Institute of Oceanology, Chinese Academy of Sciences
Article Information
- SHI Ce, GAO Xiaolong, LIU Ying, WANG Chunlin
- Long-term monitoring of the individual self-feeding behavior of rainbow trout Oncorhynchus mykiss
- Journal of Oceanology and Limnology, 37(1): 344-349
- http://dx.doi.org/10.1007/s00343-019-8020-5
Article History
- Received Feb. 1, 2018
- accepted in principle Mar. 16, 2018
- accepted for publication Mar. 27, 2018
2 Collaborative Innovation Center for Zhejiang Marine High-efficiency and Healthy Aquaculture, Ningbo 315211, China;
3 Institute of Oceanology, Chinese Academy of Sciences, Qingdao 266071, China;
4 College of Marine Technology and Environment, Dalian Ocean University, Dalian 116023, China
The feeding strategy directly affects the growth performance and welfare of the farmed fish. Inadequate feeding often leads to lower growth performance, higher coefficient of variation in growth and aggressiveness (Bureau et al., 2006), while overfeeding elevates the feed conversion ratio and environmental pollution, therefore it is quite important to choose the appropriate feeding time and proportions. However, the feeding rhythm of the fish is not only ruled by internal factor like molecular clock, but also by environment and social interaction, which makes it difficult to optimize the feeding strategy. Self-feeding employs the learning ability of the fish, allowing fish to feed according to their appetite. Fish can bite, push, pull, (Covès et al., 2006) or use external tag on dorsal (Millot et al., 2014) to activate the trigger of the selffeeding device. The device has been shown to improve the growth and welfare, reduce waste and is therefore a suitable feeding tool for commercial fish farms (Attia et al., 2012).
Indeed, most studies report the existence of an unequal distribution of trigger actuation in groups of farmed fish, including European sea bass (Covès et al., 2006), rainbow trout (Heydarnejad and Purser, 2013) and barfin flounder (Sunuma et al., 2009). Some high trigger sea bass accounts for more than 90% trigger actuation (Covès et al., 2006). The passive integrated tag (PIT) antenna had been used to monitor the self-feeding behavior of sea bass, however, for salmonids the individual self-feeding behavior has seldom been continuously observed (Heydarnejad and Purser, 2013). In contrast to sea bass, the dominance-subordination relationship is strong in salmonid. Significant correlation between self-feeding activities and growth is observed in some studies of trout (Alanärä and Brännäs, 1996) but not in others (Chen et al., 2002).
In this study we observed the individual selffeeding activity with PIT antenna to explore the relationship between group and individual selffeeding behavior and growth performance. The data can potentially help further understand the selffeeding behavior of salmoinds.
2 MATERIAL AND METHODRainbow trout (180.07±25.33 g) used in this experiment were obtained from a commercial farm in Yantai, China. The experiment started in November 5th, 2015 and last 50 d. Prior to the experiment, the fish were reared in the experimental tanks for 15 d to acclimate to the environments and learn to use the self-feeder. The fish were new to the self-feeder, the self-feeding behavior were observed in all the tanks in 48h and become comparatively stable in less than two weeks. When the experiment started, the fish were anesthetized with MS-222 (100 mg/L), individually weighted, and tagged with 12 mm conventional PIT tag (Remex®) horizontally behind the skull. Damage to the skin was minimal and no significant effect of tagging on behavior or growth was observed. Fish were held in a recirculating aquaculture system consisted of three 600-L rearing tanks (10 fish in each tank) with self-feeders, whirl separator for solids removal, biofilter, foam separator and a UV sterilizer. A photoperiod of 12 h light and 12 h dark with lights provided by LED (150 lx) turned on at 6:00 was applied. The water temperature was maintained at 16±0.5℃.
The device to operate the feeder comprised a screened type sensor (a black bead with string in a PVC cylinder coupled with the PIT tag detection antenna linked to a computer (Covès et al., 2006) (Fig. 1). The fish that activated the trigger were record, and were categorized according to consistency of the triggering activity: high trigger fish (≥25% of total actuations) (HT), low trigger fish (1%–25%) (LT), and zero-trigger fish (< 1%) (ZT). With each actuation the group were rewarded with 40 pellets (1.7 g/kg fish per trigger actuation). The reward level was a compromise between minimizing wastage, and a normal food intake, for it was found low daily ration did not meet the maximum feed intake in self-feeding fish.
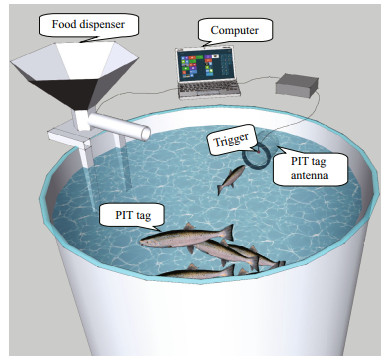
|
| Fig.1 Schematic plot of the self-feeding system |
At the end of experimentation, the fish were anesthetized in MS-222 (200 mg/L) to death and weighed. Specific growth rate (SGR), coefficient of variation (CV) ration level (RL) and feed conversion ratio (FCR) were calculated as below:
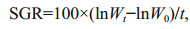
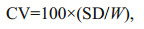


where Wt and W0 represent final and initial wet body weight (g), respectively; t is the experimental duration (days); C represents the feed intake (g); SD represent the standard deviation in body weight; W represents the mean weight of fish (g).
The data were analyzed by a SPSS for Windows (Version 17.0) statistical package. The SGR of HT, LT and ZT fish were subjected to a General Linear Model (GLM), with the fish tank set as a random factor, to test the existence of the tank effect. Then the parameters were compared with One-way analysis of variance (One-way ANOVA), followed by Tukey multiple comparison post hoc test. Spearman correlation was used to measure the relationship between initial body weight and the trigger percentage. Circadian rhythms of the feeding behavior was tested with Cosinor analysis by Acrophase software with fitted cosine wave on 95% confidence level (version 3.5, http://www.circadian.org/main.html) (Refinetti et al., 2007).
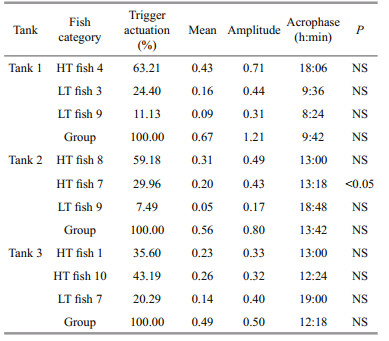
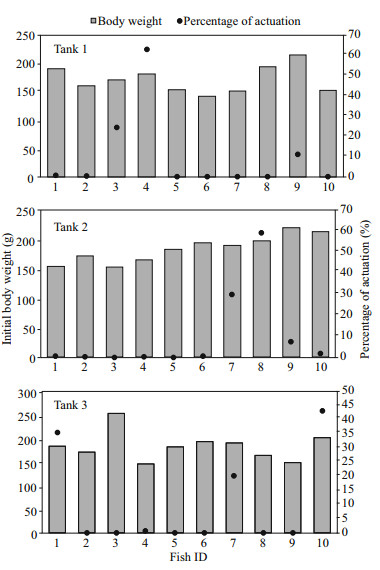
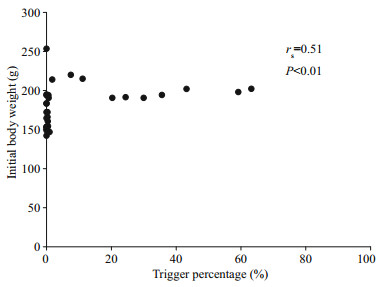
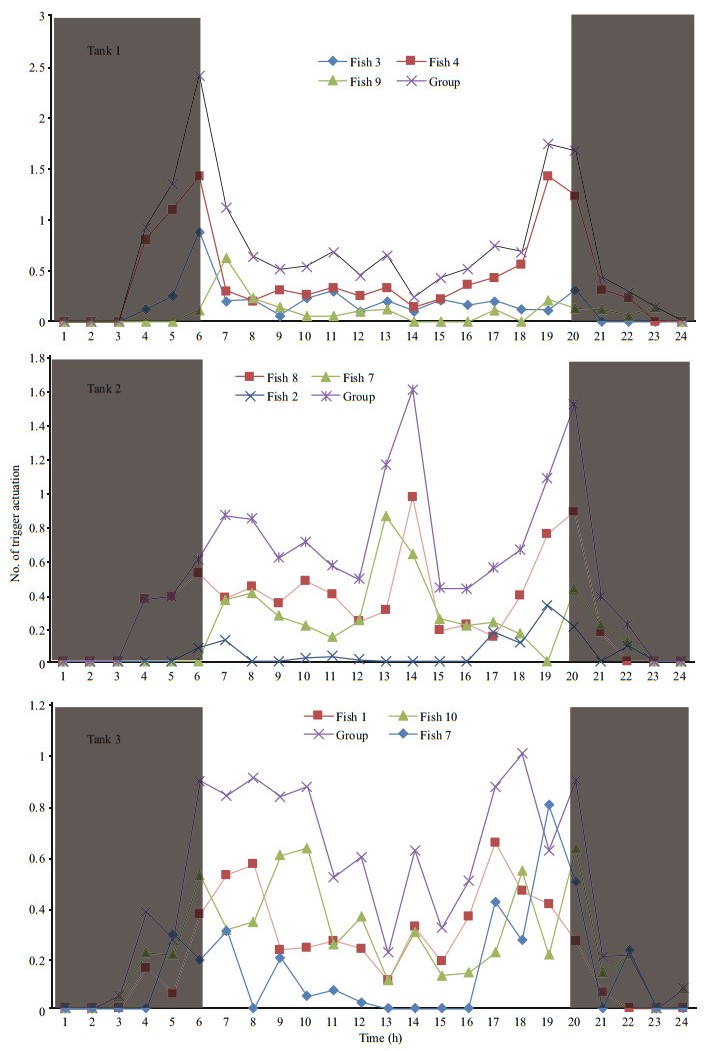
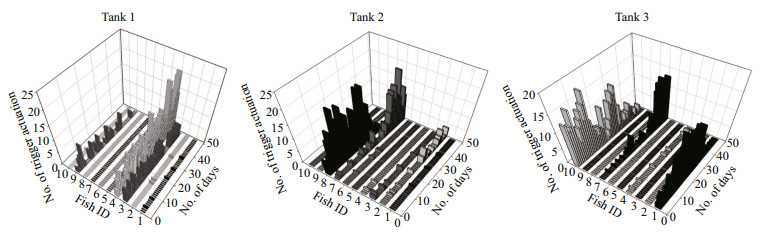
3 RESULTAt the end of the experiment the SGR of the fish, the HT and LT fish had a higher SGR ((1.5±0.17)% and (1.45±0.23)%/d, respectively) than ZT fish ((1.25±0.57)%/d), but the difference did not reach a significant level. The food wastage is less than 1% (Table 1). The HT fish is comparatively of high initial weight, there seems a threshold initial weight around approx. 200 g for triggering fish (Fig. 2). Spearman correlation showed that the individual self-feeding activity significantly correlated with initial body weight (rs=0.51, P < 0.01) (Fig. 3). Nevertheless, there is no significant difference in SGR between the HT, LT and ZT fish. The feeding rhythm of different tank varied, the feeding peak of Tank 1 occurred in dawn and dusk, however, the feeding peak of Tank 2 occurred in noon and dusk, while the Tank 3 showed multiple feeding peak during the day (Fig. 4). During the experiment the trigger actuation of HT fish range from 29.96%–63.21% (Table 2). In tank 2 and tank 3 where there are 2 HT fish, the two HT fish has similar acrophase, however, only the only the HT fish 7 in Tank 2 showed significant circadian rhythm (Cosinor; P < 0.05) (Table 2). The HT fish can be active for as long as 50 d (Tank 1), but alternation of HT fish was also observed around 40 d in Tanks 2 and 3 (Fig. 5).

|
|
| Fig.2 The relationship of initial body weight and the trigger actuation of test fish |
|
| Fig.3 The correlation analysis of trigger percentage and initial body weight |
|
| Fig.4 The group and individual circadian self-feeding rhythm of the test animals The grey part represents dark phase when light was off. |
|
| Fig.5 The daily individual self-feeding activities |
Though some studies found relatively few fish within a group account for the most of the trigger actuations, however, the characteristic of HT fish has seldom been confirmed. The high aggressiveness of HT rainbow trout was found in some studies (Heydarnejad and Purser, 2013) but not in others (Chen et al., 2002). For sea bass some studies indicate the LT and ZT individual has bolder personality (Ferrari et al., 2014) compared with HT fish, but other studies found they were suppress by social stress (Di-Poï et al., 2007). Interestingly, in present study we found that the heavier individuals were more prone to become the triggering fish (Fig. 2), and the initial body weight of the fish was significantly correlated with trigger activities (P < 0.01) (Fig. 3). In an established hierarchy the high social rank individual gain weight independent of feeding regime (Cubitt et al., 2008). Consequently, the findings partly support that the dominance hierarchies might be the main reason of different trigger activities (Jobling, 1995). Nevertheless, the fact that no significant difference in SGR between the three triggering categories, leads to the hypothesis that ⅰ) the HT fish did not gain significant advantage from their high trigger status when food resource is abundant and, ⅱ) fish can employ different strategies to achieve the same end (Chen et al., 2002; Millot et al., 2008). In contrast to (Alanärä and Brännäs, 1996), we didn't find any HT fish defending the trigger, which may be partly due to the high reward level decrease the benefit of holding a territory according to economic dependability theory (Brown, 1964).
The rainbow trout were diurnal feeders, but variability among the individuals was also evident (Fig. 4). The HT fish seemed to synchronize the feed demand acts of the low-triggering fish, thereby directing the group feeding activity pattern (Fig. 4), which was also found in sea bass (Millot and Bégout, 2009). Cosinor analysis also showed that the acrophase of the two HT fish in the same tank (Tanks 2 and 3) were quite close to each other (Table 2), indicating the possible existence of instrumental conditioning, i.e. the activation of the trigger by subordinate fish is probably based on a behavioral response as the observation of other fish feeding (Heydarnejad and Purser, 2013). In experimental period, spontaneous alternation of fish of the high trigger status was observed (Tanks 2 and 3) (Fig. 5). When a HT fish ceased triggering activity, the LT fish increased its demanding feeding behavior, as a result the group still showed normal feed intake. It seems that the high trigger function rather than the HT individual itself, is essential for the group to remain stable. Pervious study showed that in a group of 10 rainbow trout, when the HT fish is removed artificially, subordinate rainbow trout stopped demand feeding (n=10) (Heydarnejad and Purser, 2013), as well as the whole group. Nevertheless, in a larger group of sea bass (n=50) new HT fish would reappear after the former HT fish is removed (Di-Poi et al., 2008). The reason for the alternation of high trigger status in this study is still unclear, however, these results partly indicate that ⅰ) the group size is important in determining the self-feeding plasticity of the group, and ⅱ) artificial interference is a stronger influencing factor than internal variation of fish population. The physiological characteristic of the fish in different feeding category, which is important in understand the structure of fish population surrounding the selffeeding system, needs to be further studied.
5 CONCLULISONThe present study showed in a small group of rainbow trout, 1–2 fish accounts for most of the feeding behavior. The heavier individuals were more prone to become the triggering fish and the initial body weight of the fish was significantly correlated with trigger activities. The HT individuals released more food than they can consume but did not gain extra growth performance. The high trigger status of HT fish could be a synthetic effect of the individual feeding behavior and the group food demand. In the self-feeding system, the critical individual responsible for the group feeding should be closely monitored.
6 AVAILABILITIES OF SUPPORTING DATAThe data that support the findings of this study are available from the corresponding author upon reasonable request.
7 ACKNOWLEDGEMENTWe are grateful to the anonymous reviewers for their professional revision of the manuscript.
Alanärä A, Brännäs E. 1996. Dominance in demand-feeding behaviour in Arctic charr and rainbow trout:the effect of stocking density. Journal of Fish Biology, 48(2): 242-254.
DOI:10.1111/jfb.1996.48.issue-2 |
Attia J, Millot S, Di-Poï C, Bégout M L, Noble C, SanchezVazquez F J, Terova G, Saroglia M, Damsgård B. 2012. Demand feeding and welfare in farmed fish. Fish Physiology and Biochemistry, 38(1): 107-118.
|
Brown J L. 1964. The evolution of diversity in avian territorial systems. The Wilson Bulletin, 76(2): 160-169.
|
Bureau D P, Hua K, Cho C Y. 2006. Effect of feeding level on growth and nutrient deposition in rainbow trout(Oncorhynchus mykiss Walbaum) growing from 150 to 600. Aquaculture Research, 37(11): 1090-1098.
DOI:10.1111/are.2006.37.issue-11 |
Chen W M, Naruse M, Tabata M. 2002. Circadian rhythms and individual variability of self-feeding activity in groups of rainbow trout Oncorhynchus mykiss (Walbaum). Aquaculture Research, 33(7): 491-500.
DOI:10.1046/j.1365-2109.2002.00734.x |
Covès D, Beauchaud M, Attia J, Dutto G, Bouchut C, Bégout M L. 2006. Long-term monitoring of individual fish triggering activity on a self-feeding system:an example using European sea bass (Dicentrarchus labrax). Aquaculture, 253(1-4): 385-392.
DOI:10.1016/j.aquaculture.2005.08.015 |
Cubitt K F, Winberg S, Huntingford F A, Kadri S, Crampton V O, Øverli Ø. 2008. Social hierarchies, growth and brain serotonin metabolism in Atlantic salmon (Salmo salar) kept under commercial rearing conditions. Physiology & Behavior, 94(4): 529-535.
|
Di-Poï C, Attia J, Bouchut C, Dutto G, Covès D, Beauchaud M. 2007. Behavioral and neurophysiological responses of European sea bass groups reared under food constraint. Physiology & Behavior, 90(4): 559-566.
|
Di-Poi C, Beauchaud M, Bouchut C, Dutto G, Covès D, Attia J. 2008. Effects of high food-demand fish removal in groups of juvenile sea bass (Dicentrarchus labrax). Canadian Journal of Zoology, 86(9): 1015-1023.
DOI:10.1139/Z08-077 |
Ferrari S, Benhaïm D, Colchen T, Chatain B, Bégout M L. 2014. First links between self-feeding behaviour and personality traits in European seabass, Dicentrarchus labrax. Applied Animal Behaviour Science, 161: 131-141.
DOI:10.1016/j.applanim.2014.09.019 |
Heydarnejad M S, Purser J. 2013. Demand-feeding activity of rainbow trout (Oncorhynchus mykiss) in Raceways. World Journal of Zoology, 8(1): 36-46.
|
Jobling M. 1995. Simple indices for the assessment of the influences of social environment on growth performance, exemplified by studies on Arctic charr. Aquaculture International, 3(1): 60-65.
|
Millot S, Bégout M L, Person-Le Ruyet J, Breuil G, Di-Poï C, Fievet J, Pineau P, Roué M, Sévère A. 2008. Feed demand behavior in sea bass juveniles:Effects on individual specific growth rate variation and health (inter-individual and inter-group variation). Aquaculture, 274(1): 87-95.
|
Millot S, Bégout M L. 2009. Individual fish rhythm directs group feeding:a case study with sea bass juveniles(Dicentrarchus labrax) under self-demand feeding conditions. Aquatic Living Resources, 22(3): 363-370.
DOI:10.1051/alr/2009048 |
Millot S, Nilsson J, Fosseidengen J E, Bégout M L, Fernö A, Braithwaite V A, Kristiansen T S. 2014. Innovative behaviour in fish:Atlantic cod can learn to use an external tag to manipulate a self-feeder. Animal Cognition, 17(3): 779-785.
DOI:10.1007/s10071-013-0710-3 |
Refinetti R, Cornélissen G, Halberg F. 2007. Procedures for numerical analysis of circadian rhythms. Biological Rhythm Research, 38(4): 275-325.
DOI:10.1080/09291010600903692 |
Sunuma T, Amano M, Yamanome T, Yamamori K. 2009. Individual variability of self-feeding activity in groupreared barfin flounder. Fisheries Science, 75(5): 1295-1300.
DOI:10.1007/s12562-009-0143-8 |
 2019, Vol. 37
2019, Vol. 37