Institute of Oceanology, Chinese Academy of Sciences
Article Information
- SHANG Lixia, HU Zhangxi, TANG Yingzhong
- Identification of dissolved and particulate carbonyl compounds produced by marine harmful algal bloom species
- Journal of Oceanology and Limnology, 37(5): 1566-1581
- http://dx.doi.org/10.1007/s00343-019-8199-5
Article History
- Received Aug. 23, 2018
- accepted in principle Nov. 21, 2018
- accepted for publication Feb. 27, 2019
2 Laboratory for Marine Ecology and Environmental Science, Qingdao National Laboratory for Marine Science and Technology, Qingdao 266071, China;
3 Center for Ocean Mega-Science, Chinese Academy of Sciences, Qingdao 266071, China
Harmful algal blooms (HABs) have negative and sometimes disastrous impacts on marine ecosystems, wild fish stocks, aquaculture, and even human health (Landsberg, 2002; Okolodkov, 2005; Anderson et al., 2012; Pistocchi et al., 2012; Cusick and Sayler, 2013). Microalgae often produce phycotoxins to obtain a competitive advantage, which may accumulate in the marine food chain and eventually cause poisoning in humans (Landsberg, 2002; Twiner et al., 2008; Otero et al., 2011; Rasmussen et al., 2016). Algal toxins are reported to be responsible for between 50 000 and 500 000 human intoxications per year, with an overall global mortality rate of 1.5% (Cusick and Sayler, 2013). The chemical structures of some phycotoxins have been identified, such as the paralytic shellfish toxins (PST) produced by Alexandrium spp. (Taroncher-Oldenburg et al., 1997; Orlova et al., 2007), Pyrodinium bahamense (Usup et al., 1994), Gymnodinium catenatum (Hallegraeff et al., 2012) and Anabaena circinalis (Aráoz et al., 2010), diarrheic shellfish toxins (DST) produced by Dinophysis spp. (Fux et al., 2011) and Prorocentrum spp. (Gayoso et al., 2002), neurotoxic shellfish toxins (NST) produced by Karenia brevis (Mooney et al., 2007), and amnesic shellfish toxins (AST) produced by Pseudonitzschia spp. (Holland et al., 2005) and Nitzschia navisvaringica (Romero et al., 2011).
However, compared to those identified toxins, much less is known about the chemical nature of most bioactive compounds that are toxic to fish, the socalled ichthyotoxins (Zubía et al., 1993; Tang et al., 2007; Tang and Gobler, 2009; Mooney et al., 2010; Yates and Rogers, 2011; Wu et al., 2012; Shahraki et al., 2013). Up to date, only a few types of compounds, such as the karlotoxins, have been proven true ichthyotoxins with a complex structure (Van Wagoner et al., 2010; Cai et al., 2016). In the literature, a number of compounds have been proposed to be responsible for fish killing, such as hemolysin (Kozakai et al., 1982), prymnesins (Sasaki et al., 2006), fatty acid amides (Blossom et al., 2014), and even reactive oxygen species (ROS) (Kim et al., 2009). Recently, Dorantes-Aranda et al. (2015) proposed lipid peroxidation products (e.g. aldehydes) might be associated with fish killing.
Traditionally, except for a few species that have been well known for causing ASTs (Holland et al., 2005; Romero et al., 2011), diatoms have been generally regarded as providing the bulk of food to sustain the marine food chain and consequently fisheries (Dorantes-Aranda et al., 2015). However, this perception has been changed as Ianora and Poulet (1993) found that when copepods were fed on Thalassiosira rotula, hatching success was seriously impaired in 1993. Since Miralto et al. (1999) identified three aldehydes (2-trans-4-cis-7-cis-decatrienal, 2-trans-4-trans-7-cis-decatrienal and 2-trans-4-transdecadienal) isolated from diatoms are bioactive compounds, numerous studies have shown that polyunsaturated aldehydes (PUAs) produced by diatoms have toxic effects on the reproductive processes in copepods (Ianora et al., 1995; Miralto et al., 1999; Zhang and Sun, 2004), cladocerans (Carotenuto et al., 2005), sea urchins (Varrella et al., 2014), and ascidians (Castellano et al., 2015). Multiple biological and biochemical effects have been proposed for PUAs, including grazer defense, allelopathy, cell-to-cell signaling, antibacterial activity, and causing bloom termination (Vardi et al., 2006; Leflaive and Ten-Hage, 2009).
Carbonyl compounds, including aldehydes, ketones, carboxylic acids, and their derivatives (esters, amides, anhydrides, and acyl halides), are commonly found in different environments and play important roles in aquatic oxidation processes (Grimshaw, 1991; Le Lacheur et al., 1993; Bao et al., 1998). Compared to derivatizing agent 2, 4-dinitrophenylhydrazine (DNPH) with liquid chromatography (LC) method, determination of carbonyl compounds in water samples is more commonly based on derivatization with O-(2, 3, 4, 5, 6-pentafluorobenzyl) hydroxyamine hydrochloride (PFBHA) before extraction coupled with gas chromatography (GC) and electron-capture detection (ECD) or mass spectrometry (MS) detection (Le Lacheur et al., 1993; Bao et al., 1998; US Environmental Protection Agency, 1998). Carbonyl compounds could be produced by both marine macroalgae and microalgae. For instance, formaldehyde, acetaldehyde, propanal, propanone, butanal, and pentanal were released by Gelidiela acerosa and Lobophora variegata (da Silva et al., 2006). Wichard et al. (2005a) found that 36% of 51 diatom species investigated produce α, β, γ, δ-unsaturated aldehydes. Prymnesiophyte Phaeocystis pouchetii was known as a producer of trans-trans-2, 4-decadienal also (Hansen et al., 2004). Furthermore, previous studies have shown that the specific type and quantity of PUAs varied with diatom species and strains (Wichard et al., 2005a; Ianora and Miralto, 2010), nutrient regimes (Ribalet et al., 2009), and growth stages (Vidoudez and Pohnert, 2008). However, little is known about whether or not other harmful algal bloom species, e.g. dinoflagellates and raphidophyte, produce carbonyl compounds, which initiated this study.
In this study, we identified and quantified the dissolved and particulate carbonyl compounds for eight common marine HAB-forming species, which demonstrated that seven dinoflagellates and one raphidophyte could be responsible for carbonyl compounds in the marine ecosystem.
2 MATERIAL AND METHOD 2.1 ReagentHPLC-grade methanol and hexane were obtained from Merck (Darmstadt, Germany). The derivatizing reagento-(2, 3, 4, 5, 6-pentafluorobenzyl) hydroxylamine hydrochloride (PFBHA·HCl, 98.0%) was purchased from Anpel Company (Shanghai, China). Ultrapure water was obtained by distillation and filtration through a Milli-Q water purification system (Millipore, Billerica, MA, USA). Unless otherwise noted, all of the above-mentioned reagents were guaranteed reagent grade.
The standards of carbonyl compounds used in the present study included 2-methyl-2-pentenal (97%), trans-2-nonenal (95%), cis-6-nonenal (94%) and 2, 6-dimethyl-5-heptenal (≥70%) from Aldrich Chemical Company (Milwaukee, Wisconsin, USA); trans-2-hexenal (98%), trans, trans-2, 4-heptadienal (HEPTA, 90%), trans, trans-2, 4-octadienal (OCTA, 98%), and trans-2-decenal (95%) from the International Aladdin Reagent Inc. (Shanghai, China); and, trans-trans-2, 4-decadienal (DECA, 98%) from Anpel Company (Shanghai, China). Benzaldehyde (99.6%) used as the internal standard was purchased from Dr. Ehrenstorfer GmbH (Augsburg, Germany). Stock standard solutions of each carbonyl compound at 5 mg/mL were prepared with pure analyte dissolved in methanol and then diluted with ultrapure water to prepare mixed working standard solutions (1–20 μg/mL). Stock standard solutions were stored in the dark at -20℃. Aqueous working standard solutions were prepared before use. The internal standard was added to the calibrator, control, and samples.
2.2 Algal cultureWe selected seven species of dinoflagellates belonging to six genera and one species of raphidophyte to determine the carbonyl compounds production (Table 1), which represented common HAB-forming and/or toxic marine species, particularly towards fish. Sterile f/2-Si medium (Guillard, 1975) was prepared with autoclaved and 0.22 μm filtered seawater from Huiquan Bay of Qingdao, China. Triplicate 1-L flasks containing 600 mL of each culture were maintained without aeration in sterile f/2-Si medium with a salinity of 32–33, at 21℃±1℃ with a light intensity of ~100 μmol photons/(m2·s) (cool-white fluorescent lamps) and a 12 h light: 12 h dark photoperiod. Every day, 1.5 mL subsamples were taken and fixed with Lugol's solution, and cells were counted in a 1-mL counting chamber under an inverted light microscope (Olympus BX53, Tokyo, Japan). These species were harvested in the stationary growth phase for chemical analyses with the f/2-Si medium as the control.
Determination of the production of particulate and dissolved carbonyl compounds was according to protocols modified from Wichard et al. (2005b) and Vidoudez et al. (2011a), as described below: The algal cultures in the stationary growth phase were harvested and filtered through GF/C filter (pore size=1.2 μm, Whatman, Dassel, Germany) with a low vacuum pressure. The algal cells collected from 10 mL to 100 mL culture (depending on cell density) on filters were used to detect the particulate carbonyl compounds. It is important to note that some carbonyl compounds (e.g. PUA from diatoms) were commonly believed to be released after cell disruption by PUFA lypoxidation, which are considered to be detected in particulate form also (Pohnert, 2000; Barofsky and Pohnert, 2007; Vidoudez et al., 2011a). After rinsed from the filter with 5 mL of a 25-mmol/L PFBHA solution in Tris-HCl (100 mmol/L, pH 7.2), transferred into an 8 mL glass vial, and added 25 μL of internal standard (benzaldehyde, 1 mmol/L in methanol), the cells were sonicated (JY92-2D, Xinzhi Co., China) for 10 min in an ice bath to keep the sample cold. After derivatizing for 4 h at room temperature, samples were stored at -80℃ before extraction. For extraction, 2.5 mL of methanol was added to each frozen sample to make it thaw and then 5 mL of hexane was added and vortexed for 1 min. Then 30 drops of sulfuric acid were added using a glass Pasteur pipette (Anpel Company, Shanghai, China) and vortexed for 1 min. The sample was centrifuged at 1 735×g in a 5804-R centrifuge (Eppendorf, Hamburg, Germany) for 10 min at room temperature for phase separation. The hexane phase was transferred into 5-mL glass vials and dehydrated by anhydrous sodium sulphate. After the hexane phase was removed by nitrogen blowing, the samples were re-dissolved in 500 μL of hexane and stored at -80℃ until analysis by GC-MS.
For the analysis of the dissolved form of carbonyl compounds, about 500 mL cell-free culture medium (pre-filtered with a low vacuum pressure through 1.2 μm-pore sized GF/C filter) was further filtered through 0.22-μm filters (Millipore, Bedford, MA, USA). After adding 25 μL of the internal standard (benzaldehyde, 1 mmol/L in methanol), the filtrate was immediately passed through a C18 cartridge column (Anpel Company, Shanghai, China) preloaded with 1 mL of a 25-mmol/L PFBHA solution in TrisHCl 100 mmol/L pH 7.2 over a period of 5 min. After washing with deionized water, the samples were eluted with 4 mL PFBHA in methanol (5 mmol/L) and were incubated for 4 h at room temperature to ensure complete derivatization before stored at -80℃. For extraction, the samples were transferred into 25-mL glass vials. Eight milliliters of hexane and 8 mL of distilled water were added before vortexing for 1 min. Sixty drops of sulfuric acid were added with a glass Pasteur pipette and the samples were vortexed again for 1 min. After separation, the hexane phase was transferred into 10-mL glass vials. After drying over anhydrous sodium sulfate, the supernatant was dried by nitrogen. The samples were re-dissolved in 500 μL of hexane and stored in 1.5-mL vials at -80℃ before injected into the GC-MS.
2.4 Analysis of carbonyl compounds by GC-MSDerivatized carbonyl compounds were analyzed using an Agilent 7890A gas chromatograph coupled to an Agilent 5975C mass spectrometer with G4513A auto-sampler and MSD ChemStation (Version E.02.02.1431, Agilent Technologies, Inc.). The compounds were separated using an HP-5MS capillary (30 m×0.25 mm×0.25 μm) column (Agilent, USA) at a constant flow rate of 1 mL/min. Helium was used as the carrier gas. The GC temperature program for the separation was held at 60℃ for 2 min, then increased with a rate of 8℃/min to 240℃, and a rate of 15℃/min to 280℃ for 10 min. The injection temperature was fixed at 230℃ and samples were injected in split 10 mode. The electron energy was 70 eV.
2.5 Quantification of carbonyl compoundsData analysis was performed with both full scan and SIM mode. For the full scan method, the 133 largest peaks were selected in the chromatograms of all the fifty-one samples (see the results). The identification or structural proposal of the detected compounds was conducted by comparing the consistence between the mass spectrum and retention time and those of standards. A structural proposal was given when the matching degree was >85% to the mass spectral database or the mass spectrum available in the literature. The molar concentration and cell quota of detected compounds were calculated according to the concentration and peak area of the internal standard benzaldehyde added in the samples detected synchronously.
In addition to the full scan data analysis, quantitation analysis of the nine chosen aldehydes was further performed using SIM programs. The aldehydePFBHA derivatives produced by the examined species were identified by comparing to the authentic derivatized aldehyde standards and benzaldehyde (internal standard) according to their retention time and the presence of their characteristic ions at m/z: 2-methyl-2-pentenal, 181, 264 and 293; trans-2-nonenal, 181, 250 and 335; cis-6-nonenal, 181, 195 and 253; 2, 6-dimethyl-5-heptenal, 181, 253 and 72; trans-2-hexenal, 181, 250 and 293; trans-2-decenal, 181, 250 and 349; HEPTA, 181, 305 and 276; OCTA, 181, 276 and 319; DECA, 181, 276 and 347; and benzaldehyde, 181, 271 and 301. Quantification was based on the ratios of the fragment of the derivatized aldehydes to the derivatized internal standard, which was plotted against the concentrations of the aldehydes. The obtained concentrations were finally normalized to the filtered volume of culture, and the results were expressed as the molarity of aldehydes in 1 L and the molar mass per cell obtained according to the concentrated cell numbers. The detection limit, in this case, cannot be determined, as it greatly depended on the cell density and volumes of sample filtered, which were different among samples. To direct compare the PUA production in species with different cell volume, the PUA to carbon (PUA/C) ratio was also calculated according to Wichard et al. (2005a), in which the cell volume of phytoplankton was determined according to Hillebrand et al. (1999).
3 RESULT 3.1 Compounds analyzed in the GC-MS full scan modeFull scan GC/MS analysis was first used for chemical screening, as this technique provides both qualitative and semi-quantitative information. According to the GC-MS total ion-current chromatogram (e.g. Fig. 1), 133 peaks of carbonyl compound constituents were separated in all the dissolved and/or particulate form of these eight selected harmful algal species but could not be identified in the mass spectral database with matching degree below 85%. The separated peaks accounted for more than 80% of the effective peak area in all the samples.

|
| Fig.1 A representative GC-MS full scan chromatogram of dissolved derivatized carbonyl compounds produced by Scrippsiella trochoidea The retention time was marked near the separated peaks present in most samples. |
The molar concentrations were calculated for all 133 chromatographic peaks (compounds). About onethird of the peaks were detected only in dissolved form, including 29 peaks detected both in f/2-Si medium (control) and all cultures of the eight microalgae (Fig.S1), 6 peaks detected in f/2-Si medium and some cultures of the eight microalgae (Fig.S2), and 8 peaks not detected in f/2-Si medium but in some cultures of the microalgae (Fig.S3).
The rest two-third peaks were detected in the particulate and/or dissolved form of some or all of the eight studied species, with 65 peaks detected but 25 not detected in f/2-Si medium (control). For the peaks that were detected in the f/2-Si medium control, 50 of them were also detected in dissolved form in most or all of the eight microalgal cultures, but were detected in particulate form only in one (Fig.S4), two (Fig.S5), three (Fig.S6), four (Fig.S7), five (Fig.S8), six (Fig.S9) and seven (Fig.S10) species.
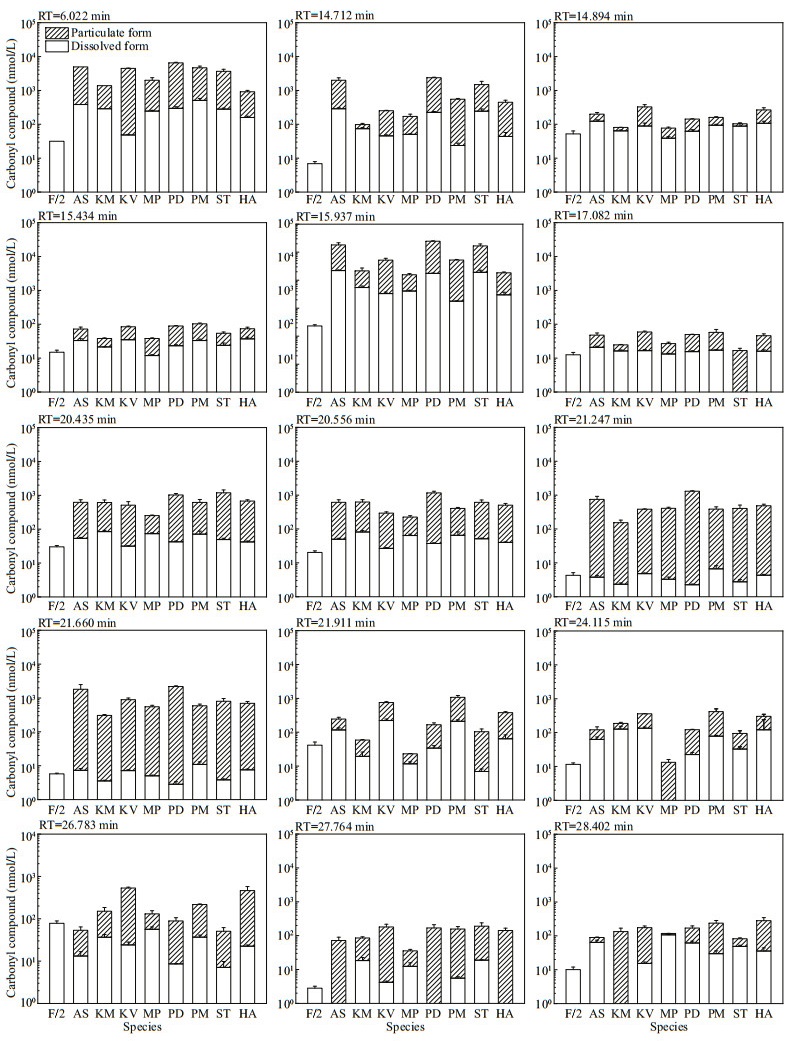
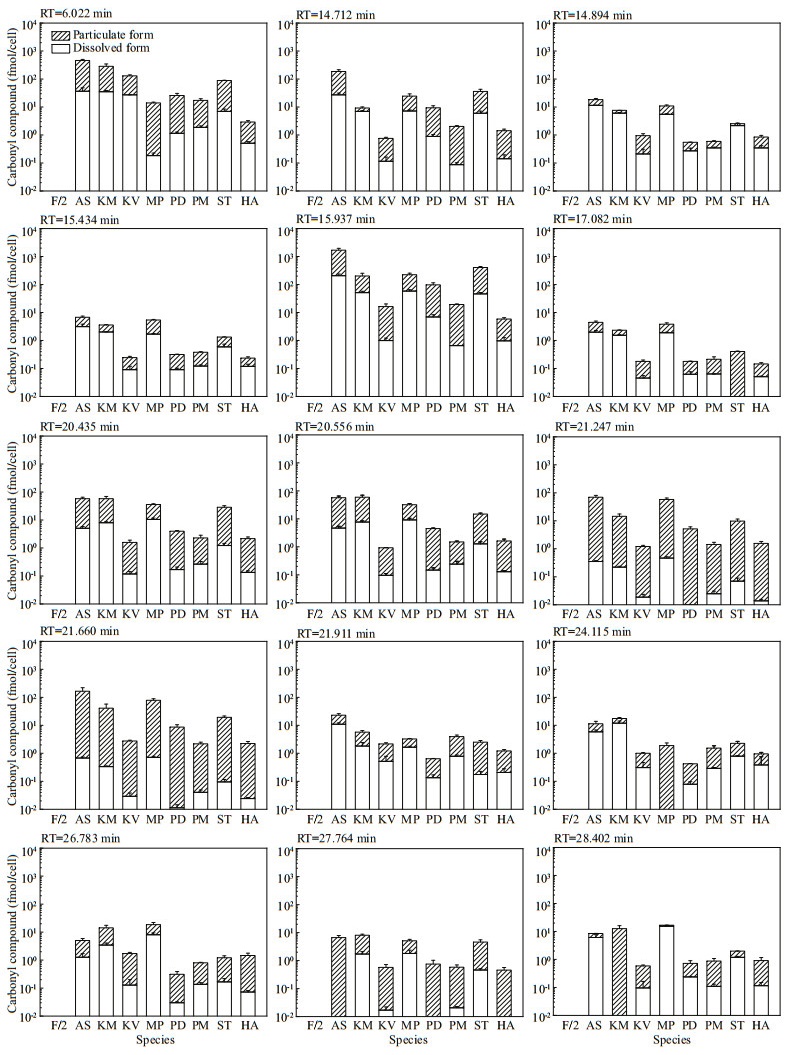
The other 15 peaks were detected in both f/2-Si medium control and in particulate form of all the eight microalgae, with the total concentrations in microalgal cells significantly exceeding that in the medium control for most of the compounds. Hence, both the molar concentrations (Fig. 2) and the cell quotas in molar mass (Fig. 3) of these 15 compounds were calculated. The production of carbonyl compounds varied significantly among the eight algal species. For those in particulate form, the concentrations and particulate content of these 15 compounds were up to 22 865.51±823.17 nmol/L (P. donghaiense) and 1 485.22±271.94 fmol/cell (A. sanguinea). For those in dissolved form, the concentrations and cell quotas of these 15 compounds were up to 2 200.63±120.61 nmol/L and 207.34± 28.92 fmol/cell, both of which were detected in A. sanguinea samples.
|
| Fig.2 The molar concentration of 15 carbonyl compounds detected in both f/2-Si medium (control) and all the eight cultures of algal species RT: retention time; AS: Akashiwo sanguinea; KM: Karenia mikimotoi; KV: Karlodinium veneficum; MP: Margalefidinium polykrikoides; PD: Prorocentrum donghaiense; PM: Prorocentrum minimum; ST: Scrippsiella trochoidea; HA: Heterosigma akashiwo. |
|
| Fig.3 The cell quotas in molar mass of 15 carbonyl compounds detected in both f/2-Si medium (control) and all the eight cultures of algal species Refer to the note of Fig. 2 for abbreviations. |
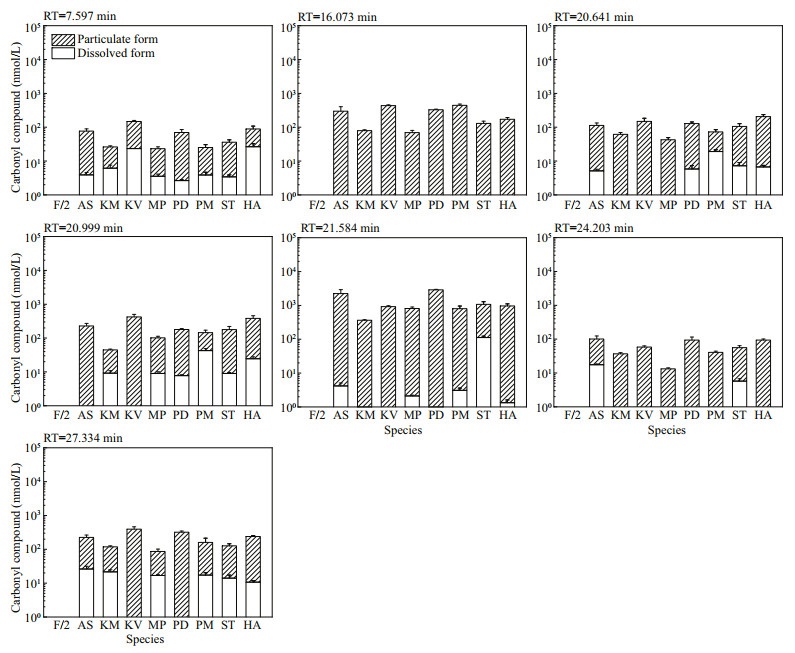
Amongst the 25 peaks not detected in f/2-Si medium, 18 of them were detected in some algal culture samples (Fig.S11). The concentrations of these analytes varied among different species. The other 7 peaks were detected in all algal cultures and most were in particulate form, which indicated the corresponding carbonyl compounds were produced by these marine algal species and held as particulate form. Both the molar concentrations (Fig. 4) and the cell quotas in molar mass (Fig. 5) were calculated for these seven compounds: the total carbonyl compounds concentrations including dissolved and particulate form ranged from 13.28±0.87 nmol/L (M. polykrikoides) to 2 887.59±92.02 nmol/L (P. donghaiense), while the total cell quotas varied from 0.01±0.00 fmol/cell (P. minimum) to 205.63± 52.50 (A. sanguinea) fmol/cell.
|
| Fig.4 The molar concentrations of seven carbonyl compounds detected in all eight cultures of algal species but not in f/2-Si medium (control) Refer to the note of Fig. 2 for abbreviations. |
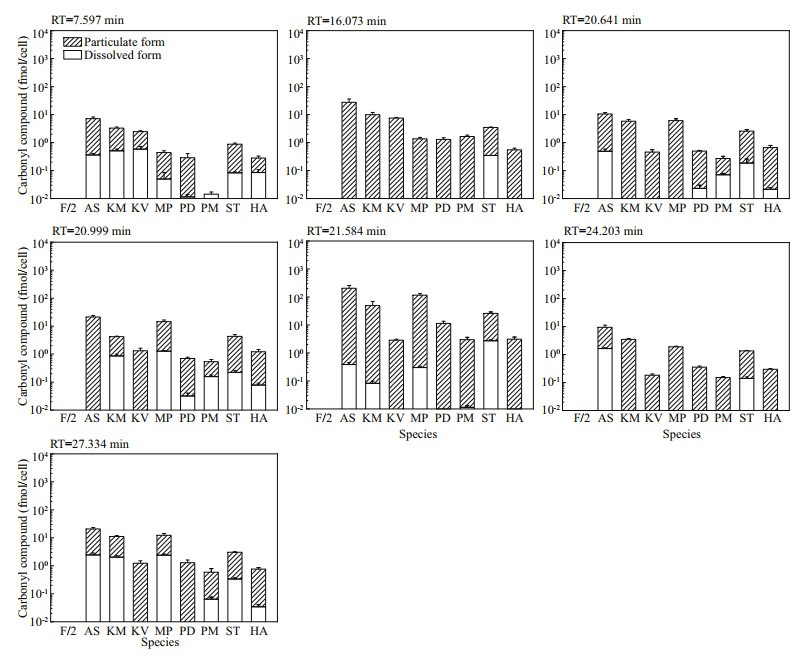
|
| Fig.5 The cell quotas in molar mass of seven carbonyl compounds detected in all the eight cultures of algal species but not in f/2-Si medium (control) Refer to the note of Fig. 2 for abbreviations. |
According to the structural proposals given in the mass spectral database for peaks described above and literature review, we chose nine aldehydes (2-methyl-2-pentenal, trans-2-nonenal, cis-6-nonenal, 2, 6-dimethyl-5-heptenal, trans-2-hexenal, trans-2-decenal, HEPTA, OCTA, and DECA) to further identify and quantify them in our algal cultures by using their corresponding authentic standards. Among them, 2, 6-dimethyl-5-heptenal and trans-2-hexenal, however, were not detected in all algal samples. The 2-methyl-2-pentenal, trans-2-nonenal, cis-6-nonenal, and trans-2-decenal were detected in all samples including the natural seawater-based culture medium control, while only trans-2-nonenal was detected in the particulate form in the species K. mikimotoi, Karl. veneficum, P. donghaiense, P. minimum, S. trochoidea and H. akashiwo (Fig. 6) with the cell quotas lower than 0.04 fmol/cell (data not shown).
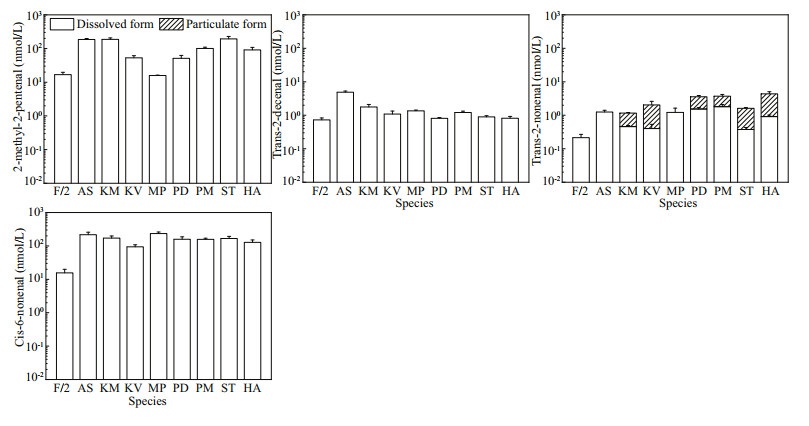
|
| Fig.6 The concentrations of 2-methyl-2-pentenal, trans-2-decenal, trans-2-nonenal and cis-6-nonenal detected in f/2-Si medium (control) and all the eight cultures of algal species Refer to the note of Fig. 2 for abbreviations. |
The three PUAs (HEPTA, OCTA, and DECA), previously reported to be produced by diatoms, were also detected in the particulate form from the five dinoflagellates A. sanguinea, K. mikimotoi, Karl. veneficum, M. polykrikoides, and S. trochoidea (Table 2). However, the concentrations of dissolved PUAs from all the five species and that of particulate PUAs from K. mikimotoi and Karl. veneficum samples were below the limit of quantitation. The cell quotas were calculated for A. sanguinea, M. polykrikoides, and S. trochoidea samples in particulate form as follows: HEPTA was 1.13±0.04, 0.68±0.08, and 0.08±0.02 fmol/cell in A. sanguinea, M. polykrikoides and S. trochoidea cells, respectively; DECA 0.68±0.10, 0.36±0.02, and 0.05±0.02 fmol/cell in A. sanguinea, M. polykrikoides, and S. trochoidea cells, respectively; and OCTA 0.01±0.00 fmol/cell in S. trochoidea cells. The three quantified PUA to carbon ratios ranged from 3±1 μg/g (OCTA in S. trochoidea) to 72±3 μg/g (HEPTA in A. sanguinea) (Table 2).
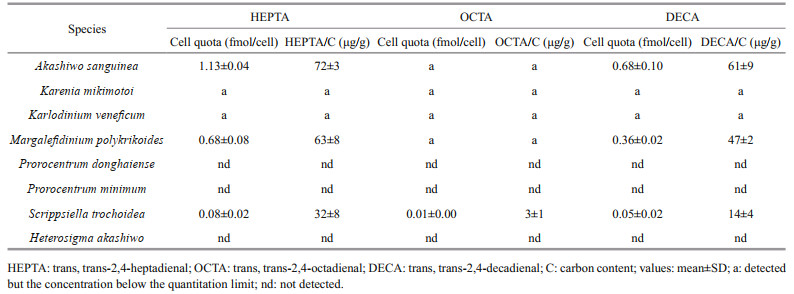
|
Carbonyl compounds are common in the aquatic environment and play an important role in oxidation processes (Grimshaw, 1991; Le Lacheur et al., 1993; Bao et al., 1998). Additional to being generated during photo-chemical reactions (Edelkraut and Brockmann, 1990), carbonyl compounds could also be released as metabolites by microbiological processes in marine waters (Miralto et al., 1999; da Silva et al., 2006). Most studies focused on the PUAs including HEPTA, OCTA, DECA, octatrienal and decatrienal produced by diatoms due to their negative influence on the reproductive success of copepods and invertebrates (Wendel and Jüttner, 1996; Miralto et al., 1999; Pohnert et al., 2002; d'Ippolito et al., 2004; Castellano et al., 2015). For other carbonyl compounds, lowmolecular-weight carbonyl compounds including formaldehyde, acetaldehyde, propanal, propanone, butanal, butanone, pentanal, hexanal and (E)-2-Hexenal were identified from 10 species of marine macroalgae (da Silva et al., 2006). However, the dissolved and particulate carbonyl compounds released by dinoflagellate and raphidophyte species were seldom reported, which might be responsible for carbonyl compounds in the marine ecosystem and toxicity/allelopathy effects on other organisms. In this study, 133 chromatographic peaks were detected in the full scan mode of GC/MS from all the dissolved and/or particulate form of these eight selected harmful-algal-blooming species. About one-third of these peaks were detected only in dissolved form, which might be because the corresponding chemicals were: 1) produced by the photodegradation of dissolved natural organic matters present in nature water ecosystem (Kieber et al., 1990); 2) released by other microbes in the marine environment (NemecekMarshall et al., 1995); and 3) released by cell death or lysis given that the cultures used in the present study were in the stationary phase. The rest peaks, which were detected in particulate form or with total concentration significantly more than those in the f/2-Si medium, were considered to be produced by the species chosen in the study, and consequently, may cause negative impacts on marine ecosystem after release.
Twenty-two carbonyl compounds shown in Figs. 2 and 4 were detected in the particulate form from all the eight algal species, demonstrating that these carbonyl compounds were produced by the studied marine HAB-forming species. The production of carbonyl compounds has been shown to be species dependent, which concentrations and cell quotas of the particulate and dissolved form varied among algal species. This was consistent with the investigation of PUAs in diatoms which showed that even different strains of the same species produced different quantities of PUAs. For instance, summer strains of Skeletonema marinoi had a higher production of PUA than strains isolated from other seasons (Taylor et al., 2009). In addition, carbonyl compounds production might also depend on factors such as nutrients and age of the culture as PUAs do (Ribalet et al., 2007). The diverse producing profiles of these compounds in different species or strains may cause different negative effects, even toxic at higher levels, which requires further investigation. The PUAs are always detected in particulate form and might be released during the wound-activated process (Pohnert, 2000). However, an active release of dissolved PUAs from Skeletonema marinoi was previously observed in laboratory culture (Vidoudez et al., 2011b). This study also found that some of the carbonyl compounds (Figs. 2 and 4) were released from cells to the medium, which might be an active secretion and/or released by cell death or lysis.
Although most of the carbonyl compounds could not be fully identified by the structural proposals given in the mass spectral database, 2-methyl-2-pentenal, trans-2-nonenal, cis-6-nonenal, and trans-2-decenal were detected according to the standards in all dissolved samples including in the f/2-Si medium control. Trans-2-nonenal was also detected in the particulate form of K. mikimotoi, Karl. veneficum, P. donghaiense, P. minimum, S. trochoidea, and H. akashiwo samples, but the cell quotas were lower than 0.04 fmol/cell in these species. The trans-2-nonenal originated from lipoxygenase-hydroperoxide lyase action, has been reported to inhibit the germination and subsequent growth of soybean (Gardner et al., 1990). Further study is needed to find out whether these aldehydes have negative effects on the organisms in the marine environment. Furthermore, it is interesting to note that HEPTA, OCTA, and DECA were identified in this study. These PUAs commonly found in the ocean, are biosynthetically derived from unsaturated fatty acids, and proposed to play an important role in chemically mediated plankton interactions (Wichard et al., 2008; Vidoudez et al., 2011a). Since Miralto et al. (1999) identified 2-trans-4-cis-7-cis-decatrienal, 2-trans-4-trans-7-cisdecatrienal, and 2-trans-4-trans-decadienal from Thalassiosira rotula, Skeletonema costatum, and Pseudo-nitzschia delicatissima, diatoms have been considered the main class of organisms in the plankton producing PUAs. In a survey of PUA-production within the Bacillariophyceae, 36% of the fifty-one diatom species (seventy-one isolates) were found to release α, β, γ, δ-unsaturated aldehydes upon cell disruption (Wichard et al., 2005a). Furthermore, the findings that marine haptophyte Phaeocystis pouchetii produces DECA indicate that production of aldehydes is not limited to diatoms only (Hansen et al., 2004). In some field surveys, hitherto unidentified picoplankton, and the prymnesiophyte Phaeocystis sp. seemed to be the sources of PUA, also indicating that PUA sources other than diatoms should be considered (Vidoudez et al., 2011a, b). Until now, no comprehensive investigation on the production of particulate and dissolved PUAs by HAB-forming species, particularly in classes Dinophyceae and Raphidophyceae have been reported. In this study, three PUAs (HEPTA, OCTA, and DECA) were detected in five dinoflagellates (Akashiwo sanguinea, Karenia mikimotoi, Karlodinium veneficum, Margalefidinium polykrikoides, and Scrippsiella trochoidea), although the contents in KM and KV were below the limit of quantification.
Our result expand the species range that could produce PUAs from diatoms and Phaeocystis sp. to dinoflagellates, and more importantly, PUA from HABs-forming species, dinoflagellate, in particular, may be dominant during HABs caused by these species. A comparison of the production of these three PUA (percentage and the total PUA) by AS, MP, and ST to that of diatoms (Wichard et al., 2005a) was performed (Table 3). Note that because the 2, 4, 7-octatrienal and 2, 4, 7-decatrienal were not detected in this study, we modified the total PUA production in Wichard et al. (2005a) as the sum of HEPTA, OCTA, and DECA and made the corresponding changes in percentage data. Except for Melosira nummuloides (7.03±0.34 fmol PUA per cell), Thalassiosira pacifica (8.73±0.06 fmol PUA per cell), and Thalassiosira rotula (CCMP 1647) (2.48±0.11 fmol PUA per cell), the PUA production by A. sanguinea (1.81±0.14 fmol PUA per cell) exceeded all other 48 investigated diatom species. The PUA content of M. polykrikoides and S. trochoidea were within the range of 0.01 fmol PUA per cell (equivalent to that of Thalassiosira nordenskioeldii) to 8.7 fmol PUA per cell (equivalent to that of Thalassiosira pacifica). Given that aldehyde contribution of species expressed in cell quota might be underestimated for the algae with low cell volume but probably high cell abundance, the PUAs to carbon (PUA/C) ratios of A. sanguinea, M. polykrikoides, and S. trochoidea were also calculated with values higher than Melosira sulcata and Thalassiosira nordenskioeldii (Table 3). Although production of secondary metabolites is always strongly modulated by physiological conditions (e.g. nutrient availability and light intensity), strain variability, and age of the cultures (Dittami et al., 2010), the PUA production in the three dinoflagellates were representative as the cultures in the stationary growth phase were used (the same as most investigations for diatoms). As PUA producers usually release a mixture of different aldehydes, the relative contents of chemicals may be characteristic in different species. According to the available data in the literature, HEPTA and OCTA are the most common PUAs produced by diatoms (Wichard et al., 2005a; Vidoudez and Pohnert, 2008; Bartual et al., 2014). However, HEPTA and DECA were the dominant PUAs in the dinoflagellates A. sanguinea, M. polykrikoides, and S. trochoidea, while a quantifiable OCTA was only found in S. trochoidea. The ratios of HEPTA: DECA were about 2:1 for A. sanguinea, M. polykrikoides, and S. trochoidea. As the most active of the known PUA, DECA is not a typical metabolite in diatoms (Miralto et al., 1999; Vidoudez et al., 2011b). Phaeocystis sp. was reported to be a source of DECA (Hansen et al., 2004). Therefore, our detection of DECA in dinoflagellates guarantees further in-depth investigations in this group of phytoplankton, especially during harmful algal blooms of dinoflagellates.
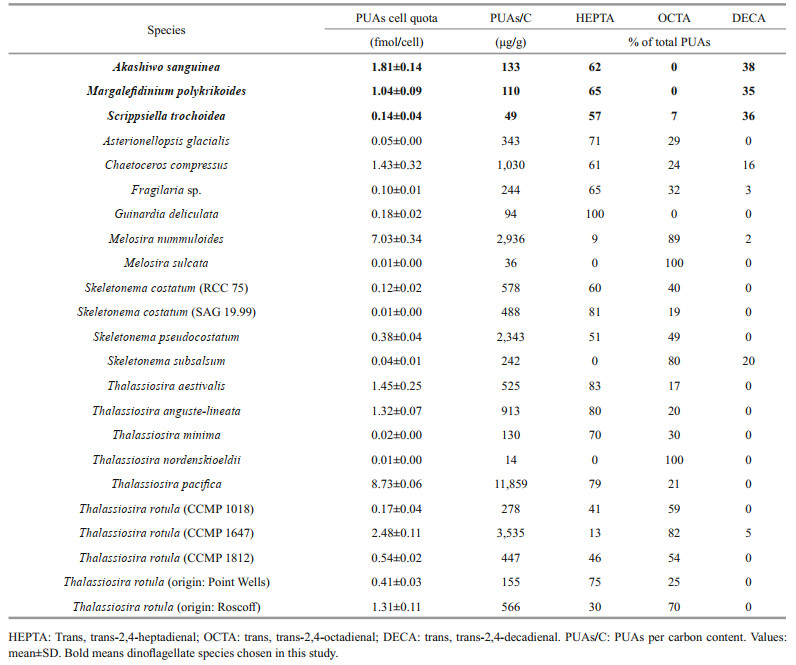
|
There are numerous functions that have been proposed for PUAs. Additional to grazer defense, cell to cell signaling, antibacterial activity, bloom termination, it is noteworthy that PUAs also have allelopathy and direct toxic effects on marine organisms (Miralto et al., 1999; Pohnert, 2000; d'Ippolito et al., 2004; Vidoudez and Pohnert, 2008; Leflaive and Ten-Hage, 2009; Dittami et al., 2010; Sansone et al., 2014). In this study, we did not detect any PUAs from the non-toxic species Prorocentrum donghaiense and P. minimum samples, although they both form HABs (Heil et al., 2005; Sun et al., 2017). The PUAs productions in the ichthyotoxic species Karenia mikimotoi and Karlodinium veneficum were also below the limit of quantitation, while the ichthyotoxins of K. veneficum have been identified as karlotoxins (Bachvaroff et al., 2008). Additionally, the other three toxic Akashiwo sanguinea (Xu et al., 2017), Margalefidinium polykrikoides (Tang and Gobler, 2009) and Scrippsiella trochoidea (Tang and Gobler, 2012), with intoxication mechanisms and responsible toxic agents far from being identified, were demonstrated to produce PUAs. Therefore, PUAs or other carbonyl compounds might be responsible for their fish-killing or/and allelopathy of these toxic species, and further research is needed to study the relationship between the quantity of PUAs and toxicity of these algal species. This study just placed a corner stone for more intensive investigations in this direction.
5 CONCLUSIONCarbonyl compounds could be produced by HABforming species, including dinoflagellates and raphidophytes (e.g. H. akashiwo), with concentrations and cell quotas of the particulate and dissolved form varied among algal species. Although most of the carbonyl compounds could not be identified, four aldehydes were demonstrated to be produced by microalgae using standards, with trans-2-nonenal produced by K. mikimotoi, Karl. veneficum, P. donghaiense, P. minimum, S. trochoidea, and H. akashiwo, 2, 4-heptadienal, and trans-trans-2, 4-decadienal produced by A. sanguinea, M. polykrikoides, and S. trochoidea, and trans, trans-2, 4-octadienal produced by S. trochoidea, respectively. It is likely that the adverse effects on the reproduction and the population dynamics of herbivorous grazers associated with PUAs observed in the marine ecosystem might be not only caused by diatoms but also other phytoplankton such as dinoflagellates. As the cell contents of many unidentified carbonyl compounds in the studied species were higher than that of the above-mentioned four PUAs, it is clear that further identifications are needed and investigations on the complex roles of carbonyl compounds in allelopathy and/or toxic effects are guaranteed.
6 DATA AVAILABILITY STATEMENTThe authors declare that all data supporting the findings of this study are available within the article and its supplementary information files.
Electronic supplementary materialSupplementary material (Supplementary Figs.S1–S11) is available in the online version of this article at https://doi.org/10.1007/s00343-019-8199-5.
Anderson D M, Cembella A D, Hallegraeff G M. 2012. Progress in understanding harmful algal blooms:paradigm shifts and new technologies for research, monitoring, and management. Annual Review of Marine Science, 4: 143-176.
DOI:10.1146/annurev-marine-120308-081121 |
Aráoz R, Molgó J, de Marsac N T. 2010. Neurotoxic cyanobacterial toxins. Toxicon, 56(5): 813-828.
DOI:10.1016/j.toxicon.2009.07.036 |
Bachvaroff T R, Adolf J E, Squier A H, Harvey H R, Place A R. 2008. Characterization and quantification of karlotoxins by liquid chromatography-mass spectrometry. Harmful Algae, 7(4): 473-484.
DOI:10.1016/j.hal.2007.10.003 |
Bao M L, Pantani F, Griffini O, Burrini D, Santianni D, Barbieri K. 1998. Determination of carbonyl compounds in water by derivatization-solid-phase microextraction and gas chromatographic analysis. Journal of Chromatography A, 809(1-2): 75-87.
DOI:10.1016/S0021-9673(98)00188-5 |
Barofsky A, Pohnert G. 2007. Biosynthesis of polyunsaturated short chain aldehydes in the diatom Thalassiosira rotula. Organic Letters, 9(6): 1017-1020.
DOI:10.1021/ol063051v |
Bartual A, Arandia-Gorostidi N, Cózar A, Morillo-García S, Jesus Ortega M, Vidal M, Cabello A M, GonzálezGordillo J I, Echevarría F. 2014. Polyunsaturated aldehydes from large phytoplankton of the Atlantic Ocean surface (42°N to 33°S). Marine Drugs, 12(2): 682-699.
DOI:10.3390/md12020682 |
Blossom H E, Rasmussen S A, Andersen N G, Larsen T O, Nielsen K F, Hansen P J. 2014. Prymnesium parvum revisited:relationship between allelopathy, ichthyotoxicity, and chemical profiles in 5 strains. Aquatic Toxicology, 157: 159-166.
DOI:10.1016/j.aquatox.2014.10.006 |
Cai P J, He S, Zhou C X, Place A R, Haq S, Ding L J, Chen H M, Jiang Y, Guo C, Xu Y R, Zhang J R, Yan X J. 2016. Two new karlotoxins found in Karlodinium veneficum(strain GM2) from the East China Sea. Harmful Algae, 58: 66-73.
DOI:10.1016/j.hal.2016.08.001 |
Carotenuto Y, Wichard T, Pohnert G, Lampert W. 2005. Lifehistory responses of Daphnia pulicaria to diets containing freshwater diatoms:effects of nutritional quality versus polyunsaturated aldehydes. Limnology and Oceanography, 50(2): 449-454.
DOI:10.4319/lo.2005.50.2.0449 |
Castellano I, Ercolesi E, Romano G, Ianora A, Palumbo A. 2015. The diatom-derived aldehyde decadienal affects life cycle transition in the ascidian Ciona intestinalis through nitric oxide/ERK signalling. Open Biology, 5(3): 140-182.
|
Cusick K D, Sayler G S. 2013. An overview on the marine neurotoxin, saxitoxin:genetics, molecular targets, methods of detection and ecological functions. Marine Drugs, 11(4): 991-1.
|
da Silva V M, da Cunha Veloso M C, Teixeira Sousa E, Santos G V, Accioly MC, de P Pereira P A, de Andrade J B. 2006. Determination of 11 low-molecular-weight carbonyl compounds in marine algae by high-performance liquid chromatography. Journal of Chromatographic Science, 44(5): 233-238.
DOI:10.1093/chromsci/44.5.233 |
d'Ippolito G, Tucci S, Cutignano A, Romano G, Cimino G, Miralto A, Fontana A. 2004. The role of complex lipids in the synthesis of bioactive aldehydes of the marine diatom Skeletonema costatum. Biochimica et Biophysica ActaMolecular and Cell Biology of Lipids, 1686(1-2): 100-107.
|
Dittami S M, Wichard T, Malzahn A M, Pohnert G, Boersma M, Wiltshire K H. 2010. Culture conditions affect fatty acid content along with wound-activated production of polyunsaturated aldehydes in Thalassiosira rotula(Coscinodiscophyceae). In: Proceedings of the 7th International Chrysophyte Symposium. Nova Hedwigia, New London. p.231-248.
|
Dorantes-Aranda J J, Seger A, Mardones J I, Nichols P D, Hallegraeff G M. 2015. Progress in understanding algal bloom-mediated fish kills:the role of superoxide radicals, phycotoxins and fatty acids. PLoS One, 10(7): e01336549.
|
Edelkraut F, Brockmann U. 1990. Simulataneous determination of carboxylic acids and carbonyl compounds in estuaries by HPLC. Chromatographia, 30(7-8): 432-435.
DOI:10.1007/BF02328512 |
Fux E, Smith J L, Tong M M, Guzmán L, Anderson D M. 2011. Toxin profiles of five geographical isolates of Dinophysis spp. from North and South America. Toxicon, 57(2): 275-287.
|
Gardner H W, Dornbos Jr D L, Desjardins A E. 1990. Hexanal, trans-2-hexenal, and trans-2-nonenal inhibit soybean, Glycine max, seed germination. Journal of Agricultural and Food Chemistry, 38(6): 1316-1320.
DOI:10.1021/jf00096a005 |
Gayoso A M, Dover S L, Morton S L, Busman M, Moeller P D R, Fulco V K, Maranda L. 2002. Diarrhetic shellfish poisoning associated with Prorocentrum lima(Dinophyceae) in Patagonian Gulfs (Argentina). Journal of Shellfish Research, 21(2): 461-463.
|
Grimshaw J. 1991. Hydroxy-aldehydes and -ketones and related compounds: dicarbonyl compounds. In: Sainsbury M ed. Second Supplements to the 2nd Edition of Rodd's Chemistry of Carbon Compounds. Elsevier, Amsterdam.p.53-107.
|
Guillard R R L. 1975. Culture of phytoplankton for feeding marine invertebrates. In: Smith W L, Chanley M H eds.Culture of Marine Invertebrate Animals. Plenum Press, New York. p.26-60.
|
Hallegraeff G M, Blackburn S I, Doblin M A, Bolch C J S. 2012. Global toxicology, ecophysiology and population relationships of the chainforming PST dinoflagellate Gymnodinium catenatum. Harmful Algae, 14: 130-143.
DOI:10.1016/j.hal.2011.10.018 |
Hansen E, Ernstsen A, Eilertsen H C. 2004. Isolation and characterisation of a cytotoxic polyunsaturated aldehyde from the marine phytoplankter Phaeocystis pouchetii(Hariot) Lagerheim. Toxicology, 199(2-3): 207-217.
DOI:10.1016/j.tox.2004.02.026 |
Heil C A, Glibert P M, Fan C L. 2005. Prorocentrum minimum(Pavillard) Schiller:a review of a harmful algal bloom species of growing worldwide importance. Harmful Algae, 4(3): 449-470.
DOI:10.1016/j.hal.2004.08.003 |
Hillebrand H, Dürselen C D, Kirschtel D, Pollingher U, Zohary T. 1999. Biovolume calculation for pelagic and benthic microalgae. Journal of Phycology, 35(2): 403-424.
DOI:10.1046/j.1529-8817.1999.3520403.x |
Holland P T, Selwood A I, Mountfort D O, Wilkins A L, McNabb P, Rhodes L L, Doucette G J, Mikulski C M, King K L. 2005. Isodomoic acid C, an unusual amnesic shellfish poisoning toxin from Pseudo-nitzschia australis. Chemical Research in Toxicology, 18(5): 814-816.
DOI:10.1021/tx0496845 |
Ianora A, Miralto A. 2010. Toxigenic effects of diatoms on grazers, phytoplankton and other microbes:a review. Ecotoxicology, 19(3): 493-511.
DOI:10.1007/s10646-009-0434-y |
Ianora A, Poulet S A, Miralto A. 1995. A comparative study of the inhibitory effect of diatoms on the reproductive biology of the copepod Temora stylifera. Marine Biology, 121(3): 533-539.
DOI:10.1007/BF00349463 |
Ianora A, Poulet S A. 1993. Egg viability in the copepod Temora stylifera. Limnology and Oceanography, 38(8): 1615-1626.
DOI:10.4319/lo.1993.38.8.1615 |
Kieber R J, Zhou X L, Mopper K. 1990. Formation of carbonyl compounds from UV-induced photodegradation of humic substances in natural waters:fate of riverine carbon in the sea. Limnology and Oceanography, 35(7): 1503-1515.
DOI:10.4319/lo.1990.35.7.1503 |
Kim D, Yamasaki Y, Yamatogi T, Yamaguchi K, Matsuyama Y, Kang Y S, Lee Y, Oda T. 2009. The possibility of reactive oxygen species (ROS)-independent toxic effects of Cochlodinium polykrikoides on damselfish (Chromis caerulea). Bioscience, Biotechnology, and Biochemistry, 73(3): 613-618.
DOI:10.1271/bbb.80693 |
Kozakai H, Oshima Y, Yasumoto T. 1982. Isolation and structural elucidation of hemolysin from the phytoflagellate Prymnesium parvum. Agricultural and Biological Chemistry, 46(1): 233-236.
|
Landsberg J H. 2002. The effects of harmful algal blooms on aquatic organisms. Reviews in Fisheries Science, 10(2): 113-390.
DOI:10.1080/20026491051695 |
Le Lacheur R M, Sonnenberg L B, Singer P C, Christman R F, Charles M J. 1993. Identification of carbonyl compounds in environmental samples. Environmental Science & Technology, 27(13): 2745-2753.
|
Leflaive J, Ten-Hage L. 2009. Chemical interactions in diatoms:role of polyunsaturated aldehydes and precursors. New Phytologist, 184(4): 794-805.
DOI:10.1111/j.1469-8137.2009.03033.x |
Miralto A, Barone G, Romano G, Poulet S A, Ianora A, Russo G L, Buttino I, Mazzarella G, Laabir M, Cabrini M, Giacobbe M G. 1999. The insidious effect of diatoms on copepod reproduction. Nature, 402(6758): 173-176.
DOI:10.1038/46023 |
Mooney B D, Hallegraeff G M, Place A R. 2010. Ichthyotoxicity of four species of gymnodinioid dinoflagellates(Kareniaceae, Dinophyta) and purified karlotoxins to larval sheepshead minnow. Harmful Algae, 9(6): 557-562.
DOI:10.1016/j.hal.2010.04.005 |
Mooney B D, Nichols P D, de Salas M F, Hallegraeff G M. 2007. Lipid, fatty acid, and sterol composition of eight species of Kareniaceae (Dinophyta):chemotaxonomy and putative lipid phycotoxins. Journal of Phycology, 43(1): 101-111.
DOI:10.1111/j.1529-8817.2006.00312.x |
Nemecek-Marshall M, Wojciechowski C, Kuzma J, Silver G M, Fall R. 1995. Marine vibrio species produce the volatile organic compound acetone. Applied and Environmental Microbiology, 61(1): 44-47.
|
Okolodkov Y B. 2005. The global distributional patterns of toxic, bloom dinoflagellates recorded from the Eurasian Arctic. Harmful Algae, 4(2): 351-369.
DOI:10.1016/j.hal.2004.06.016 |
Orlova T Y, Selina M S, Lilly E L, Kulis D M, Anderson D M. 2007. Morphogenetic and toxin composition variability of Alexandrium tamarense (Dinophyceae) from the east coast of Russia. Phycologia, 46(5): 534-548.
DOI:10.2216/06-17.1 |
Otero A, Chapela M J, Atanassova M, Vieites J M, Cabado A G. 2011. Cyclic imines:chemistry and mechanism of action:a review. Chemical Research in Toxicology, 24(11): 1817-1829.
DOI:10.1021/tx200182m |
Pistocchi R, Guerrini F, Pezzolesi L, Riccardi M, Vanucci S, Ciminiello P, Dell'Aversano C, Forino M, Fattorusso E, Tartaglione L, Milandri A, Pompei M, Cangini M, Pigozzi S, Riccardi E. 2012. Toxin levels and profiles in microalgae from the North-Western Adriatic Sea-15 years of studies on cultured species. Marine Drugs, 10(1): 140-162.
|
Pohnert G, Lumineau O, Cueff A, Adolph S, Cordevant C, Lange M, Poulet S A. 2002. Are volatile unsaturated aldehydes from diatoms the main line of chemical defence against copepods?. Marine Ecology Progress Series, 245: 33-45.
DOI:10.3354/meps245033 |
Pohnert G. 2000. Wound-activated chemical defense in unicellular planktonic algae. Angewandte Chemie International Edition, 39(23): 4352-4354.
|
Rasmussen S A, Andersen A J C, Andersen N G, Nielsen K F, Hansen P J, Larsen T O. 2016. Chemical diversity, origin, and analysis of phycotoxins. Journal of Natural Products, 79(3): 662-673.
DOI:10.1021/acs.jnatprod.5b01066 |
Ribalet F, Vidoudez C, Cassin D, Pohnert G, Ianora A, Miralto A, Casotti R. 2009. High plasticity in the production of diatom-derived polyunsaturated aldehydes under nutrient limitation:physiological and ecological implications. Protist, 160(3): 444-451.
DOI:10.1016/j.protis.2009.01.003 |
Ribalet F, Wichard T, Pohnert G, Ianora A, Miralto A, Casotti R. 2007. Age and nutrient limitation enhance polyunsaturated aldehyde production in marine diatoms. Phytochemistry, 68(15): 2059-2067.
DOI:10.1016/j.phytochem.2007.05.012 |
Romero M L J, Kotaki Y, Lundholm N, Thoha H, Ogawa H, Relox J R, Terada R, Takeda S, Takata Y, Haraguchi K, Endo T, Lim P T, Kodama M, Fukuyo Y. 2011. Unique amnesic shellfish toxin composition found in the South East Asian diatom Nitzschia navis-varingica. Harmful Algae, 10(5): 456-462.
DOI:10.1016/j.hal.2011.02.006 |
Sansone C, Braca A, Ercolesi E, Romano G, Palumbo A, Casotti R, Francone M, Ianora A. 2014. Diatom-derived polyunsaturated aldehydes activate cell death in human cancer cell lines but not normal cells. PLoS One, 9(7): e10122.
|
Sasaki M, Takeda N, Fuwa H, Watanabe R, Satake M, Oshima Y. 2006. Synthesis of the JK/LM-ring model of prymnesins, potent hemolytic and ichthyotoxic polycyclic ethers isolated from the red tide alga Prymnesium parvum:confirmation of the relative configuration of the K/L-ring juncture. Tetrahedron Letters, 47(32): 5687-5691.
DOI:10.1016/j.tetlet.2006.06.032 |
Shahraki J, Motallebi A, Aghvami M, Pourahmad J. 2013. Ichthyotoxic Cochlodinium polykrikoides induces mitochondrial mediated oxidative stress and apoptosis in rat liver hepatocytes. Iranian Journal of Pharmaceutical Research, 12(4): 829-844.
|
Sun K, Qiu Z F, He Y J, Fan W, Wei Z X. 2017. Vertical development of a Prorocentrum donghaiense bloom in the coastal waters of the East China Sea:coupled biophysical numerical modeling. Acta Oceanologica Sinica, 36(6): 23-33.
DOI:10.1007/s13131-016-0965-z |
Tang Y Z, Gobler C J. 2009. Characterization of the toxicity of Cochlodinium polykrikoides isolates from Northeast US estuaries to finfish and shellfish. Harmful Algae, 8(3): 454-462.
DOI:10.1016/j.hal.2008.10.001 |
Tang Y Z, Gobler C J. 2012. Lethal effects of Northwest Atlantic Ocean isolates of the dinoflagellate, Scrippsiella trochoidea, on Eastern oyster (Crassostrea virginica) and Northern quahog (Mercenaria mercenaria) larvae. Marine Biology, 159(1): 199-210.
DOI:10.1007/s00227-011-1800-x |
Tang Y Z, Kong L S, Holmes M J. 2007. Dinoflagellate Alexandrium leei (Dinophyceae) from Singapore coastal waters produces a water-soluble ichthyotoxin. Marine Biology, 150(4): 541-549.
|
Taroncher-Oldenburg G, Kulis D M, Anderson D M. 1997. Toxin variability during the cell cycle of the dinoflagellate Alexandrium fundyense. Limnology and Oceanography, 42(5): 1178-1188.
|
Taylor R L, Abrahamsson K, Godhe A, Wängberg S Å. 2009. Seasonal variability in polyunsaturated aldehyde production potential among strains of Skeletonema marinoi (Bacillariophyceae). Journal of Phycology, 45(1): 46-53.
DOI:10.1111/j.1529-8817.2008.00625.x |
Twiner M J, Rehmann N, Hess P, Doucette G J. 2008. Azaspiracid shellfish poisoning:a review on the chemistry, ecology, and toxicology with an emphasis on human health impacts. Marine Drugs, 6(2): 39-72.
DOI:10.3390/md6020039 |
US Environmental Protection Agency. 1998. EPA Method 556 Determination of carbonyl compounds in drinking water by pentafluorobenzylhidroxylamine derivatization and capillary gas chromatography with electron capture detection. EPA, Cincinatti, OH.
|
Usup G, Kulis D M, Anderson D M. 1994. Growth and toxin production of the toxic dinoflagellate Pyrodinium bahamense var. compressum in laboratory cultures. Natural Toxins, 2(5): 254-262.
DOI:10.1002/nt.2620020503 |
Van Wagoner R M, Deeds J R, Tatters A O, Place A R, Tomas C R, Wright J L C. 2010. Structure and relative potency of several karlotoxins from Karlodinium veneficum. Journal of Natural Products, 73(8): 1360-1365.
DOI:10.1021/np100158r |
Vardi A, Formiggini F, Casotti R, De Martino A, Ribalet F, Miralto A, Bowler C. 2006. A stress surveillance system based on calcium and nitric oxide in marine diatoms. PLoS Biology, 4(3): e60.
DOI:10.1371/journal.pbio.0040060 |
Varrella S, Romano G, Ianora A, Bentley M G, Ruocco N, Costantini M. 2014. Molecular response to toxic diatomderived aldehydes in the sea urchin Paracentrotus lividus. Marine Drugs, 12(4): 2089-2113.
DOI:10.3390/md12042089 |
Vidoudez C, Casotti R, Bastianini M, Pohnert G. 2011a. Quantification of dissolved and particulate polyunsaturated aldehydes in the Adriatic Sea. Marine Drugs, 9(4): 500-513.
DOI:10.3390/md9040500 |
Vidoudez C, Nejstgaard J C, Jakobsen H H, Pohnert G. 2011b. Dynamics of dissolved and particulate polyunsaturated aldehydes in Mesocosms inoculated with different densities of the diatom Skeletonema marinoi. Marine Drugs, 9(3): 345-358.
DOI:10.3390/md9030345 |
Vidoudez C, Pohnert G. 2008. Growth phase-specific release of polyunsaturated aldehydes by the diatom Skeletonema marinoi. Journal of Plankton Research, 30(11): 1305-1313.
DOI:10.1093/plankt/fbn085 |
Wendel T, Jüttner F. 1996. Lipoxygenase-mediated formation of hydrocarbons and unsaturated aldehydes in freshwater diatoms. Phytochemistry, 41(6): 1445-1449.
DOI:10.1016/0031-9422(95)00828-4 |
Wichard T, Poulet S A, Boulesteix A L, Ledoux J B, Lebreton B, Marchetti J, Pohnert G. 2008. Influence of diatoms on copepod reproduction. Ⅱ. Uncorrelated effects of diatomderived α, β, γ, δ-unsaturated aldehydes and polyunsaturated fatty acids on Calanus helgolandicus in the field. Progress in Oceanography, 77: 30-44.
DOI:10.1016/j.pocean.2008.03.002 |
Wichard T, Poulet S A, Halsband-Lenk C, Albaina A, Harris R, Liu D Y, Pohnert G. 2005a. Survey of the chemical defence potential of diatoms:screening of fifty species for α,β,γ,δ-unsaturated aldehydes. Journal of Chemical Ecology, 31(4): 949-958.
DOI:10.1007/s10886-005-3615-z |
Wichard T, Poulet S A, Pohnert G. 2005b. Determination and quantification of α,β,γ,δ-unsaturated aldehydes as pentafluorobenzyl-oxime derivates in diatom cultures and natural phytoplankton populations:application in marine field studies. Journal of Chromatography B, 814(1): 155-161.
DOI:10.1016/j.jchromb.2004.10.021 |
Wu N, Jiang T J, Jiang T. 2012. Analyses of hemolytic toxin from ichthyotoxic phytoplankton Chattonella marina(Hong Kong Strain) by high performance liquid chromatography. Chinese Journal of Analytical Chemistry, 40(8): 1181-1186.
(in Chinese with English abstract) |
Xu N, Wang M, Tang Y Z, Zhang Q, Duan S, Gobler C J. 2017. Acute toxicity of the cosmopolitan bloom-forming dinoflagellate Akashiwo sanguinea to finfish, shellfish, and zooplankton. Aquatic Microbial Ecology, 80: 209-222.
DOI:10.3354/ame01846 |
Yates B S, Rogers W J. 2011. Atrazine selects for ichthyotoxic Prymnesium parvum, a possible explanation for golden algae blooms in lakes of Texas, USA. Ecotoxicology, 20(8): 2003-2010.
DOI:10.1007/s10646-011-0742-x |
Zhang G T, Sun S. 2004. Effects of diatoms on reproduction and growth of marine copepods. Progress in Natural Science, 14(10): 841-847.
DOI:10.1080/10020070412331344431 |
Zubía E, Gavagnin M, Crispino A, Martinez E, Ortea J, Cimino G. 1993. Diasteroisomeric ichthyotoxic acylglycerols from the dorsum of two geographically distinct populations of Archidoris nudibranchs. Experientia, 49(3): 268-271.
DOI:10.1007/BF01923538 |
 2019, Vol. 37
2019, Vol. 37