Institute of Oceanology, Chinese Academy of Sciences
Article Information
- SONG Minpeng, WANG Jinhai, ZHENG Xiaodong
- Prey preference of the common long-armed octopus Octopus minor (Cephalopoda: Octopodidae) on three different species of bivalves
- Journal of Oceanology and Limnology, 37(5): 1595-1603
- http://dx.doi.org/10.1007/s00343-019-8217-7
Article History
- Received Aug. 20, 2018
- accepted in principle Oct. 8, 2018
- accepted for publication Dec. 13, 2018
2 Institute of Evolution and Marine Biodiversity, Ocean University of China, Qingdao 266003, China
Predators and prey are important components of ecological communities. There is a series of established relationships between them and predation is one of the most important interactions (Hu et al., 2016). Predators often hunt different prey in their natural surroundings. Under conditions of multiple prey availability, however, predators have many prey preferences (Ambrose, 1984; Wong and Barbeau, 2005). A thorough understanding of prey preferences by predators is essential to understand the ecological significance of predation. A predator's selection of prey is affected by many factors, including detectability, accessibility, ease of capture, prey energy content, prey handling time and the time required for non-predatory behaviors (Hughes and Dunkin, 1984). Therefore, it is necessary to use the optimal foraging theory in order to model and predict this decision made by animals during foraging (McQuaid, 1994). To date, optimal strategies have been established by which predators select prey to maximize the net rate of energy per unit foraging time (Schoener, 1971; Hughes, 1980). Although cephalopods are common predators, there are limited information on their individual predation strategies (Ambrose, 1984; Iribarne et al., 1991).
Octopuses are important predators in the habitats they occupy. They have a higher metabolic rate than many other benthic predators, suggesting that they may be consuming more prey per unit time than most other predators in their habitat (Seibel and Drazen, 2007; Onthank and Cowles, 2011). They are generalist predators with behavioral and morphologic adaptations that enable them to search, capture and handle different types of prey (Portela et al., 2014); they can also be further named "switching predators" (Vincent et al., 1998) that vary their diets based on the abundance of prey and as a result, can stabilize prey populations (Murdoch, 1969).
Octopus minor is widely distributed on the coast of China, the Korean Peninsula, and the coast of Japan (Yamamoto, 1942; Dong, 1988). In recent years, its capture has decreased rapidly (Kim et al., 2008), and, as a dominant species in aquaculture, many researchers have carried out in-depth studies of its histology (Iwakoshi et al., 2000), physiology (Seol et al., 2007), nutritionology (Qian et al., 2010), embryonic development (Kim and Kim, 2006; Qian et al., 2016), breeding (Zheng et al., 2014) and genetics (Bo et al., 2016; Gao et al., 2016; Xu et al., 2018). However, to date, there is no publication in the literature on its prey selection.
It is well known that octopods prefer to ingest a wide variety of crustaceans and mollusks, and when offered, tend to hold as many crabs as possible (Guerra, 1978; Hartwick et al., 1981; Hanlon and Messenger, 1996). During octopus's artificial breeding, however, crabs are more difficult to obtain than bivalve mollusks. Therefore, it is of practical value to understand the bivalve prey selection of O. minor. In this study, we fed O. minor with three species of bivalves, namely, Ruditapes philippinarum, Mactra chinensis, and Mytilus galloprovincialis and we observed and analyzed the selection of prey under laboratory conditions.
Here we address the following questions. Of the three prey bivalves provided, which of these species do O. minor show a preference for? Do these prey preferences by O. minor follow the optimal Foraging Theory and if so, what are the possible driving forces that predicate these choices? The answers to these questions will ultimately provide additional insights into understanding the prey selection process of O. minor.
2 MATERIAL AND METHOD 2.1 Experimental materialThis investigation was conducted at the Ocean University of China, Shandong, China, from October to November 2017. All experimental animals (O. minor, R. philippinarum, M. chinensis and M. galloprovincialis) were obtained from the local sea. Fifty active, undamaged O. minor (mix of gender) were collected from the Moon Lake and separated in two rectangular cement pools (10 m×1 m×0.6 m) containing PVC pipes shelters (diameter, 90 mm; length, 700 mm), with open water circulation (temperature, 17.4–20.5℃; salinity, 29–30) and a water depth of 50 cm to maintain oxygenation. R. philippinarum, M. chinensis and M. galloprovincialis were individually maintained in a similar tank until they were used. During the acclimation period, O. minor were fed with fresh Manila clams which were collected from the local sea. Only healthy animals were used for the experiments and the biological indices of the experimental animals was shown in Table 1.
Predation rates and prey preferences were examined in single-prey (no choice), two-prey (choice) and three-prey (choice) experiments. These experiments were conducted in a white cuboid plastic tank (70 cm×60 cm×60 cm), a water depth of 50 cm, a water temperature of 18.0±1.0℃, a salinity of 29– 30 and a photoperiod of 14 h light and 10 h dark. Two days prior to the experiments, a single O. minor was randomly selected and transferred to the tank to adapt. On a daily basis with prior cleaning of the sundries, 50% of the water volume was changed at 5:00 pm, with prior cleaning of the sundries. The prey were randomly offered at the bottom of the tank at 5:00 pm on the first day, and the consumed prey was collected and replaced with the same species at 7:00 am and 5:00 pm on the next day.
To determine the predation rates, single-prey experiments were implemented. In the single-prey experiment, 12 individuals of each prey species (R. philippinarum, M. chinensis or M. galloprovincialis) were offered to one octopus. The predator-prey grouping are designated as OP, OC and OG, respectively. Three octopuses were selected to repeat this treatment and lasted for 5 days (Table 2). To examine prey selection, experiments with two and three different prey were designed. In the two-prey experiment, prey were provided in three treatment combinations as follows: six R. philippinarum plus six M. chinensis, six R. philippinarum plus six M. galloprovincialis, and six M. chinensis plus six M. galloprovincialis, represented by OPC, OPG, and OCG, respectively. Three octopuses were selected to repeat this treatment and lasted for 5 days (Table 2). In the three-prey experiment, one octopus was provided with four R. philippinarum, four M. chinensis, and four M. galloprovincialis, represented by OPCG and this experiment was repeated three times and lasted 5 days (Table 2).
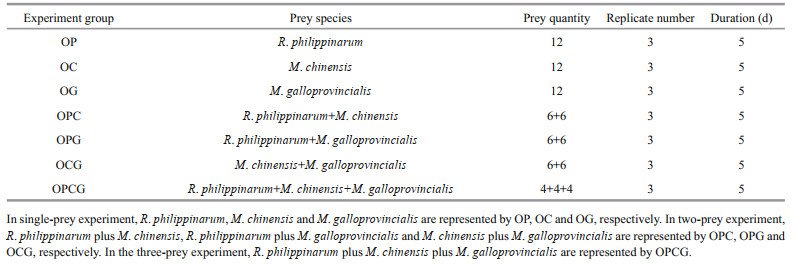
In the single-prey experiment, the number of prey consumed per day by O. minor was calculated by the predation rate of each replicate. In the two- or threeprey experiments, the selection index was calculated by:
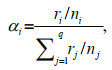 (1)
(1)where α was the selection index, i and j were the prey types, r was the number of prey consumed by the predator, n was the total number of prey in the tank, and q was the total number of prey types in the experiment (Chesson, 1978).
In the two-prey experiment (choice), the number of each prey type consumed was treated as the observed frequency. In the single-prey experiment (no choice), the number of prey consumed was treated as the expected frequency. To analyze the selection of prey, we examined the active selection by comparing the observed frequencies with the expected frequencies using the χ2 test with Yate's correction for continuity (Liszka and Underwood, 1990; Zar, 1996). The expected frequencies were calculated by the following equations:
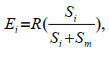 (2)
(2)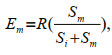 (3)
(3)where Ei and Em were the expected number of prey type i and prey type m consumed, respectively, R was the number of prey type i plus the number of prey type m consumed when presented together, and Si and Sm were the number of prey type i and prey type m consumed under single-prey conditions (Liszka and Underwood, 1990).
2.3.2 Characteristics data of the preyTo further understand the prey selection of the predators, we evaluated the characteristics of the prey by including the prey energy content, handling time, prey profitability and prey adductor muscle tension. To measure the energy content per prey, R. philippinarum, M. chinensis and M. galloprovincialis (n=10 each) soft tissues were dried for 48 h at 80℃. The average dry weight per prey specimen was equal to the total dry weight divided by the number of prey specimens. The average energy per unit dry weight was measured using an oxygen bomb calorimeter (Parr 6400). The energy content per prey specimen was equal to the average dry tissue weight multiplied by the mean energy content per unit dry weight. The handling time was calculated as the total time spent by the predator to capture and consume a single prey, from the encounter to the end of consumption. During the single-prey experiment, the handling time of prey was recorded by videos during the daytime with a camera (Brinno TLC-200), which was placed at a height of 1.5 m above the water surface. The prey profitability was equal to the average energy content divided by the average handling time per prey (Stephens and Krebs, 1986). The entry techniques used by O. minor to consume bivalves which had been eaten were judged by the integrity of the shells. The pulling force, which was used to separate the bivalve shells, was measured with a "clam rack" (Anderson and Mather, 2007) that employed rubber suction cups to adhere to the center of each shell after washing and drying. One rubber suction cup was fixed to the scale, and the other one to the clam rack platform. The tension was increased until a gape (approximately 1 mm) was visible at the ventral margin of the valves and the resulting pull tension data was recorded.
2.4 Statistical analysisStatistical analysis of data was performed using IBM SPSS Statistics Software. An independent sample t-test was used to analyze the selection index. Other predation and selectivity data, as well as the prey characteristics data, were analyzed using one way analysis of variance (ANOVA). Levene's test was used to test the assumption of homogeneity of variances. When this assumption was violated, Square root transformation was performed to obtain homogeneity of variance. For multiple comparisons, Tukey's test was used to compare the means. All means were presented±standard deviation, and statistical significance was defined as P < 0.05.
3 RESULT 3.1 Predation and selectivity data 3.1.1 Predation rateIn single-prey experiments, the predation rates (Fig. 1) of O. minor on R. philippinarum, M. chinensis and M. galloprovincialis were 1.73±0.50, 1.27±0.42 and 0.8±0.2 per day, respectively. There was a significant difference (ANOVA, F=8.65, P=0.02) among R. philippinarum, M. chinensis and M. galloprovincialis consumed by O. minor. The predation rate on R. philippinarum was significantly higher (Tukey, P=0.01) than that on M. galloprovincialis, but there was no significant difference (Tukey, P>0.05) in the predation rates between M. chinensis and R. philippinarum or M. galloprovincialis.
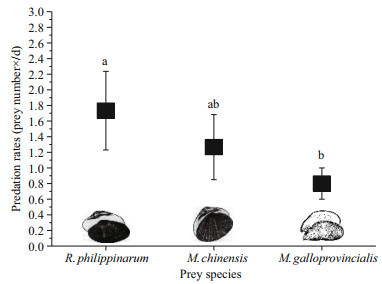
|
| Fig.1 The average (±SD) predation rates of O. minor on R. philippinarum, M. chinensis and M. galloprovincialis Different letters indicate significant differences (Tukey, P<0.05) |
In experiments performed with different prey combinations, the selectivity index was estimated (Fig. 2). In the OPC combination group, the selectivity index for R. philippinarum (0.84±0.30) was significantly higher (t-test, F=33.048, P < 0.000 1) than that for M. chinensis (0.16±0.30). In the OPG and OCG combination group, O. minor only consumed R. philippinarum or M. chinensis, respectively. In the OPCG combination group, where three different prey were provided at the same time. O. minor did not consume M. galloprovincialis, and the selectivity index for R. philippinarum (0.78±0.29) was significantly higher (ANOVA, F=26.923, P < 0.001) than that for M. chinensis (0.22±0.29). Consequently, O. minor preferred R. philippinarum as their first choice of prey, followed by M. chinensis and M. galloprovincialis, a distant third.
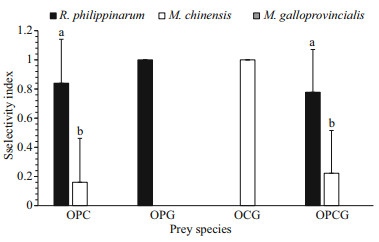
|
| Fig.2 The prey selectivity index of O. minor (O) in the presence of different R. philippinarum (P), M. chinensis (C), and M. galloprovincialis (G) prey species combinations Values in the same group with different letters indicate significant differences (P<0.05). |
In the two-prey experiments, the number of consumed prey differed significantly than the expected number calculated from the single-prey experiment (Table 3). The octopuses preferred one type of prey when provided with a choice. In the OPC and OPG combination groups, O. minor preferentially selected R. philippinarum over the other two bivalves. In the OCG combination group, O. minor actively selected M. chinensis than M. galloprovincialis. When R. philippinarum were provided together with M. chinensis or M. galloprovincialis, O. minor actively selected R. philippinarum. In the presence of M. chinensis and M. galloprovincialis, O. minor actively selected M. chinensis.
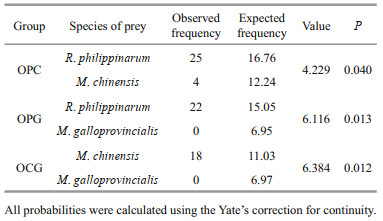
|
Regarding the handling time (Fig. 3), there was a significant difference (ANOVA, F=8.976, P=0.001) among R. philippinarum, M. chinensis and M. galloprovincialis. O. minor spent 0.22±0.05 h to handle R. philippinarum, which was significantly lower (Tukey, P < 0.000 1) than that of M. galloprovincialis (0.31±0.06 h). O. minor spent 0.26±0.04 h to handle M. chinensis, which was not significantly different (Tukey, P>0.05) from R. philippinarum or M. galloprovincialis. The order of the handling time for the three different preys was as follows: M. galloprovincialis > M. chinensis > R. philippinarum.
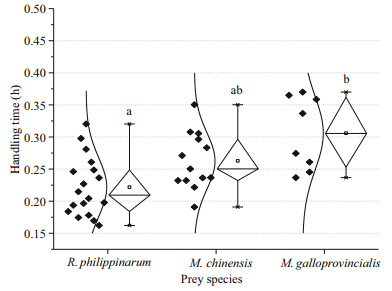
|
| Fig.3 Handling time of O. minor on R. philippinarum, M. chinensis and M. galloprovincialis Different letters indicate significant differences (P<0.05). Box-plot presenting average, median, 25 and 75 percentiles, minimum and maximum time taken to handle the three types of prey by octopuses, black diamonds indicate an individual prey item. |
For all bivalve prey species, O. minor consumed them by pulling apart the shells. The pulling force used by O. minor to separate R. philippinarum, M. chinensis and M. galloprovincialis was 24.84±3.67, 35.22±6.48 and 17.80±2.33 N, respectively (Fig. 4). There were significant differences (ANOVA, F=30.26, P < 0.000 1) in the strength used by O. minor to open the three different prey. According to the rankings (Fig. 4), the bivalves are more easily opened from M. galloprovincialis to R. philippinarum to M. chinensis. The energy contents (Fig. 4) were significantly different (ANOVA, F=1 313.2, P < 0.000 1) among the three different preys. The energy content of R. philippinarum (5.94±0.13 KJ) was significantly higher (Tukey, P < 0.000 1) than that of M. chinensis (4.45±0.11 KJ), which, in turn was significantly higher (Tukey, P < 0.000 1) than that of M. galloprovincialis (3.22±0.12 KJ). As for the profitability, there were significant differences (ANOVA, F=3 030.7, P < 0.000 1) among the three different preys. The profitability of R. philippinarum (26.78±0.60 KJ/(prey·h)) was significantly higher than that of M. chinensis (16.95±0.40 KJ/(prey·h)) and M. galloprovincialis (10.52±0.39 KJ/(prey·h)). In summary, both the energy content and profitability of R. philippinarum were the highest relative to the other two prey species.
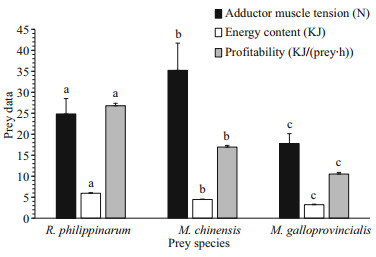
|
| Fig.4 The average (±SD) of adductor muscle tension, energy content and profitability of R. philippinarum, M. chinensis and M. galloprovincialis Values in the same group with a different letter indicate significant differences (Tukey, P<0.05). |
A predator's diet consists of many prey species, but each predator has a preference for a specific prey. For instance, O. bimaculatus consume more than 55 prey species and Ambrose (1984) through laboratory experiments, found that octopuses prefer to consume crabs the most, In contrast, Rapana venosa (a gastropod) prefers Anadara (Scapharca) inaequivalvis when M. galloprovincialis, R. philippinarum and A. inaequivalvis were provided at the same time (Savini and Occhipinti-Ambrogi, 2006). In this study, O. minor consumed different prey, although they had an apparent preference for R. philippinarum. Carlsson et al. (2009) and Robinson et al. (2015) suggests that predators may avoid consuming new species of prey due to unfamiliarity. In this research, we found that R. philippinarum is abundant in the moon lake where experimental octopuses were collected, but M. chinensis and M. galloprovincialis could not be found. We speculated that O. minor's high preference for R. philippinarum is related to its familiarity with feeding on this prey. This phenomenon is consistent with a high preference for native mussels Semimytilus algosus, when Jasus lalandii (a lobster) and Marthasterias africanaa (a starfish) are offered with familiar native mussels S. algosus and unfamiliar invading mussels M. galloprovincialis as prey (Skein et al., 2018).
When bivalve preys were presented to octopods, there were two entry methods to open the prey: "drilling" or "pulling" (Anderson and Mather, 2007). In general, the octopods first try to use a quick method requiring enormous energy, that is, pulling the prey apart by the suckers on its arms (Fiorito and Gherardi, 1999). If this method is not successful, the octopuses drill holes into the shell with the help of salivary papilla, which involves little energy but is time consuming (Nixon, 1980; Casey, 1999). In this experiment, we collected the shells of bivalves consumed by O. minor and found that all the shells were intact without any evidence of drill holes, suggesting that O. minor only used the former method. These results are different from those of O. dierythraeus and Enteroctopus dofleini that consume bivalves using these two entry methods (Steer and Semmens, 2003; Anderson and Mather, 2007). McQuaid (1994) found that when O. vulgaris consume different sizes of brown mussel, Perna perna, they adopt different entry methods, that is, they adopt the pulling entry method to consume small sizes of mussels and they utilize the drilling entry method to consume large mussels (McQuaid, 1994). We speculate that in this experiment, O. minor is much larger than the bivalves, and it has enough strength to pull the bivalves open. However, further experimental research on larger bivalves is required to determine whether O. minor can use other entry methods.
Active prey selection was studied because O. minor always selected R. philippinarum when offered a choice of prey species (Table 3). There are many factors that influence the prey selection of the predator, but the most important index to affect prey selection is the energy intake rate (Pyke et al., 1977; Pyke, 1984). This means that the predator will consume the higher profitability prey and ignore the lower profitability prey (Wong and Barbeau, 2005). In this study, we collected and analyzed the profitability for O. minor of three species of bivalves (Fig. 4). We found that the order of profitability, from highest to lowest, was R. philippinarum, M. chinensis and M. galloprovincialis. These results were consistent with the prey selection of octopuses, and the prey selection of O. minor is consistent with the optimal foraging theory. In marine invertebrates, "time minimization" has been clearly demonstrated to be an efficient predation strategy (Hughes and Seed, 1981; Leite et al., 2009), which can optimize foraging and reduce handling time, thereby decreasing its exposure to other predators and risks (Mascaró and Seed, 2001). Handling time was measured in this study (Fig. 3) and the relationship between handling time and prey preference was determined; high preference prey had a short handling time and low preference prey had a long handling time and these results are consistent with the "time minimization" strategy. However, these results were different from Hu et al. (2016) who studied R. venosa (a gastropod). In this case, high prey preference had long handling time and low prey preference had the shorter handling time such that, there was no obvious relationship between the prey preference and the handling time. This study, we also analyzed the relationship between prey selection and adductor muscle tension, and found that these results were not consistent with prey preference; M. galloprovincialis were most easily opened, but O. minor had the lowest preference for it. This suggests that the difficulty of consumption was not the main reason for the prey preference of O. minor in this study. In contrast to these results, the Cancer crab species refused clams (Protothaca staminea) because of predation failure (Boulding, 1984). We concluded that the pulling force required to open the three different bivalves was significantly different, but O. minor can provide enough pulling force to open them all. Therefore, the adductor muscle tension of bivalves did not affect the prey selection of O. minor.
5 CONCLUSIONIn this study, we provided O. minor with three different bivalves. We observed and analyzed the prey preferences and found that O. minor preferred R. philippinarum, followed by M. chinensis and M. galloprovincialis. Moreover, the prey preference was consistent with the prey's profitability and handling time, regardless of the adductor muscle tension. Several studies (Nixon, 1980; Casey, 1999; Fiorito and Gherardi, 1999; Anderson and Mather, 2007) have reported that octopods use two different methods (drilling and pulling) to open bivalves; however, we found that O. minor only used the pulling entry method.
6 DATA AVAILABILITY STATEMENTAll data included in this research are available from the corresponding author on reasonable request.
7 ACKNOWLEDGMENTWe thank Dr. Diego Orol Gómez, Dr. YU Zhenlin, Master HU Nan and Master XIN Xiaoke for their valuable advice and suggestion, and Mr. CAI Bing, Mr. CAI Hui for the sample collection. We also thank three reviewers for their useful ideas and criticism.
Ambrose R F. 1984. Food preferences, prey availability, and the diet of Octopus bimaculatus Verrill. Journal of Experimental Marine Biology and Ecology, 77(1-2): 29-44.
DOI:10.1016/0022-0981(84)90049-2 |
Anderson R C, Mather J A. 2007. The packaging problem:bivalve prey selection and prey entry techniques of the octopus Enteroctopus dofleini. Journal of Comparative Psychology, 121(3): 300-305.
DOI:10.1037/0735-7036.121.3.300 |
Bo Q K, Zheng X D, Gao X L, Li Q. 2016. Multiple paternity in the common long-armed octopus Octopus minor(Sasaki, 1920) (Cephalopoda:Octopoda) as revealed by microsatellite DNA analysis. Marine Ecology, 37(5): 1073-1078.
DOI:10.1111/maec.12364 |
Boulding E G. 1984. Crab-resistant features of shells of burrowing bivalves:decreasing vulnerability by increasing handling time. Journal of Experimental Marine Biology and Ecology, 76(3): 201-223.
DOI:10.1016/0022-0981(84)90189-8 |
Carlsson N O L, Sarnelle O, Strayer D L. 2009. Native predators and exotic prey-an acquired taste?. Frontiers in Ecology and the Environment, 7(10): 525-532.
DOI:10.1890/080093 |
Casey E. 1999. Intelligent predation by the California two-spot octopus. The Festivus, 31(2): 21-24.
|
Chesson J. 1978. Measuring preference in selective predation. Ecology, 59(2): 211-215.
DOI:10.2307/1936364 |
Dong Z Z. 1998. Chinese Journal of Animal (Mollusca:Cephalopoda). Science Press, Beijing, China. p.181-182.
(in Chinese)
|
Fiorito G, Gherardi F. 1999. Prey-handling behaviour of Octopus vulgaris (Mollusca, Cephalopoda) on bivalve preys. Behavioural Processes, 46(1): 75-88.
DOI:10.1016/S0376-6357(99)00020-0 |
Gao X L, Zheng X D, Bo Q K, Li Q. 2016. Population genetics of the common long-armed octopus Octopus minor(Sasaki, 1920) (Cephalopoda:Octopoda) in Chinese waters based on microsatellite analysis. Biochemical Systematics and Ecology, 66: 129-136.
DOI:10.1016/j.bse.2016.03.014 |
Guerra Á. 1978. Sobre la alimentación y el comportamiento alimentario de Octopus vulgaris. Investigacion Pesquera, 42(2): 351-364.
|
Hanlon R T, Messenger J B. 1996. Cephalopod Behaviour. ambridge University Press, Cambridge, New York. p.47-65.
|
Hartwick B, Tulloch L, MacDonald S. 1981. Feeding and growth of Octopus dofleini (Wulker). The Veliger, 24(2): 129-138.
|
Hu N, Wang F, Zhang T, Song H, Yu Z L, Liu D P. 2016. Prey selection and foraging behavior of the whelk Rapana venosa. Marine Biology, 163(11): 233.
DOI:10.1007/s00227-016-3006-8 |
Hughes R N, Dunkin S D B. 1984. Behavioural components of prey selection by dogwhelks, Nucella lapillus (L.), feeding on mussels, Mytilus edulis L., in the laboratory. Journal of Experimental Marine Biology and Ecology, 77(1-2): 45-68.
DOI:10.1016/0022-0981(84)90050-9 |
Hughes R N, Seed R. 1981. Size selection of mussels by the blue crab Callinectes sapidus:energy maximizer or time minimizer?. Marine Ecology Progress Series, 6(1): 83-89.
|
Hughes R N. 1980. Optimal foraging theory in the marine context. Oceanography and Marine Biology-An Annual Review, 18: 423-481.
|
Iribarne O O, Fernandez M E, Zucchini H. 1991. Prey selection by the small Patagonian octopus Octopus tehuelchus d'Orbigny. Journal of Experimental Marine Biology and Ecology, 148(2): 271-282.
|
Iwakoshi E, Hisada M, Minakata H. 2000. Cardioactive peptides isolated from the brain of a Japanese octopus, Octopus minor. Peptides, 21(5): 623-630.
DOI:10.1016/S0196-9781(00)00201-1 |
Kim D H, An H C, Lee K H, Hwang J W. 2008. Optimal economic fishing efforts in Korean common octopus Octopus minor trap fishery. Fisheries Science, 74(6): 1215-1221.
DOI:10.1111/j.1444-2906.2008.01645.x |
Kim D S, Kim J M. 2006. Sexual maturity and growth characteristics of Octopus minor. Korean Journal of Fisheries and Aquatic Sciences, 39(5): 410-418.
DOI:10.5657/kfas.2006.39.5.410 |
Leite T S, Haimovici M, Mather J. 2009. Octopus insularis(Octopodidae), evidences of a specialized predator and a time-minimizing hunter. Marine Biology, 156(11): 2355-2367.
DOI:10.1007/s00227-009-1264-4 |
Liszka D, Underwood A J. 1990. An experimental design to determine preferences for gastropod shells by a hermitcrab. Journal of Experimental Marine Biology and Ecology, 137(1): 47-62.
DOI:10.1016/0022-0981(90)90059-L |
Mascaró M, Seed R. 2001. Foraging behavior of juvenile Carcinus maenas (L.) and Cancer pagurus L. Marine Biology, 139(6): 1135-1145.
DOI:10.1007/s002270100677 |
McQuaid C D. 1994. Feeding behaviour and selection of bivalve prey by Octopus vulgaris Cuvier. Journal of Experimental Marine Biology and Ecology, 177(2): 187-202.
DOI:10.1016/0022-0981(94)90236-4 |
Murdoch W W. 1969. Switching in general predators:experiments on predator specificity and stability of prey populations. Ecological Monographs, 39(4): 335-354.
DOI:10.2307/1942352 |
Nixon M. 1980. The salivary papilla of Octopus as an accessory radula for drilling shells. Journal of Zoology, 190(1): 53-57.
|
Onthank K L, Cowles D L. 2011. Prey selection in Octopus rubescens:possible roles of energy budgeting and prey nutritional composition. Marine Biology, 158(12): 2795-2804.
DOI:10.1007/s00227-011-1778-4 |
Portela E, Simões N, Rosas C, Mascaró M. 2014. Can preference for crabs in juvenile Octopus maya be modified through early experience with alternative prey?. Behaviour, 151(11): 1597-1616.
DOI:10.1163/1568539X-00003206 |
Pyke G H, Pulliam H R, Charnov E L. 1977. Optimal foraging:a selective review of theory and tests. The Quarterly Review of Biology, 52(2): 137-154.
|
Pyke G H. 1984. Optimal foraging theory:a critical review. Annual Review of Ecology and Systematics, 15: 523-575.
DOI:10.1146/annurev.es.15.110184.002515 |
Qian Y S, Zheng X D, Wang P, Li Q. 2010. Analysis and evaluation of nutritive composition of Octopus minor in Lake Swan. Marine Sciences, 34(12): 14-18.
(in Chinese with English abstract) |
Qian Y S, Zheng X D, Wang W J, Yang J M, Li Q. 2016. Ultrastructure of spermatozoa and spermatogenesis in Octopus minor (Sasaki, 1920) (Cephalopoda:Octopoda). Journal of Natural History, 50(31-32): 2037-2047.
DOI:10.1080/00222933.2016.1184343 |
Robinson T B, Pope H P, Hawken L, Binneman C. 2015. Predation-driven biotic resistance fails to restrict the spread of a sessile rocky shore invader. Marine Ecology Progress Series, 522: 169-179.
DOI:10.3354/meps11167 |
Savini D, Occhipinti-Ambrogi A. 2006. Consumption rates and prey preference of the invasive gastropod Rapana venosa in the Northern Adriatic Sea. Helgoland Marine Research, 60(2): 153-159.
DOI:10.1007/s10152-006-0029-4 |
Schoener T W. 1971. Theory of feeding strategies. Annual Review of Ecology and Systematics, 2: 369-404.
DOI:10.1146/annurev.es.02.110171.002101 |
Seibel B A, Drazen J C. 2007. The rate of metabolism in marine animals:environmental constraints, ecological demands and energetic opportunities. Philosophical Transactions of the Royal Society B:Biological Sciences, 362(1487): 2061-2078.
DOI:10.1098/rstb.2007.2101 |
Seol D W, Lee J, Im S Y, Park I S. 2007. Clove oil as an anaesthetic for common octopus (Octopus minor, Sasaki). Aquaculture Research, 38(1): 45-49.
|
Skein L, Robinson T B, Alexander M E. 2018. Impacts of mussel invasions on the prey preference of two native predators. Behavioral Ecology, 29(2): 353-359.
|
Steer M A, Semmens J M. 2003. Pulling or drilling, does size or species matter? An experimental study of prey handling in Octopus dierythraeus (Norman, 1992). Journal of Experimental Marine Biology and Ecology, 290(2): 165-178.
DOI:10.1016/S0022-0981(03)00076-5 |
Stephens D W, Krebs J R. 1986. Foraging Theory. Journal of Ecology, 49(5): 247.
|
Vincent T L S, Scheel D, Hough K R. 1998. Some aspects of diet and foraging behavior of Octopus dofleini Wülker, 1910 in its Northernmost Range. Marine Ecology, 19(1): 13-29.
|
Wong M C, Barbeau M A. 2005. Prey selection and the functional response of sea stars (Asterias vulgaris Verrill)and rock crabs (Cancer irroratus Say) preying on juvenile sea scallops (Placopecten magellanicus (Gmelin)) and blue mussels (Mytilus edulis Linnaeus). Journal of Experimental Marine Biology and Ecology, 327(1): 1-21.
|
Xu R, Bo Q K, Zheng X D. 2018. A divergent lineage among Octopus minor (Sasaki, 1920) populations in the Northwest Pacific supported by DNA barcoding. Marine Biology Research, 14(4): 335-344.
DOI:10.1080/17451000.2018.1427866 |
Yamamoto T. 1942. On the ecology of Octopus variabilis typicus (Sasaki), with special reference to its breading habits. The Malacological Society of Japan, 12(1-2): 9-20.
|
Zar J H. 1996. Biostatistical Analysis. 3rd ed. Prentice-Hall Inc, Englewood Cliffs NJ, USA.
|
Zheng X D, Qian Y S, Liu C, Li Q. 2014. On the ecology of Octopus variabilis typicus (Sasaki), with special reference to its breading habits. Springer, Dordrecht, New York. 9-20.
DOI:10.1007/978-94-017-8648-5_22
|
 2019, Vol. 37
2019, Vol. 37