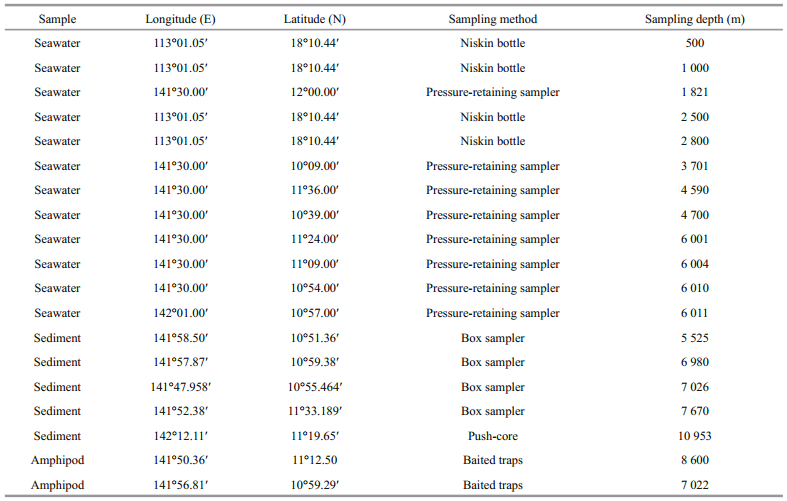
3 RESULT 3.1 Molecular identification of the deep-sea bacterial isolatesIn total 237 strains were isolated from the seawater, sediments and deep-sea amphipods collected from the South China Sea and the Mariana Trench at the depth ranged from 500 m to over 10 000 m. Phylogenetic analysis based on the 16S rRNA gene sequences revealed their affiliation to 50 species of 23 genera, 4 phyla, using 97% and 95% sequence identity as the thresholds for classification of species and genus, respectively (Tindall et al., 2010). Proteobacteria accounted for 81.4% of all the isolates (193 strains), with the majority belonged to Gammaproteobacteria (149 strains, 77% of Proteobacteria), and the rest belonged to Alphaproteobacteria (44 strains, 23% of Proteobacteria). In addition, we also obtained 8 isolates (3.4%) affiliated to Bacteroidetes, 14 isolates (5.9%) affiliated to Firmicutes, and 22 isolates (9.3%) affiliated to Actinobacteria (Fig. 1).
|
| Fig.1 Phylogenetic tree of bacteria isolated from the South China Sea and Mariana Trench Neighbor-joining phylogenetic tree based on bacterial 16S rRNA genes from the South China Sea and the Mariana Trench, numbers on the nodes are the bootstrap values (percentages) based on 1 000 replicates and values of above 20% were presented. Black solid lines represent strains of Proteobacteria, red dash line represents strains of Firmicutes, blue double dot dash line represents strains of Actinobacteria, and violet dash-dotted line represents strains of Bacteroidetes. Number in braces indicates the number of isolates. |
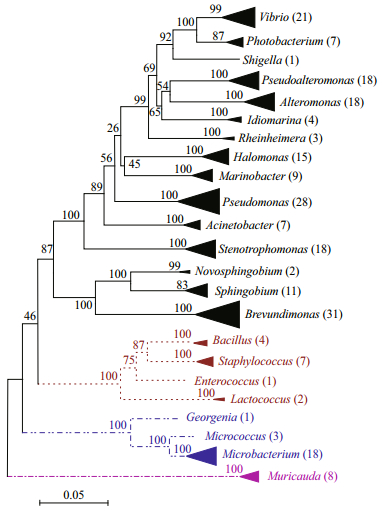
Twenty-eight strains from 4 genera were isolated from sublittoral zone (200–1 000 m), dominated by Pseudoalteromonas (43%) and Vibrio (46%). Seventy-eight strains were isolated from the bathyal zone (1 000–4 000 m), consisting of Pseudomonas (24%), Halomonas (8%), Pseudoalteromonas (8%), Stenotrophomonas (8%), Photobacterium (8%) and other 12 genera. Over a hundred strains belonged to 14 genera were isolated from abyssal zone (4 000– 6 000 m), and one fourth of them were Brevundimonas. From the hadal zone (over 6 000 m), we obtained twenty-six strains belonging to 12 genera, dominated by Staphylococcus (23%) and Alteromonas (19%) (Fig. 2). Among all the isolates, Alteromonas was the only genus that had been isolated from all the four zones, while Staphylococcus, Shigella, Enterococcus, Lactococcus, Muricauda and Novosphingobium were obtained only from the samples deeper than 4 000 m (Fig. 2).
|
| Fig.2 The composition and distribution of strains isolated from different depth Sublittoral (200 to 1 000 m depth) includes samples collected from the depth of 500 and 1 000 m; bathyal (1 000 to 4 000 m) includes samples collected from the depth of 1 821 m, 2 500 m, 2 800 m and 3 701 m; abyssal (4 000 to 6 000 m) includes samples collected from the depth of 4 590 m, 4 700 m, 5 525 m, 6 001 m, 6 004 m, 6 010 m and 6 011 m; hadal (over 6 000 m) includes samples collected from the depth of 6 980 m, 7 022 m, 7 026 m, 7 670 m, 8 600 m and 10 953 m. |
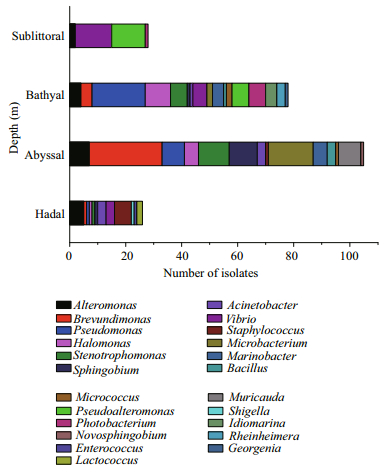
Based on the sensitivity to HHP, bacteria can be divided into four types: a) the piezo-sensitive bacteria that have higher growth rates at atmospheric pressure (0.1 MPa); b) the piezo-tolerant bacteria, whose growth rate is stable under atmospheric pressure and HHP condition; c) the piezophilic bacteria that grow better under HHP condition; and d) the obiligate piezophilic bacteria that grow only under HHP condition (Kato et al., 2008). An accurate definition of the pressure tolerance of a bacterial strain is based on the comparison of the maximal growth rates under different pressure conditions, and thus requires the measurement of plenty of growth curves. However, measuring the growth curve, especially under HHP conditions, is restrained by availability of cultivation equipment because sampling at each time point sacrifices at least one high pressure vessel. Therefore, to have a primary evaluation of the pressure tolerance of the bacteria isolates more efficiently, we first compared their growth at atmospheric pressure (0.1 MPa) and HHP (30 MPa, under which condition the growth of typical piezo-sensitive strain such as Escherichia coli is known to be obviously impeded, whereas most cultivated piezo-tolerant strains are not affected and piezophiles demonstrate improved growth compared to 0.1 MPa) conditions by measuring the absorbtion at 600 nm at the stationary phase, and calculated the ratio of biomass at 30 MPa to 0.1 MPa (ratio +/- pressure). The isolates with biomass ratio (+/- pressure) below 0.80 are preliminarily defined as pressure sensitive strains, that is, high pressure restrained their growth. Those with a ratio above 1.20 are probably piezophiles, since they grow better under high-pressure conditions. And those in between (≥0.80 and < 1.20) are likely to be pressure tolerant bacteria.
By applying these criteria, only two of the seventyfour isolates, Photobacterium angustum QY26 and Photobacterium leiognathi W214 are defined as piezophiles, with the ratio +/- pressure of 1.30 and 1.40, respectively (Fig. 3 and Table 2). Fifty-three strains (72% of all the isolates) are sensitive to high pressure, and 19 isolates (26% of all the isolates) are not remarkably affected by increased pressure (Fig. 3 and Table 2).
|
| Fig.3 The effects of high pressure on growth of isolates from different depth Ratio of biomass +/- pressure represents the ratio of biomass at 30 MPa versus 0.1 MPa. Left dash line represents the ratio +/- pressure of 0.8, the cut-off value of piezo-sensitive and piezotolerant characterization. The right dash line represents the biomass ratio +/- pressure of 1.2, the cut-off value of piezo-tolerant and piezophilic characterization. |
The biomass ratio (+/- pressure) reflects only the difference of biomass at the stationary phase. To ascertain their pressure tolerance, several isolates were further selected for detailed analysis of growth curve under 0.1, 10, 20, 30, 40 and 50 MPa conditions. In total 49 isolates were analyzed, and three types of pressure tolerance were observed (Fig. 4). Thirty isolates affiliated to 14 species of 10 genera (QY7-10, QY14, QY18-22, QY24, QY25, QY27, QY31, QY33, QY35, QY38 - 40, QY42, S32, W111, W117, W119, W124, W125, W211, W226, W228 and W239) were pressure sensitive strains. As represented by Vibrio furnissii QY42, their growth rates kept decreasing when the pressure increased from 0.1 MPa to 50 MPa (Fig. 4a). Seventeen isolates are pressure-tolerant, as their growth rates were stable when cultivated under from 0.1 MPa to 30 MPa, and decreased at 40 MPa and 50 MPa (Fig. 4b). Pseudoalteromonas shioyasakiensis QY32 and other sixteen isolates (QY1-5, QY23, QY15, QY16, QY28 - 30, QY32, A17, S33, W105, W107 and W116) from 8 species of 5 genera belong to this type. The Photobacterium leiognathi W214 and P. angustum QY26 were the only piezophilic strains isolated in this study. Their growth rate increased remarkably when the pressure elevated from 0.1 MPa to 30 MPa, and dropped afterwards (Fig. 4c).
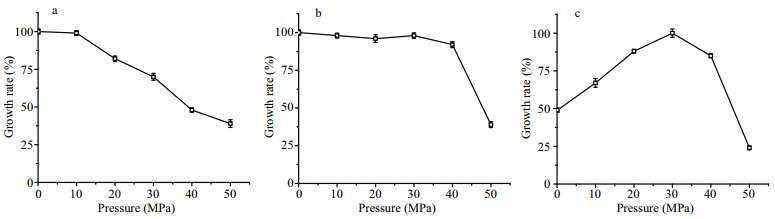
|
| Fig.4 The effect of pressure to the maximal growth rate of strains isolated in this study The maximal growth rates under different pressure conditions of a. piezo-sensitive strains, represented here by Vibrio furnissii QY42; b. piezo-tolerant strains, represented here by Pseudoalteromonas shioyasakiensis QY32; and c. piezophilic strain, represented here by Photobacterium angustum QY26. The maximal growth rates at 0.1 MPa (a and b) or 30 MPa (c) were set as 100%. |
The two piezophiles, QY26 and W214, are isolated from seawater of 500 m and 1 821 m depth, respectively. Meanwhile, piezo-tolerant and piezosensitive strains can be obtained from 10 000 m depths. It further confirmed our presumption that there is no apparent strict correlation between the depth where the bacteria inhabit and its pressure tolerance, regarding to these isolates.
3.4 Utilization of TMAO by deep-sea bacteriaTMAO is commonly found in the tissues of marine organisms, especially in deep-sea species where it serves to protect against the adverse effect of HHP. Meantime, high concentration of TMAO implies a locally and temporally abundant substrate disposable for bacterial metabolism. To evaluate the potential of TMAO metabolism in the isolates, 74 strains as listed in Table 2 were cultivated under atmospheric pressure with or without the supplementation of TMAO, and the ratio of the biomass (+/- TMAO) was calculated. We found that the addition of TMAO improved the growth of 29 strains belonging to 13 species of 7 genera as indicated by the biomass ratio (+/- TMAO) > 1.20, whereas it had no or negative effect on the growth of 45 strains from 28 species of 14 genera with the biomass ratio (+/- TMAO) ≤1.20 (Table 2).
We then monitored the growth curves of 32 strains, in order to further ascertain their capacity of TMAO utilization. The P. angustum QY26 and other 13 strains from 10 species of 6 genera (S33, QY9, QY15, QY16, QY20, QY39, QY40, QY42, W111, W124, W211, W214 and W228) had a similar response to TMAO. Both the growth rate and the biomass increased when they were cultivated with the addition of TMAO, indicating they were able to utilize TMAO (Fig. 5a). On the contrary, the growth of the rest 18 strains (A17, S32, QY1, QY3, QY10, QY18, QY28– 29, QY32, QY35, W105, W107, W116–117, W119, W125, W226 and W239) were not affected or even slightly repressed with the addition of TMAO (Fig. 5b). The results obtained by growth curve measurement were consistent to the comparison of biomass at stationary phase, further supported the preliminary classification of TMAO-utilizing and TMAO-not-utilizing strains.
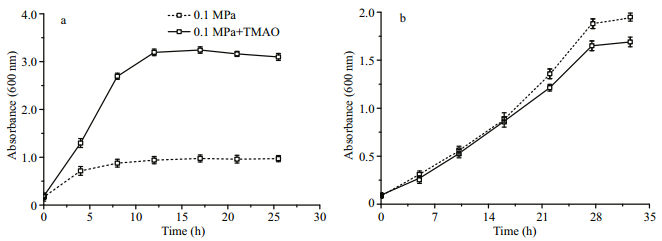
|
| Fig.5 The effect of TMAO to the growth of bacterial isolates at atmospheric pressure a. the growth of 29 strains are significantly improved by addition of TMAO, represented by Photobacterium angustum QY26; b. the growth of 45 strains are not affected by addition of TMAO, represented by Pseudoalteromonas shioyasakiensis QY32. The solid line represents growth with a supplement of 1% TMAO (w/v), the dash line represents growth without TMAO. |
Seventy-four strains whose biomass ratio (+/- TMAO) have been analyzed belong to 36 species of 16 genera, including at least 5 strains of Alteomonas, Brevundimonas, Microbacterium, Pseudoalteromonas and Vibrio, respectively. Almost all the isolates of Vibrio are capable of TMAO utilization, whereas the growth of over 70% of Pseudoalteromonas and Alteromonas strains were not improved by addition of TMAO (Table 2), indicating that the TMAO utilization is a species-specific feature.
To further understand the TMAO utilization at deep-sea environment, we sought for relationship between the depth where bacterium inhabits and its ability of TMAO utilization. Nine strains of V. neocaledonicus have been isolated from different depth from 500 m to 2 800 m. Most of them were able to utilize TMAO. Addition of TMAO had the most significant impact on the growth of QY15 and QY16 isolated from 2 800 m depths, with over 4-fold rising of biomass. Whereas the growth of other isolates is moderately improved, with the ratio of biomass (+/- TMAO) ranged from 1.11 to 1.53. It suggests that the ability of TMAO utilization might be in positive correlation with the depth of in situ habits. However, whether this rule can be applied to all the deep-sea bacteria that are capable of TMAO utilization remains to be confirmed by the increasing number of samples in the future.
3.5 The effect of TMAO metabolism on bacterial pressure toleranceOur previous study demonstrated addition of TMAO in the culture medium improves the pressure tolerance of strain QY27 and changes the piezosensitive phenotype into a piezophilic one (Yin et al., 2018). Whether this intriguing phenomenon is universal in deep-sea bacteria or restrained in certain species requires further investigation.
Thus, we tested the effect of TMAO to the growth of thirty-two isolates under HHP conditions. As represented by the strain QY28, eighteen strains that are unable to utilize TMAO under both atmospheric pressure and 30 MPa (Fig. 6a, Table 2). For those utilizing TMAO under atmospheric pressure, their growth was also improved by TMAO under HHP, but the pressure tolerance remained the same. With the presence of TMAO, 12 piezo-sensitive or piezotolerant strains grew better at the atmospheric pressure than at high pressure (Fig. 6b, black line versus red line, Table 2), whereas the piezophilic strain QY26 and W214 had longer exponential growth phase and higher biomass at the end of growth at high pressure compared to atmospheric pressure (Fig. 6c, Table 2). Collectively, for the strains analyzed in this study, the metabolism of TMAO may improve their growth, but have no impact on the pressure tolerance, and the TMAO-improved pressure tolerance might be a feature of only few species such as Vibrio fluvialis.
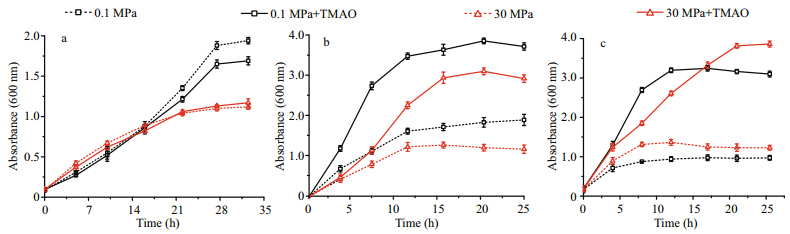
|
| Fig.6 Combination effects of hydrostatic pressure and supplementation of TMAO on the growth The growth curves at 0.1 MPa or 30 MPa, with or without supplementation of TMAO of a. eighteen strains that are unable to utilize TMAO, represented by the piezo-tolerant strain A. macleodii QY28; b. 12 piezo-sensitive or piezo-tolerant strains that utilize TMAO, represented by the piezo-sensitive strain V. alginolyticus QY20; and c. two piezophilic strains, represented by the piezophilic strain P. angustum QY26. The dash lines represent culture without TMAO, the solid lines represent culture with supplementation of TMAO. Line in black, 0.1 MPa; line in red, 30 MPa. |
The discrepancy between the number of cells in seawater samples and the colonies formed on agar plates has been noticed decades ago (Jannasch and Jones, 1959). However, the low efficiency in seawater bacteria isolation remains an unsolved problem. Several hypotheses have been proposed, such as the dormancy state of microorganisms in natural environment, the incapable of forming colonies on agar plates, or certain microbes have specialized requirements for media that are difficult to meet in laboratory. Regarding to deep-sea bacteria, the in situ HHP might be the major factor inhibiting their cultivation (Tamburini et al., 2013). The application of pressure-retaining sampler and cultivation system (such as DEEP-BATH) leaded to isolation of several piezophiles and even obligate piezophiles (Parkes et al., 2009). Apart from those isolated from hydrothermal vent, most piezophiles are psychropilic and cannot be cultured at temperatures higher than 20℃. Yet, isolation of meso-piezophilic (Demacoccus abyssi MT1.1) and thermo-piezophilic bacteria (Marinitoga piezophila KA3, Thioprofundum lithotrophica strain 106 and Piezobacter thermophilus strain 108) from the Challenger Deep of the Mariana Trench or the East-Pacific Rise have been reported previously (Pathom-Aree et al., 2006; Takai et al., 2009; Lucas et al., 2012; Ji et al., 2013). In this study, typical psychropiezo-tolerants and psychro-piezophiles such as Shewanella, Moritella and Psychromonas that have been previously isolated from the Mariana Trench are absent in our isolates which is possibly due to the relatively high temperature conditions (23–25℃) utilized for bacterial isolation. High temperature is favored for mesophiles but impedes the growth of psychrophiles.
Low temperature and HHP have similar physical effect to biological molecules, both lead to a reduction in volume that influence the structure and function of macromolecules. Therefore, although a pre-incubation at HHP condition was performed in the attempt to enrich for pressure tolerant strains, the effect of HHP might have been neutralized by the relatively high temperature comparing to the in situ condition (23℃ vs 3℃) and enables the survival and growth of pressure sensitive strains.
As has been demonstrated in deep-sea model strains, the physiological condition and metabolism capacity of deep-sea bacteria can be influenced by both temperature and pressure (Jian et al., 2016; Xiong et al., 2016). In our study, the Photobacterium leiognathi W214 and Photobacterium angustum QY26 are the only two piezophiles among the 74 isolates with experimentally confirmed pressure tolerance. Some of the isolates from bathyal zone demonstrated even better growth at HHP condition comparing to isolates from abyssal zone, such as QY29 isolated from 1 000 m, QY28 from 2 500 m and QY15 from 2 800 m that had biomass ratio 30 MPa/0.1 MPa without TMAO over 1.0. Even the four strains isolated from over 10 000 m depth showed better growth at 0.1 MPa than at 30 MPa (Fig. 3 and Table 2). It suggests that tolerance to HHP may not be a required feature of deep-sea bacteria. However, it reflects only the bacterial pressure tolerance at room temperature conditions, but not that under in situ conditions with lower temperature. Growth experiments at different temperature conditions are on-going and are thus expected to bring novel knowledge regarding the pressure tolerance and TMAO metabolism of these isolates.
Forty-one strains affiliated to 21 species of 9 genera showed the ability of TMAO utilization, some of them were isolated from the abyssal and hadal zone. Our results demonstrated that the ability of TMAO utilization is species-dependent at certain level. All the isolates of Vibrio are capable of using TMAO for growth, as the biomass increased when TMAO is present, whereas the growth of Pseudoalteromonas spp. are not improved with the addition of TMAO. TMAO reduction has been reported in species of Alteromonas and Pseudomonas (Ringø et al., 1984; Barrett and Kwan, 1985; Ge et al., 2017). However, we noticed that TMAO didn't promote the growth for most of the isolates from the two genera in our study. As was pointed out by E. L. Barrett and H. S. Kwan, certain species of Pseudomonas have been reclassified into Alteromonas when the G+C content was taken into consideration (Barrett and Kwan, 1985). Therefore, a careful examination of the early reports and experimental confirmation might be necessary before a conclusion could be made for these two genera. With regarding to Bacillus, Brevundimonas, Halomonas, Marinobacter, Muricauda, Novosphingobium, Rheinheimera, Sphingobium and Stenotrophomonas, the number of strains analyzed in this study is still inadequate for a conclusion.
Among the strains capable of TMAO utilization, 15 strains affiliated to 8 species of 5 genera are closely related to pathogen or symbiotic bacteria. The Brevundimonas vesicularis, Pseudomonas stutzeri, Vibrio furnissii, V. fluvialis, V. alginolyticus and V. campbellii are reported as pathogen or opportunistic pathogen (Lee et al., 1981; Brenner et al., 1983; Wang et al., 2007; Yoo et al., 2012; Bashar et al., 2017; Dong et al., 2017). While Halomonas meridian and Photobacterium leiognathi are commonly found as symbiotic bacteria (Courtenay et al., 2000; Meyer et al., 2015). TMAO could be generated by oxygenation of TMA in human, or synthesized endogenously in diverse marine animals (Ufnal et al., 2015). It's plausible that comparing to the free-living bacteria inhabiting oceanic environment, pathogenic and symbiotic bacteria are more likely to encounter with higher concentration TMAO (Proctor and Gunsalus, 2000; Lee et al., 2012).
Apart from one of the most important osmolytes which counteract the adverse effect of low temperature and urea, TMAO is also known as piezolyte. High concentration of TMAO has been observed in multiple deep-sea animals and is speculated to prevent the effect of HHP on protein structures and enzyme function by protecting the protein interior from invasion of water molecules (Gillett et al., 1997; Saad-Nehme, et al., 2001; Yancey et al., 2001, 2014; Petrov et al., 2012). Accumulation of TMAO has been reported in diverse deep-sea animals but not microorganisms dwelling in the deep-sea environment. Our recent study demonstrated that TMAO anaerobic respiration is favorable for bacterial acclimation to HHP conditions. Both expression of TMAO reductase and kinetics of TMAO reduction are increased under HHP condition, which eventually result in improved pressure tolerance in deep-sea bacteria V. fluvialis QY27 when TMAO is presented (Yin et al., 2018). Pressure inducible TMAO reductase has been reported in several deep-sea bacteria (Vezzi et al., 2005; Le Bihan et al., 2013; Zhang et al., 2014, 2016), it is plausible to deduce that TMAO improved pressure tolerance may be present in other strains as well. However, none of the 32 strains analyzed in this study exhibited improved pressure tolerance at 30 MPa when supplemented with TMAO, indicating the remarkable phenomenon might be a species-specific feature of Vibrio fluvialis.
5 CONCLUSIONIn this study, 237 bacterial strains affiliated to 50 species, 23 genera of Proteobacteria, Bacteroidetes, Firmicutes and Actinobacteria were isolated from deep-sea environment. We observed no strict correlation between the depth where the bacterium inhabit and its pressure tolerance under ambient temperature conditions. Strains from the genera of Alteromonas, Halomonas, Marinobacter, Photobacterium and Vibrio showed capacity of TMAO utilization, but none of strains from Acinebacter, Bacillus, Brevundimonas, Muricauda, Novosphingobium, Rheinheimera, Sphingobium and Stenotrophomonas did, indicating the utilization of TMAO is a species-specific feature. Taken together, the results described for the first time the TMAO utilization in deep-sea bacterial strains, and expanded our understanding of the physiological characteristic of marine bacteria.
6 DATA AVAILABILITY STATEMENTThe 16S rRNA sequences of this study have been deposited in GenBank with the accession codes KP676698-676706, KU321307-321339 and MF554596-554628.
7 ACKNOWLEDGEMENTWe thank Dr. HE Lisheng for sharing the South China Sea samples, Prof. Zhang Xiaohua for Vibrio fluvialis type strain ATCC33809.
Barrett E L, Kwan H S. 1985. Bacterial reduction of trimethylamine oxide. Annual Review of Microbiology, 39: 131-149.
DOI:10.1146/annurev.mi.39.100185.001023 |
Bashar S, Sanyal S K, Sultana M, Hossain M A. 2017. Emergence of IntI1 associated blaVIM-2 gene cassettemediated carbapenem resistance in opportunistic pathogen Pseudomonas stutzeri. Emerging Microbes & Infections, 6(5): e29.
|
Brenner D J, Hickman-Brenner F W, Lee J V, Steigerwalt A G, Fanning G R, Hollis D G, Farmer Ⅲ J J, Weaver R E, Joseph S W, Seidler R J. 1983. Vibrio furnissii (formerly aerogenic biogroup of vibrio fluvialis), a new species isolated from human feces and the environment. Journal of Clinical Microbiology, 18(4): 816-824.
|
Courtenay E S, Capp M W, Anderson C F, Record M T. 2000. Vapor pressure osmometry studies of osmolyte-protein interactions:implications for the action of osmoprotectants in vivo and for the interpretation of "osmotic stress" experiments in vitro. Biochemistry, 39(15): 4455-4471.
DOI:10.1021/bi992887l |
DeLong E F, Preston C M, Mincer T, Rich V, Hallam S J, Frigaard N U, Martinez A, Sullivan M B, Edwards R, Brito B R, Chisholm S W, Karl D M. 2006. Community genomics among stratified microbial assemblages in the ocean's interior. Science, 311(5760): 496-503.
DOI:10.1126/science.1120250 |
Dong X, Wang H L, Zou P Z, Chen J Y, Liu Z, Wang X P, Huang J. 2017. Complete genome sequence of Vibrio campbellii strain 20130629003S01 isolated from shrimp with acute hepatopancreatic necrosis disease. Gut Pathogens, 9: 31.
DOI:10.1186/s13099-017-0180-2 |
Dos Santos J P, Iobbi-Nivol C, Couillault C, Giordano G, Méjean V. 1998. Molecular analysis of the trimethylamine N-oxide (TMAO) reductase respiratory system from a Shewanella species. Journal of Molecular Biology, 284(2): 421-433.
DOI:10.1006/jmbi.1998.2155 |
Dunn A K, Stabb E V. 2008. Genetic analysis of trimethylamine N-oxide reductases in the light organ symbiont Vibrio fischeri ES114. Journal of Bacteriology, 190(17): 5814-5823.
DOI:10.1128/JB.00227-08 |
Fang J S, Zhang L, Bazylinski D A. 2010. Deep-sea piezosphere and piezophiles:geomicrobiology and biogeochemistry. Trends in Microbiology, 18(9): 413-422.
DOI:10.1016/j.tim.2010.06.006 |
Ge X L, Wexler A S, Clegg S L. 2011. Atmospheric amines-part Ⅰ. A review. Atmospheric Environment, 45(3): 524-546.
DOI:10.1016/j.atmosenv.2010.10.012 |
Ge Y, Zhu J, Ye X, Yang Y. 2017. Spoilage potential characterization of Shewanella and Pseudomonas isolated from spoiled large yellow croaker (Pseudosciaena crocea). Letters in Applied Microbiology, 64(1): 86-93.
DOI:10.1111/lam.12687 |
Gibb S W, Hatton A D. 2004. The occurrence and distribution of trimethylamine-N-oxide in Antarctic coastal waters. Marine Chemistry, 91(1-4): 65-75.
DOI:10.1016/j.marchem.2004.04.005 |
Gillett M B, Suko J R, Santoso F O, Yancey P H. 1997. Elevated levels of trimethylamine oxide in muscles of deep-sea gadiform teleosts:a high-pressure adaptation?. Journal of Experimental Zoology, 279(4): 386-391.
DOI:10.1002/(ISSN)1097-010X |
He H L, Chen X L, Zhang X Y, Sun C Y, Zou B C, Zhang Y Z. 2009. N-ovel use for the osmolyte trimethylamine Noxide:retaining the psychrophilic characters of coldadapted protease deseasin MCP-01 and simultaneously improving its thermostability. Marine Biotechnology, 11(6): 710-716.
DOI:10.1007/s10126-009-9185-2 |
Jannasch H W, Jones G E. 1959. Bacterial populations in sea water as determined by different methods of enumeration. Limnology and Oceanography, 4(2): 128-139.
DOI:10.4319/lo.1959.4.2.0128 |
Ji B Y, Gimenez G, Barbe V, Vacherie B, Rouy Z, Amrani A, Fardeau M L, Bertin P, Alazard D, Leroy S, Talla E, Ollivier B, Dolla A, Pradel N. 2013. Complete genome sequence of the piezophilic, mesophilic, sulfate-reducing bacterium Desulfovibrio hydrothermalis AM13T. Genome Announcements, 1(1): e00226-12.
|
Jian H H, Li S K, Tang X X, Xiao X. 2016. A transcriptome resource for the deep-sea bacterium Shewanella piezotolerans WP3 under cold and high hydrostatic pressure shock stress. Marine Genomics, 30: 87-91.
DOI:10.1016/j.margen.2016.09.004 |
Kato C, Nogi Y, Arakaw S. 2008. Isolation, cultivation, and diversity of deep-sea piezophiles. In: Michiels C, Bartlett D, Aersten A eds. High-pressure Microbiology. ASM Press, Washington, DC. p.203-217.
|
Lauro F M, Bartlett D H. 2008. Prokaryotic lifestyles in deep sea habitats. Extremophiles, 12(1): 15-25.
DOI:10.1007/s00792-006-0059-5 |
Le Bihan T, Rayner J, Roy M M, Spagnolo L. 2013. Photobacterium profundum under pressure:a MS-based label-free quantitative proteomics study. PLoS One, 8(5): e60897.
DOI:10.1371/journal.pone.0060897 |
Lee J V, Shread P, Furniss A L, Bryant T N. 1981. Taxonomy and description of Vibrio fluvialis sp. nov. (synonym group F vibrios, group EF6). Journal of Applied Bacteriology, 50(1): 73-94.
DOI:10.1111/jam.1981.50.issue-1 |
Lee K M, Park Y, Bari W, Yoon M Y, Go J, Kim S C, Lee H I, Yoon S S. 2012. Activation of cholera toxin production by anaerobic respiration of trimethylamine N-oxide in Vibrio cholerae. Journal of Biological Chemistry, 287(47): 39742-39752.
DOI:10.1074/jbc.M112.394932 |
Lidbury I D, Murrell J C, Chen Y. 2015. Trimethylamine and trimethylamine N-oxide are supplementary energy sources for a marine heterotrophic bacterium:implications for marine carbon and nitrogen cycling. The ISME Journal, 9(3): 760-769.
DOI:10.1038/ismej.2014.149 |
Lidbury I, Murrell J C, Chen Y. 2014. Trimethylamine N-oxide metabolism by abundant marine heterotrophic bacteria. Proceedings of the National Academy of Sciences of the United States of America, 111(7): 2710-2715.
DOI:10.1073/pnas.1317834111 |
Lucas S, Han J, Lapidus A, Cheng J F, Goodwin L A, Pitluck S, Peters L, Mikhailova N, Teshima H, Detter J C, Han C, Tapia R, Land M, Hauser L, Kyrpides N C, Ivanova N, Pagani I, Vannier P, Oger P, Bartlett D H, Noll K M, Woyke T, Jebbar M. 2012. Complete Genome Sequence of the Thermophilic, Piezophilic, Heterotrophic Bacterium Marinitoga piezophila KA3. Journal of Bacteriology, 194(21): 5974-5975.
|
Martini S, Al Ali B, Garel M, Nerini D, Grossi V, Pacton M, Casalot L, Cuny P, Tamburini C. 2013. Effects of hydrostatic pressure on growth and luminescence of a moderately-piezophilic luminous bacteria Photobacterium phosphoreum ANT-2200. PLoS One, 8(6): e66580.
DOI:10.1371/journal.pone.0066580 |
McCrindle S L, Kappler U, McEwan A G. 2005. Microbial dimethylsulfoxide and trimethylamine-N-oxide respiration. Advances in Microbial Physiology, 50: 147-198.
DOI:10.1016/S0065-2911(05)50004-3 |
Meyer J L, Dillard B A, Rodgers J M, Ritchie K B, Paul V J, Teplitski M. 2015. Draft genome sequence of Halomonas meridiana R1t3 isolated from the surface microbiota of the Caribbean Elkhorn coral Acropora palmata. Standards in Genomic Sciences, 10(1): 75.
DOI:10.1186/s40793-015-0069-y |
Nunoura T, Takaki Y, Hirai M, Shimamura S, Makabe A, Koide O, Kikuchi T, Miyazaki J, Koba K, Yoshida N, Sunamura M, Takai K. 2015. Hadal biosphere:insight into the microbial ecosystem in the deepest ocean on Earth. Proceedings of the National Academy of Sciences of the United States of America, 112(11): E1230-E1236.
DOI:10.1073/pnas.1421816112 |
Ohke Y, Sakoda A, Kato C, Sambongi Y, Kawamoto J, Kurihara T, Tamegai H. 2013. Regulation of cytochrome c- and quinol oxidases, and piezotolerance of their activities in the deep-sea piezophile Shewanella violacea DSS12 in response to growth conditions. Bioscience, Biotechnology, and Biochemistry, 77(7): 1522-1528.
DOI:10.1271/bbb.130197 |
Parkes R J, Sellek G, Webster G, Martin D, Anders E, Weightman A J, Sass H. 2009. Culturable prokaryotic diversity of deep, gas hydrate sediments:first use of a continuous high-pressure, anaerobic, enrichment and isolation system for subseafloor sediments (DeepIsoBUG). Environmental microbiology, 11(12): 3140-3153.
DOI:10.1111/emi.2009.11.issue-12 |
Pathom-Aree W, Nogi Y, Sutcliffe I C, Ward A C, Horikoshi K, Bull A T, Goodfellow M. 2006. Dermacoccus abyssi sp.nov., a piezotolerant actinomycete isolated from the Mariana Trench. International Journal of Systematic and Evolutionary Microbiology, 56(6): 1233-1237.
DOI:10.1099/ijs.0.64133-0 |
Petrov E, Rohde P R, Cornell B, Martinac B. 2012. The protective effect of osmoprotectant TMAO on bacterial mechanosensitive channels of small conductance MscS/MscK under high hydrostatic pressure. Channels (Austin), 6(4): 262-271.
DOI:10.4161/chan.20833 |
Proctor L M, Gunsalus R P. 2000. Anaerobic respiratory growth of Vibrio harveyi, Vibrio fischeri and Photobacterium leiognathi with trimethylamine N-oxide, nitrate and fumarate:ecological implications. Environmental Microbiology, 2(4): 399-406.
DOI:10.1046/j.1462-2920.2000.00121.x |
Ringø E, Stenberg E, Strøm A R. 1984. Amino acid and lactate catabolism in trimethylamine oxide respiration of alteromonas putrefaciens NCMB-1735. Applied and Environmental Microbiology, 47(5): 1084-1089.
|
Saad-Nehme J, Silva J L, Meyer-Fernandes J R. 2001. Osmolytes protect mitochondrial F0F1-ATPase complex against pressure inactivation. Biochimica et Biophysica Acta (BBA)-Protein Structure and Molecular Enzymology, 1546(1): 164-170.
DOI:10.1016/S0167-4838(01)00137-6 |
Salazar G, Cornejo-Castillo F M, Benítez-Barrios V, FraileNuez E, Álvarez-Salgado X A, Duarte C M, Gasol J M, Acinas S G. 2016. Global diversity and biogeography of deep-sea pelagic prokaryotes. The ISME Journal, 10(3): 596-608.
DOI:10.1038/ismej.2015.137 |
Seibel B A, Walsh P J. 2002. Trimethylamine oxide accumulation in marine animals:relationship to acylglycerol storage. The Journal of Experimental Biology, 205(Pt 3): 297-306.
|
Simon-Colin C, Raguénès G, Cozien J, Guezennec J G. 2008. Halomonas profundus sp. nov., a new PHA-producing bacterium isolated from a deep-sea hydrothermal vent shrimp. Journal of Applied Microbiology, 104(5): 1425-1432.
DOI:10.1111/j.1365-2672.2007.03667.x |
Smith R S, Pineiro S A, Singh R, Romberg E, Labib M E, Williams H N. 2004. Discrepancies in bacterial recovery from dental unit water samples on R2A medium and a commercial sampling device. Current Microbiology, 48(4): 243-246.
DOI:10.1007/s00284-003-4130-5 |
Sogin M L, Morrison H G, Huber J A, Mark Welch D, Huse S M, Neal P R, Arrieta J M, Herndl G J. 2006. Microbial diversity in the deep sea and the underexplored "rare biosphere". Proceedings of the National Academy of Sciences of the United States of America, 103(32): 12115-12120.
DOI:10.1073/pnas.0605127103 |
Takai K, Miyazaki M, Hirayama H, Nakagawa S, Querellou J, Godfroy A. 2009. Isolation and physiological characterization of two novel, piezophilic, thermophilic chemolithoautotrophs from a deep-sea hydrothermal vent chimney. Environmental Microbiology, 11(8): 1983-1997.
DOI:10.1111/emi.2009.11.issue-8 |
Tamburini C, Boutrif M, Garel M, Colwell R R, Deming J W. 2013. Prokaryotic responses to hydrostatic pressure in the ocean-a review. Environmental Microbiology, 15(5): 1262-1274.
DOI:10.1111/emi.2013.15.issue-5 |
Tamegai H, Nishikawa S, Haga M, Bartlett D H. 2012. The respiratory system of the piezophile Photobacterium profundum SS9 grown under various pressures. Bioscience, Biotechnology, and Biochemistry, 76(8): 1506-1510.
DOI:10.1271/bbb.120237 |
Tarn J, Peoples L M, Hardy K, Cameron J, Bartlett D H. 2016. Identification of free-living and particle-associated microbial communities present in hadal regions of the mariana trench. Frontiers in Microbiology, 7: 665.
|
Tindall B J, Rosselló-Móra R, Busse H J, Ludwig W, Kämpfer P. 2010. Notes on the characterization of prokaryote strains for taxonomic purposes. International Journal of Systematic and Evolutionary Microbiology, 60(1): 249-266.
DOI:10.1099/ijs.0.016949-0 |
Ufnal M, Zadlo A, Ostaszewski R. 2015. TMAO:a small molecule of great expectations. Nutrition, 31(11-12): 1317-1323.
DOI:10.1016/j.nut.2015.05.006 |
Vezzi A, Campanaro S, D'Angelo M, Simonato F, Vitulo N, Lauro F M, Cestaro A, Malacrida G, Simionati B, Cannata N, Romualdi C, Bartlett D H, Valle G. 2005. Life at depth:Photobacterium profundum genome sequence and expression analysis. Science, 307(5714): 1459-1461.
DOI:10.1126/science.1103341 |
Wang Q Y, Liu Q, Ma Y, Zhou L Y, Zhang Y X. 2007. Isolation, sequencing and characterization of cluster genes involved in the biosynthesis and utilization of the siderophore of marine fish pathogen Vibrio alginolyticus. Archives of Microbiology, 188(4): 433-439.
DOI:10.1007/s00203-007-0261-6 |
Xiong L, Jian H H, Zhang Y X, Xiao X. 2016. The two sets of DMSO respiratory systems of Shewanella piezotolerans WP3 are involved in deep sea environmental adaptation. Frontiers in Microbiology, 7: 1418.
|
Yancey P H, Clark M E, Hand S C, Bowlus R D, Somero G N. 1982. Living with water stress:evolution of osmolyte systems. Science, 217(4566): 1214-1222.
DOI:10.1126/science.7112124 |
Yancey P H, Fyfe-Johnson A L, Kelly R H, Walker V P, Aunon M T. 2001. Trimethylamine oxide counteracts effects of hydrostatic pressure on proteins of deep-sea teleosts. Journal of Experimental Zoology, 289(3): 172-176.
DOI:10.1002/(ISSN)1097-010X |
Yancey P H, Gerringer M E, Drazen J C, Rowden A A, Jamieson A. 2014. Marine fish may be biochemically constrained from inhabiting the deepest ocean depths. Proceedings of the National Academy of Sciences of the United States of America, 111(12): 4461-4465.
DOI:10.1073/pnas.1322003111 |
Yin Q J, Zhang W J, Qi X Q, Zhang S D, Jiang T, Li X G, Chen Y, Santini C L, Zhou H, Chou I M, Wu L F. 2018. High hydrostatic pressure inducible trimethylamine N-oxide reductase improves the pressure tolerance of piezosensitive bacteria Vibrio fluvialis. Frontiers in Microbiology, 8: 2646.
DOI:10.3389/fmicb.2017.02646 |
Yoo S H, Kim M J, Roh K H, Kim S H, Park D W, Sohn J W, Yoon Y K. 2012. Liver abscess caused by Brevundimonas vesicularis in an immunocompetent patient. Journal of Medical Microbiology, 61(10): 1476-1479.
|
Yoon S H, Ha S M, Kwon S, Lim J, Kim Y, Seo H, Chun J. 2017. Introducing EzBioCloud:a taxonomically united database of 16S rRNA gene sequences and whole-genome assemblies. International Journal of Systematic and Evolutionary Microbiology, 67(5): 1613-1617.
DOI:10.1099/ijsem.0.001755 |
Zhang S D, Barbe V, Garel M, Zhang W J, Chen H T, Santini C L, Murat D, Jing H M, Zhao Y, Lajus A, Martini S, Pradel N, Tamburini C, Wu L F. 2014. Genome Sequence of Luminous Piezophile Photobacterium phosphoreum ANT-2200. Genome Announcement, 2(2): e00096-14.
|
Zhang S D, Santini C L, Zhang W J, Barbe V, Mangenot S, Guyomar C, Garel M, Chen H T, Li X G, Yin Q J, Zhao Y, Armengaud J, Gaillard J C, Martini S, Pradel N, Vidaud C, Alberto F, Médigue C, Tamburini C, Wu L F. 2016. Genomic and physiological analysis reveals versatile metabolic capacity of deep-sea Photobacterium phosphoreum ANT-2200. Extremophiles, 20(3): 301-310.
DOI:10.1007/s00792-016-0822-1 |
Zhu Y J, Jameson E, Parslow R A, Lidbury I, Fu T T, Dafforn T R, Schäfer H, Chen Y. 2014. Identification and characterization of trimethylamine N-oxide (TMAO)demethylase and TMAO permease in Methylocella silvestris BL2. Environmental Microbiology, 16(10): 3318-3330.
DOI:10.1111/emi.2014.16.issue-10 |
Zou Q, Bennion B J, Daggett V, Murphy K P. 2002. The molecular mechanism of stabilization of proteins by TMAO and its ability to counteract the effects of urea. Journal of the American Chemical Society, 124(7): 1192-1202.
DOI:10.1021/ja004206b |
 2019, Vol. 37
2019, Vol. 37