Institute of Oceanology, Chinese Academy of Sciences
Article Information
- GUO Shujin, ZHU Mingliang, ZHAO Zengxia, LIANG Junhua, ZHAO Yongfang, DU Juan, SUN Xiaoxia
- Spatial-temporal variation of phytoplankton community structure in Jiaozhou Bay, China
- Journal of Oceanology and Limnology, 37(5): 1611-1624
- http://dx.doi.org/10.1007/s00343-019-8249-z
Article History
- Received Sep. 20, 2018
- accepted in principle Nov. 13, 2018
- accepted for publication Feb. 27, 2019
2 Laboratory for Marine Ecology and Environmental Science, Pilot National Laboratory for Marine Science and Technology(Qingdao), Qingdao 266237, China;
3 Center for Ocean Mega-Science, Chinese Academy of Sciences, Qingdao 266071, China;
4 University of Chinese Academy of Sciences, Beijing 100049, China
Marine phytoplankton is responsible for approximately 50% of global productivity (Falkowski and Raven, 2007). They convert dissolved inorganic carbon into particulate organic carbon via photosynthesis and allow the carbon fixed to be transferred to higher trophic levels via zooplankton grazing, supporting the food web in waters ranging from coastal seas to open oceans (Gasol et al., 1997). The sinking export of phytoplankton cells at the termination of phytoplankton blooms is also an important pathway by which primary-produced organic carbon is transferred from the surface waters to the benthos (Turner, 2015). Therefore, spatial and temporal variability in phytoplankton community structure is of fundamental importance to aquatic environments, and it is essential to further our understanding of phytoplankton community structure for the overall ecological function of phytoplankton.
The Jiaozhou Bay, located on the western coast of the Yellow Sea (35°38′–36°18′N, 120°04′–120°23′N), is a semi-enclosed bay with an area of approximately 367 km2. The average depth is 7 m, and it is connected to the Yellow Sea via a narrow opening approximately 2.5 km wide. At least 10 small rivers with varying water and sediment loads empty into the bay, notably the Yanghe, Dagu, Moshui, Loushan, Licun, and Haibo Rivers (Liu et al., 2005). Due to the advancement of economic activities and the increase in population of Qingdao City in recent years, these rivers have become discharge trenches for industrial and domestic wastewaters (Shen, 2001; Liu et al., 2005). Therefore, Jiaozhou Bay has been eutrophicated in recent 30 years (Jiao, 2001; Shen, 2001; Han et al., 2011). Study on phytoplankton in the bay began more than 30 years ago, and the publications before 1990s are mainly taxonomic reports on phytoplankton (Li and Huang, 1956; Qian et al., 1983). Since the 1990s, studies on the phytoplankton community structure have been increasing, and provided valuable historical data of the phytoplankton community structure in the bay (Guo and Yang, 1992; Wu et al., 2004; Sun et al., 2011c; Yang et al., 2014; Zheng et al., 2014; Luo et al., 2016). However, most of the studies focused on the species composition and cell abundance of phytoplankton community. At present, studies on the seasonal variation in the phytoplankton community and its determining factors in Jiaozhou Bay are very limited. The spatial-temporal variation in the phytoplankton community structure and its relationship with the physico-chemical environmental parameters remain unclear, which is not beneficial for our comprehensive understanding of the marine ecosystem in this area. Furthermore, as a typical shallow coastal bay, the physico-chemical parameters vary greatly in season (Chen et al., 1999; Liu et al., 2005), which would have significant effects on phytoplankton community structure, and calls for comprehensive in-depth studies.
To clarify the spatial and temporal variation in the phytoplankton community structure in the Jiaozhou Bay, explore the relationship between the phytoplankton community structure and various environmental parameters in recent years, four sampling cruises once in each season were carried out in 2017. The results of this study with historical studies were compared. The species composition, cell abundance distribution, and seasonal variation were analyzed. We believe that this study can provide fundamental data for marine environmental protection and management in Jiaozhou Bay in the future.
2 MATERIAL AND METHOD 2.1 Study area and sampling stationsSampling was performed in Jiaozhou Bay once every season in 2017. Fourteen sampling stations were set deployed with the logistic help of R/V Chuangxin (Fig. 1). Each sampling was carried out for two days (May 2 to 3, August 17 to 18, November 8 to 9, and February 13 to 14). Six rivers (Dagu River, Yanghe River, Moshui River, Loushan River, Licun River, and Haibo River) were the major inputs of the anthropological wastes (Liu et al., 2005).
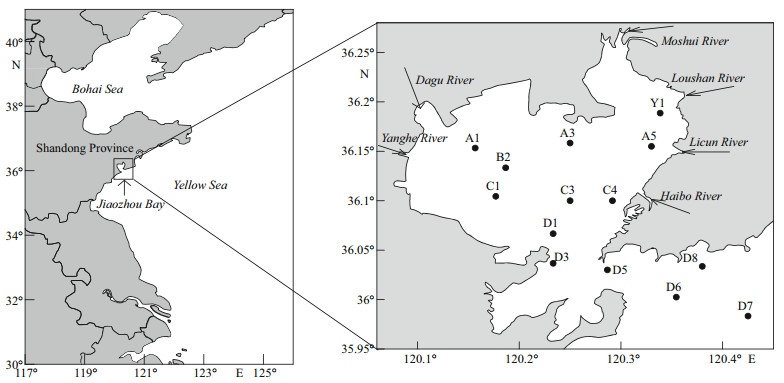
|
| Fig.1 Study area and sampling stations in Jiaozhou Bay in 2017 |
The phytoplankton samples were collected vertically from the bottom to the surface using a phytoplankton net (mesh size, 76 μm; the inner diameter of the net mouth, 37 cm; length, 140 cm). All the samples collected were fixed with 5% formalin in 500-mL plastic bottles and stored in dark. In the laboratory, the phytoplankton cells were identified and counted on a scaled slide (0.5 mL) with an Olympus BX51 microscope at 200× or 400× magnification according to Tomas and Hasle (1997).
The vertical profiles of temperature (T), salinity (S), and pH were measured using an YSI 6600 Sonde. The stratification index (I) is defined as the temperature difference between the surface and near the sea bottom to express the turbulence level of the water. The water transparency (P) was measured with a Secchi disk. The surface (0.5 m) waters were sampled with a 10-L bucket at each station to determine Chl a, nutrients and particulate matter (PM).
The chlorophyll a (Chl a) samples are filtrated through 25 mm GF/F filters (WhatmanTM) and stored in dark at -20℃ for analysis. In the laboratory, Chl a is extracted with 90% acetone at 4℃ in dark for 24 h and then measured with a Turner-Designs TrilogyTM laboratory fluorimeter (Welschmeyer, 1994).
The samples for the dissolved inorganic nutrient concentration determination are filtered through acidwashed acetate cellulose filters (0.45 μm pore size). The filtrates are stored at 0–4℃ in dark after being poisoned in HgCl2 solution. In the laboratory, the nutrients (NO3-, NO2-, PO43-, SiO32-, and NH4+) are analyzed with an autoanalyzer (model: SkalarSANplus, Skalar Analysis, the Netherlands). The dissolved inorganic nitrogen (DIN) is defined as the sum of the NO3-, NO2-, and NH4+ concentrations (Liu et al., 2005). For the particulate matter (PM) analysis, 300 mL samples are filtered by polycarbonate filters (0.45 μm pore size, DHI) that are previously desiccated and weighed. After filtration, the filters are dried for 24 h and weighed again. The difference in weight afterbefore filtration is divided by the filtered volume, and the result is the PM concentration.
2.3 Data analysisThe Shannon-Wiener diversity index (H′) (Shannon and Weaver, 1949) and dominance index (Y) are calculated, and the formulas are given below:
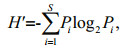
where Pi is the proportion of species i's cell abundance in the total cell abundance in a sample, and S is the species richness in the sample.
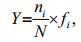
where ni is the sum of species i's cell abundances in all the samples; N is the sum of all the species cell abundances, and fi is the species i occurrence frequency in all the samples. SPSS14.0 is used to carry out the 2-tailed Pearson correlation analysis (PCA) between the phytoplankton abundance and various environmental parameters. MVSP 3.13 is used to carry out the canonical correspondence analysis (CCA) between the dominant species abundances and the environmental parameters. The comparison of the environmental variables between seasons is conducted with ANOVA followed by Duncan's multiple range test.
3 RESULT 3.1 Hydrographic conditionsThe temperature, salinity, DIN, and Chl a concentrations in the surface layer in the four seasons are presented in Fig. 2. The surface temperature was higher in the northern part than in the southern part of the bay in spring (Fig. 2a), but it was higher in the southern part than in the northern part of the bay in other three cruises (Fig. 2b–d). The surface salinity was the lowest at stations Y1 and A5 in spring, summer, and winter, indicating the evident influence of the Loushan River and the Licun River in these three seasons (Fig. 2e, f, h). The distribution pattern of the DIN concentrations was quite similar in the four seasons, with high values always appearing in the northwestern and northeastern part of the bay (Fig. 2i–l). The distribution pattern of the Chl a concentrations is consistent with that of the DIN, with higher values in the northwestern and northeastern parts of the bay in four seasons (Fig. 2m–p).

|
| Fig.2 Distribution of temperature (T, ℃) (a–d), salinity (S) (e–h), DIN (μmol/L) (i–l) and Chl a (μg/L) (m–p) in the surface layer in Jiaozhou Bay in four seasons in 2017 Black dots mark the sampling stations. |
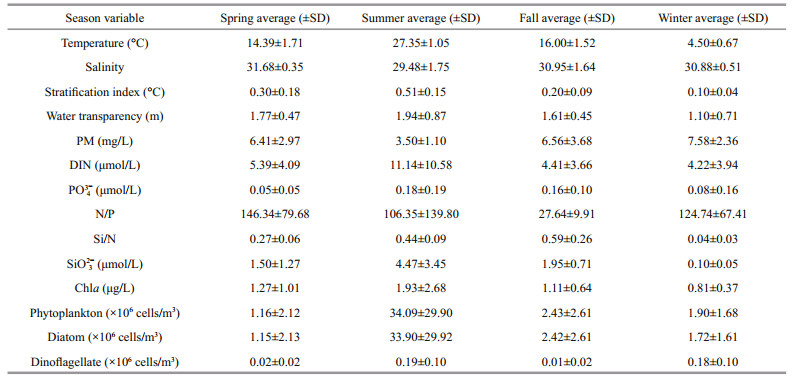
The mean values of the various environmental parameters in each cruise are shown in Table 1. The temperature was highest in summer, followed by spring and fall, and lowest in winter. The nutrient and Chl a concentrations were also highest in summer. In spring and fall, the nutrient and Chl a concentrations were lower than in summer (Duncan's multiple range test, P < 0.05). In winter, a low stratification index indicated that the water was turbulent. The water transparency was lowest and the PM concentration was highest in winter. The Chl a concentration was the lowest of the four seasons in winter. The N/P ratios were higher in spring and winter than in summer and fall (Duncan's multiple range test, P < 0.05), while the Si/N ratios were lower in spring and winter than in summer and fall (Duncan's multiple range test, P < 0.05).
|
In total, 140 taxa belonging to 3 phyla and 53 genera were identified in the four seasons in Jiaozhou Bay (Table 2). Diatoms and dinoflagellates were the dominant groups, and the species richness of the diatoms was higher than that of the dinoflagellates in all four seasons. Most of these species were neritic or cosmopolitan, and some brackish species were also observed. The most species-rich genera in Jiaozhou Bay were Chaetoceros, Coscinodiscus, Pleurosigma, and Rhizosolenia for the diatoms and Ceratium and Protoperidinium for the dinoflagellates.
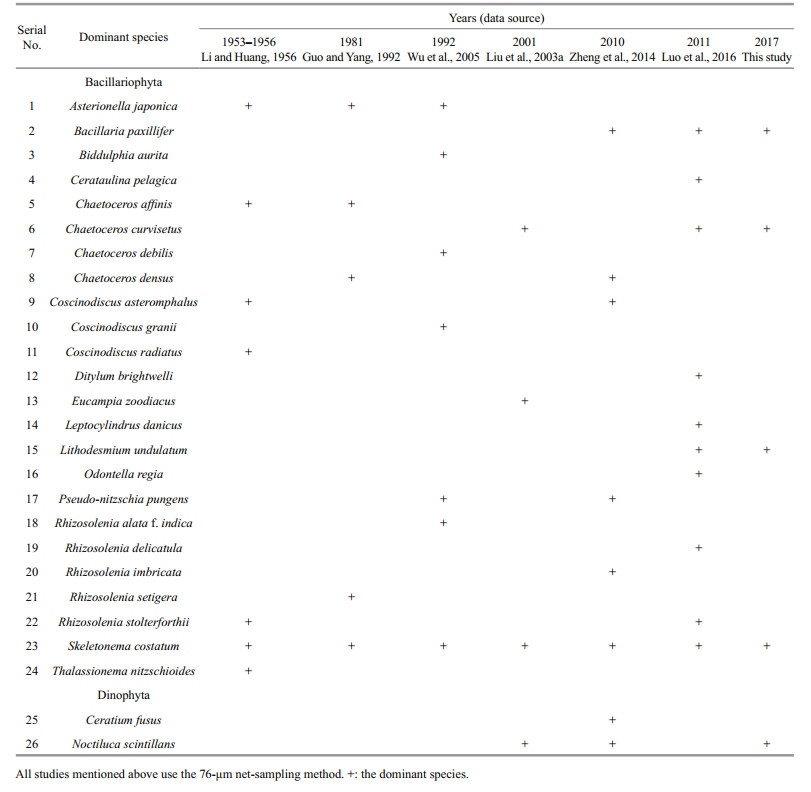
The dominant species of phytoplankton in Jiaozhou Bay were mostly diatoms in the four seasons (Table 3). For the dinoflagellates, only Noctiluca scintillans and Ceratium fusus in spring and N. scintillans in winter were found to be the dominant species. Several species showed a high dominance index in all four seasons, such as Lithodesmium undulatum, Bacillaria paxillifer, Coscinodiscus oculus-iridis, Chaetoceros sp., and Coscinodiscus asteromphalus.
The phytoplankton abundances in Jiaozhou Bay ranged from 0.03×106 to 8.38×106 cells/m3 (mean 1.16×106 cells/m3) in spring, 1.03×106 to 107.22×106 cells/m3 (mean 34.09×106 cells/m3) in summer, 0.43×106 to 10.74×106 cells/m3 (mean 2.43×106 cells/m3) in fall and 0.89×106 to 6.21×106 cells/m3 (mean 1.90×106 cells/m3) in winter. The horizontal distributions of the phytoplankton abundances in the study area are shown in Fig. 3. The distribution of the phytoplankton abundance in Jiaozhou Bay shows dominance of diatoms in all seasons, and the abundance was always high in the northeastern and northwestern parts of the bay. The phytoplankton cell abundance in the central and outer parts of the bay was lower than those in the northern part. The dinoflagellate abundance was quite low in the bay, and the distribution of the dinoflagellate abundance is quite different from that of the total phytoplankton and diatoms.
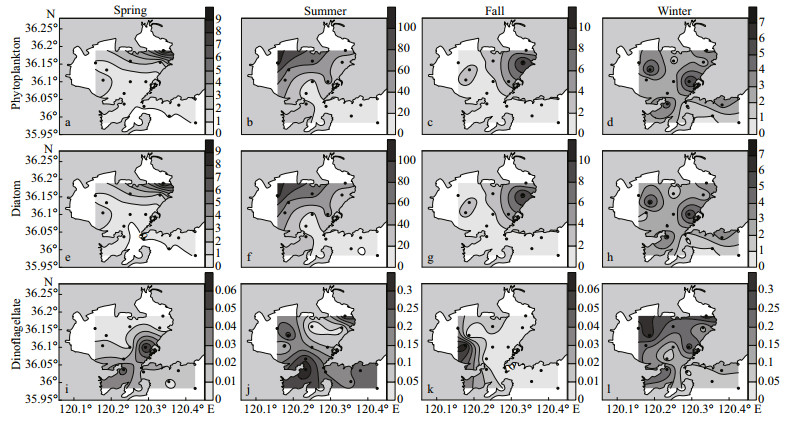
|
| Fig.3 Horizontal distributions of phytoplankton abundance (×106 cells/m3) in four seasons in the study area a–d. total phytoplankton; e–h. diatoms; i–l. dinoflagellates; black dot: sampling stations. |
In spring, a significant positive correlation was observed between the phytoplankton abundance and the temperature, and a significant negative correlation was observed between the phytoplankton abundance and the salinity (Table 4). In other three seasons, no significant correlation was observed between phytoplankton abundance and temperature/salinity. In summer, a significant positive correlation was observed between phytoplankton abundance and PM concentration. A significant positive correlation was observed between dinoflagellate abundance and stratification index in spring, fall, and winter. In terms of nutrients, a significant positive correlation was observed between PO43- concentration and diatoms in spring and fall, and a significant negative correlation between PO43- concentration and dinoflagellate abundance in summer. A significant negative correlation was observed between dinoflagellate abundance and DIN in summer, and a significant positive correlation was observed between diatom abundances and DIN in fall.
|
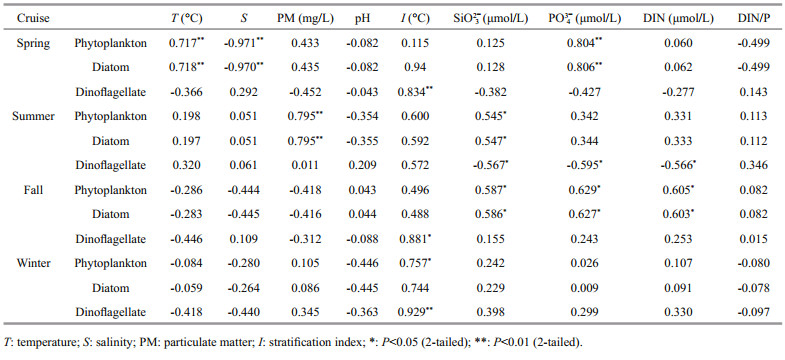
The CCA biplots of the dominant species and environmental parameters are presented in Fig. 4. In spring, L. undulatum correlated positively with phosphate concentration significantly (Fig. 4a). Most of the other dominant species correlated negatively with phosphate concentration and positively with the transparency, N/P ratio, and salinity. As the only dinoflagellate dominant species, N. scintillans correlated positively with stratification index and transparency. In summer, Skeletonema costatum, as the most dominant species, correlated positively with nutrient concentrations (Fig. 4b). Most of the other dominant species, however, correlated negatively with nutrient concentrations and positively with transparency, stratification index, and salinity. In fall, B. paxillifer correlated positively with N/P ratio (Fig. 4c). Most of the other dominant species, correlated positively with stratification index, temperature, and nutrient concentrations. In winter, B. paxillifer, Detonula pumila, and Rhizosolenia setigera correlated positively with temperature, transparency, and stratification index (Fig. 4d). Most of the other species correlated positively with nutrient concentration.

|
| Fig.4 CCA of the dominant phytoplankton species and the environmental parameters in four seasons in Jiaozhou Bay in 2017 a. spring; b. summer; c. fall; d. winter. |
The distributions of the Shannon-Wiener diversity index (H′) in the four seasons in the study area are shown in Fig. 5. H′ ranged from 0.08 to 3.50 (mean=2.01±1.18) in spring, 0.32 to 3.64 (mean=1.35±1.13) in summer, 1.22 to 3.98 (mean=2.90±0.74) in fall, and 2.54 to 4.18 (mean=3.26±0.44) in winter. In spring, the H′ values were highest in the southern and outer parts of the bay and were relatively low in the northern part (Fig. 5a). In summer, the H′ values were highest in the outer part of the bay and low in the northern and central parts (Fig. 5b). In fall, the H′ values were higher in the western part of the bay, and the lowest value appeared at station C4 (Fig. 5c). In winter, the distribution of H′ was almost flat in the study area (Fig. 5d).
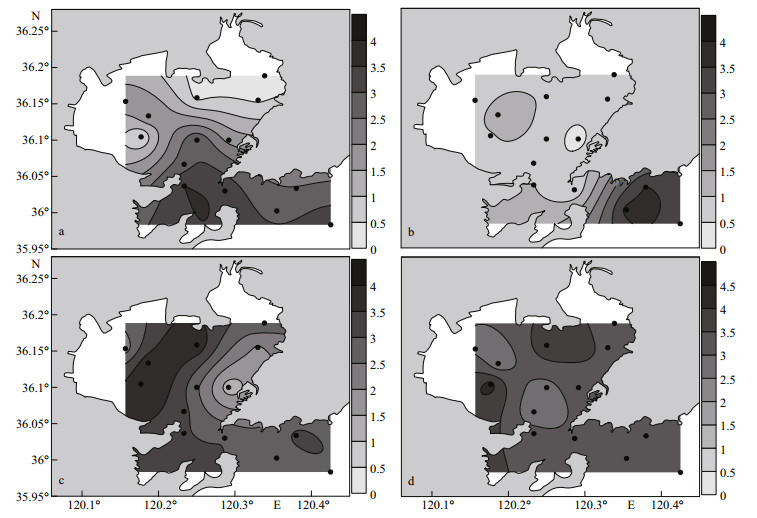
|
| Fig.5 Distribution of the Shannon-Wiener diversity index (H′) values for the phytoplankton community in Jiaozhou Bay, 2017 a. spring; b. summer; c. fall; d. winter. |
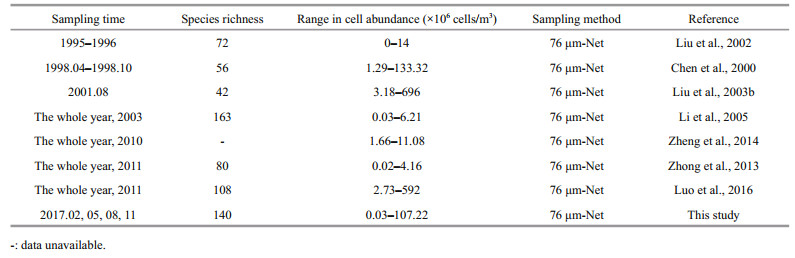
The phytoplankton cell abundance in this study ranged from (0.03–107.22)×106 cells/m3, which fall into the range reported in previous studies (Table 5). It is clear that the phytoplankton cell abundance in historical studies fluctuated highly among years, the highest to 696×106 cells/m3 in 2011 (Liu et al., 2003a) and lowest to only 4.16×106 cells/m3 in 2011 (Zhong et al., 2013) (Table 5). Several studies have addressed the variation in phytoplankton cell abundance in Jiaozhou Bay in different periods. Wang and Li (2006) found that the phytoplankton cell abundance showed a decreasing pattern from the 1960s to the 2000s in Jiaozhou Bay. Wu et al. (2005) found that phytoplankton cell abundance showed an increasing pattern in the last 50 years. Sun et al. (2011c) found that the phytoplankton cell abundance first decreased before the 1990s, and then increased due to the increase in silicate concentration in Jiaozhou Bay.
|
Our analyses of the historical data on the dominant phytoplankton species in Jiaozhou Bay show that the dominant phytoplankton species composition has changed in recent years (Table 6). Some species belonging to Chaetoceros, Coscinodiscus, and Skeletonema are still the dominant species in Jiaozhou Bay. Asterionella japonica was a frequent dominant species before the 2000s. However, after the 2000s, A. japonica lost its dominant position. Other species, such as B. paxillifer, L. undulatum, and N. scintillans, have become the new dominant species since the 2010s. B. paxillifer is an epipelic/periphytic taxon that is probably found in brackish habitats with great fluctuations in salinity and high nutrient concentrations (Schmid, 2007). Fluctuation in salinity due to the freshwater input from rivers around Jiaozhou Bay and the increasing nutrient concentrations in recent years makes the bay an ideal habitat for B. paxillifer. L.undulatum is a warm-water species and was found in the southwest sea area of the Xisha Islands (Wu et al., 2005). Since the 2010s, it has become one of the dominant species in Jiaozhou Bay. It has been found that the water temperature in Jiaozhou Bay has increased over the past several decades (Sun et al., 2011c), which is similar to other coastal seas in the world (Sheppard and Loughland, 2002; Chen et al., 2013; Rubal et al., 2013). The rising temperatures in Jiaozhou Bay in the past several decades would favor the growth of L. undulatum. As a large heterotrophic and omnivorous dinoflagellate, N. scintillans has become prominent in and around Jiaozhou Bay since the 1990s (Wu et al., 2005; Sun et al., 2011c; Zheng et al., 2014; Luo et al., 2016). It is widely distributed over temperate and tropical coastal waters and frequently forms harmful algal blooms. An N. scintillans bloom was considered the result of seawater eutrophication (Xu, 2009). In the last 40 years, the nutrient concentrations in Jiaozhou Bay have increased greatly due to the advancement of economic activity and the increase in population around the bay (Sun et al., 2011c), which could be responsible for the frequent occurrence of N. scintillans blooms in recent years.
|
In the present study, the phytoplankton community structure was different in the four seasons (Tables 1 and 3). This could be attributed to seasonal variations in the environmental parameters. It was reported that temperature could greatly affect the seasonal dynamics of phytoplankton in the ocean (Dupuis and Hann, 2009). In this study, the Chl a concentration was lowest in winter because of the weaker metabolic activities of the phytoplankton cells in low water temperatures (Table 1). As the water temperature increased, the growth of the phytoplankton cells accelerated, and the Chl a concentration peaked in summer when water temperature was highest in the whole year (Table 1). Nutrient concentrations could also have an important role in affecting the seasonal variation of phytoplankton (Ward et al., 2011). In this study, the nutrient concentrations were also highest in summer, followed by spring and fall, and lowest in winter, which is consistent with the seasonal variation pattern of Chl a and the phytoplankton cell abundance in the study area (Table 1).
The dominant phytoplankton species were mostly diatoms in the four seasons, except in spring and winter, when N. scintillans and C. fusus species took over in the study area (Table 3). As the cell volume of N. scintillans is much larger than those of other dominant diatoms, N. scintillans should constitute a significant portion of the phytoplankton biomass in spring and winter. Therefore, the phytoplankton community structure was diatom-dominated in summer and fall and diatoms and dinoflagellates were dominant in spring and winter. This seasonal difference in phytoplankton community structure in summer/fall and spring/winter could be closely related to the Si/N ratio in the study area. Several studies have found that a high Si/N ratio favors the growth of diatoms in the ocean (Turner et al., 2007; Grosse et al., 2010). In this study, the Si/N ratio was much higher in summer and fall than in spring and winter (Table 1), which could be responsible for the higher dominance of diatoms in summer and fall than in spring and winter.
4.3 Correlations between the phytoplankton cell abundances and the environmental parametersIn this study, the dinoflagellate cell abundances positively correlated significantly with the stratification index in spring, fall, and winter, indicating that dinoflagellates would prefer to accumulate in less turbulent water (Table 4). Several studies have revealed that the water turbulence level has a direct effect on phytoplankton community structure, and dinoflagellates are not favored in highly turbulent waters (Margalef, 1978; Juhl and Latz, 2002; Guo et al., 2014b). Dinoflagellates have been found more susceptive to water turbulence than diatoms, and the growth of dinoflagellate cells would be inhibited when the turbulence level increases due to morphological damage, the inhibition of cell division, and the disruption of nutrient-retrieval migrations (Thomas and Gibson, 1990; Smayda and Reynolds, 2003). Pollingher and Zemel (1981) found that the division rate of Peridinium gatunense was inhibited when the water turbulence level increased in Lake Kinneret, Israel. Sullivan et al. (2003) studied the vertical distributions of Alexandrium catenella in East Sound, Washington, USA, and found that A.catenella actively concentrated at depths with low turbulence levels. Our CCA analysis also revealed that N. scintillans and C. fusus correlated positively with the water stratification index in spring (Fig. 4a), indicating that less-turbulent water favors the growth of these dinoflagellates. In contrast, diatoms are less susceptible to turbulence due to protection from their silicified cell walls (Hamm et al., 2003). Therefore, no significant correlation was observed between the diatoms cell abundance and the stratification index in the four seasons (Table 4).
The diatom cell abundances were found to be significantly correlated positively with the silicate concentrations in summer and fall, significantly positively correlated with the phosphate concentrations in spring and fall, and significantly correlated positively with the DIN concentrations in fall (Table 4). Generally, the diatom cell abundances correlated positively with the nutrient concentrations in the study area, and this could also be concluded from the distribution pattern of the diatom cell abundances (Fig. 4e–h) and the DIN concentration (Fig. 2i–h). The dinoflagellates, however, were found to have a significant negative correlation with the silicate, phosphate and DIN concentrations in summer and had no significant correlation with the nutrient concentrations in other seasons (Table 4). This discrepancy is mainly due to the different survival strategies of diatoms and dinoflagellates. Diatoms are totally autotrophic, and they can easily become dominant when growing in non-limiting conditions (Smayda, 1997). In this study, high values of diatoms cell abundances were observed mainly in the nutrients sufficient areas (Fig. 4e–h). When nutrients are limited, however, diatoms are affected more easily than dinoflagellates (Egge, 1998). It has been found that dinoflagellates have the ability to be mixotrophic, and the mixotrophic modes of dinoflagellates provide dinoflagellates with sufficient nutrients under oligotrophic conditions (Smayda, 1997; Stoecker, 1999). Therefore, dinoflagellates possess a survival advantage over diatoms in waters with low nutrient concentrations, which could explain the negative correlation between the dinoflagellate cell abundances and the nutrient concentrations in this study (Table 4).
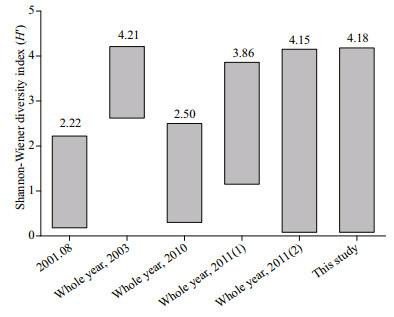
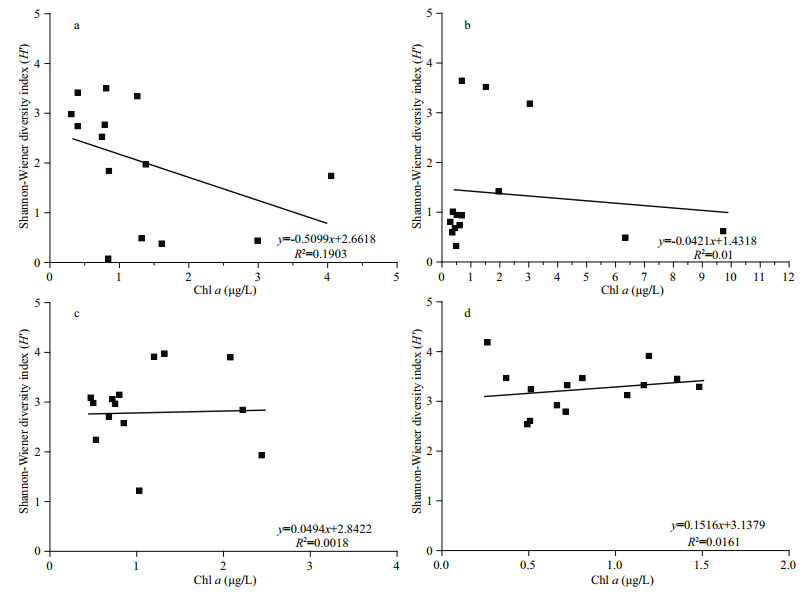
4.4 Diversity index of the phytoplankton community structure in Jiaozhou BayAs one of the parameters used for the environmental assessment, the Shannon-Wiener diversity index (H′) has been widely used in studies on phytoplankton community structure (Sun and Liu, 2004), and a higher biodiversity index indicates a higher stability of the phytoplankton community and its resistance to environmental oppression (Gao et al., 2013). The H′ values in this study fell in the range reported in historical studies in Jiaozhou Bay (Fig. 6), with the lowest value (H′=0.08) observed at station Y1 in spring and the highest value (H′=4.18) observed at station C1 in winter. In spring and summer, the H′ value was higher in the outer and central parts of the bay than that in the northern part of the bay (Fig. 5a & b), which was opposite to the distribution pattern of Chl a (Fig. 2m & n) and phytoplankton cell abundance (Fig. 3a & b). In fall and winter, the H′ value showed a patchy distribution pattern, which was quite different from that of the Chl a (Fig. 2o & p) and phytoplankton cell abundances (Fig. 3c & d). A scatter plot between Chl a and H′ in this study revealed that no significant correlation was observed between Chl a and H′ in the four seasons in the study area (Fig. 7), and even a negative correlation was observed between Chl a and H′ in spring and summer (Fig. 7a & b). Therefore, the phytoplankton community structure might show a high biomass with a low diversity index at some stations in the study area. The reason why phytoplankton community structure has high biomass but low diversity is usually attributed to relatively high nutrient availability (Chen et al., 2016). High nutrients may favor the growth of a few phytoplankton species and result in the much higher biomasses of these species than other non-dominant species. Therefore, a phytoplankton community will become simplified with a low diversity index of the phytoplankton community (Guo et al., 2014a). A phytoplankton community with high biomass but a low diversity index was also observed in eutrophic Bohai Bay, China (Chen et al., 2016).
|
|
Fig.6
Range of Shannon-Wiener diversity index (H′) values observed in Jiaozhou Bay in different years
Historical data are from Liu et al. (2003b; 2001.08), Li et al. (2005; whole year, 2003), Zheng et al. (2014; whole year, 2010), |
|
| Fig.7 Scatter plot of the Shannon-Wiener diversity index values and the surface Chl a concentrations (μg/L) in four seasons in Jiaozhou Bay in 2017 a. spring; b. summer; c. fall; d. winter. |
Net phytoplankton community structure in Jiaozhou Bay was studied in four seasonal cruises in 2017, and its relationship with various environmental parameters was explored. The phytoplankton community in Jiaozhou Bay was diatom-dinoflagellate co-dominated in spring and winter and diatomdominated in summer and fall, mainly due to the seasonal variation of Si/N ratios in the area. The phytoplankton cell abundance was higher in the northern part of the bay than in the central and outer parts of the bay in all seasons, which is consistent with the distribution pattern of nutrients. Diatoms and dinoflagellates showed different relationships with the environmental parameters: with diatoms showing significant positive correlations with the nutrient concentrations, while the dinoflagellates showed no correlation or negative correlations with the nutrient concentrations but significant positive correlations with the stratification index. This discrepancy was mainly due to different survival strategies of these two phytoplankton groups. The Shannon-Wiener diversity index (H′) values ranged from 0.08 to 4.18, which fell in the range reported in historical studies. The distribution pattern of H′ values was quite different from that of Chl a, indicating that the phytoplankton community structure might show a high biomass with a low diversity index in the study area. Compared with historical data, the dominant phytoplankton species have changed, mainly due to the rising temperatures and increased eutrophication in the study area in the last 30 years. The historical variation pattern of phytoplankton cell abundances in Jiaozhou Bay differed among several studies, which should be clarified in the future.
6 DATA AVAILABILITY STATEMENTData available on request from the authors.
7 ACKNOWLEDGMENTWe thank the crew and captain of the R/V Chuangxin for the logistic support in the cruises.
Chen B J, Chen J F, Yuan Y X, Cui Y, Qu K M. 2000. Study on the ecological characteristics of phytoplankton in the northern coast of Jiaozhou Bay. Mar. Fisheries Res., 21(2): 34-40.
(in Chinese with English abstract) |
Chen C S, Ji R B, Zheng L Y, Zhu M Y, Rawson M. 1999. Influences of physical processes on the ecosystem in Jiaozhou Bay:a coupled physical and biological model experiment. J. Geophys. Res., 1042(C12): 29 925-29 949.
|
Chen T R, Li S, Yu K F, Zheng Z Y, Wang L R, Chen T G. 2013. Increasing temperature anomalies reduce coral growth in the Weizhou Island, northern South China Sea. Estuar. Coast. Shelf Sci., 130: 121-126.
DOI:10.1016/j.ecss.2013.05.009 |
Chen Y H, Gao Y H, Chen C P, Liang J R, Sun L, Zhen Y, Qiao L. 2016. Seasonal variations of phytoplankton assemblages and its relation to environmental variables in a scallop culture sea area of Bohai Bay, China. Mar. Pollut. Bull., 113(1-2): 362-370.
DOI:10.1016/j.marpolbul.2016.10.025 |
Dupuis A P, Hann B J. 2009. Warm spring and summer water temperatures in small eutrophic lakes of the Canadian prairies:potential implications for phytoplankton and zooplankton. J. Plankton Res., 31(5): 489-502.
DOI:10.1093/plankt/fbp001 |
Egge J K. 1998. Are diatoms poor competitors at low phosphate concentrations? J. Mar. Syst., 16(3-4): 191-198.
DOI:10.1016/S0924-7963(97)00113-9 |
Falkowski P G, Raven J A. 2007. Aquatic Photosynthesis. 2thedn. Princeton University Press, Princeton. p.70-73.
|
Gao Y, Jiang Z B, Liu J J, Chen Q Z, Zeng J N, Huang W. 2013. Seasonal variations of net-phytoplankton community structure in the Southern Yellow Sea. J. Ocean Univ.China, 12(4): 557-567.
DOI:10.1007/s11802-013-2258-x |
Gasol J M, delGiorgio P A, Duarte C M. 1997. Biomass distribution in marine planktonic communities. Limnol. Oceanogr., 42(6): 1 353-1 363.
DOI:10.4319/lo.1997.42.6.1353 |
Grosse J, Bombar D, Doan H N, Nguyen L N, Voss M. 2010. The Mekong River plume fuels nitrogen fixation and determines phytoplankton species distribution in the South China Sea during low and highdischarge season. Limnol. Oceanogr., 55(4): 1 668-1 680.
DOI:10.4319/lo.2010.55.4.1668 |
Guo S J, Feng Y Y, Wang L, Dai M H, Liu Z L, Bai Y, Sun J. 2014b. Seasonal variation in the phytoplankton community of a continental-shelf sea:the East China Sea. Mar. Ecol.Prog. Ser., 516: 103-126.
DOI:10.3354/meps10952 |
Guo S J, Li Y Q, Zhang C X, Zhai W D, Huang T, Wang L F, Ma W, Jin H L, Sun J. 2014a. Phytoplankton community in the Bohai Sea and its relationship with environmental factors. Mar. Sci. Bull., 33(1): 95-105.
(in Chinese with English abstract) |
Guo Y J, Yang Z Y. 1992. Phytoplankton ecology. In: Liu R Y ed. Ecosystem of the Jiaozhou Bay. Science Press, Beijing, China. p.136-169. (in Chinese)
|
Hamm C E, Merkel R, Springer O, Jurkojc P, Maier C, Prechtel K, Smetacek V. 2003. Architecture and material properties of diatom shells provide effective mechanical protection. Nature, 421(6925): 841-843.
DOI:10.1038/nature01416 |
Han H Y, Li K Q, Wang X L, Shi X Y, Qiao X D, Liu J. 2011. Environmental capacity of nitrogen and phosphorus pollutions in Jiaozhou Bay, China:modeling and assessing. Mar. Pollut. Bull., 63(5-12): 262-266.
DOI:10.1016/j.marpolbul.2010.12.017 |
Jiao N Z. 2001. The ecological processes and sustainable development in the Bay. Science Press, Beijing, China. p. 241-253.
(in Chinese)
|
Juhl A R, Latz M I. 2002. Mechanisms of fluidshear-induced inhibition of population growth in a red-tide dinoflagellate. J. Phycol., 38(4): 683-694.
DOI:10.1046/j.1529-8817.2002.00165.x |
Li G G, Huang S M. 1956. Seasonal variation of planktonic diatoms at Tsingtao. J. Shandong Univ., 2(4): 119-143.
(in Chinese with English abstract) |
Li Y, Li R X, Wang Z L, Zhu M Y, Sun P X, Xia B. 2005. A preliminary study on phytoplankton community structure and its changes in the Jiaozhou Bay. Adv. Mar. Sci., 23(3): 328-334.
(in Chinese with English abstract) |
Liu D Y, Sun J, Chen H T, Zhang L Y. 2003b. The phytoplankton community in summer 2001 in Jiaozhou Bay, China. J. Ocean Univ. Qingdao, 33(3): 366-374.
(in Chinese with English abstract) |
Liu D Y, Sun J, Qian S B. 2002. Study on the phytoplankton in Jiaozhou Bay Ⅱ:influence of the environmental factors to phytoplankton community. J. Ocean Univ. Qingdao, 32(3): 415-421.
(in Chinese with English abstract) |
Liu D Y, Sun J, Zhang L Y. 2003a. Structural characteristics of phytoplankton community during harmful algae bloom in Jiaozhou Bay. Chin. J. Appl. Ecol., 14(11): 1 963-1 966.
(in Chinese with English abstract) |
Liu S M, Li R H, Zhang G L, Wang D R, Du J Z, Herbeck L S, Zhang J, Ren J L. 2005. The impact of anthropogenic activities on nutrient dynamics in the tropical Wenchanghe and Wenjiaohe Estuary and Lagoon system in East Hainan, China. Mar. Chem., 125(1-4): 49-68.
|
Luo X, Sun X X, Zheng S, Zhao Y F. 2016. On phytoplankton community structure and causative factors in the Jiaozhou Bay in 2011. Oceanol. Limnol. Sin., 47(5): 915-923.
(in Chinese with English abstract) |
Margalef R. 1978. Life-forms of phytoplankton as survival alternatives in an unstable environment. Oceanol. Acta, 1(4): 493-509.
|
Pollingher U, Zemel E. 1981. In situ and experimental evidence of the influence of turbulence on cell division processes of Peridinium cinctum forma westii (Lemm) Lefèvre. Brit. Phycol. J., 16(3): 281-287.
DOI:10.1080/00071618100650301 |
Qian S B, Wang X Q, Chen G W. 1983. The phytoplankton of the Jiaozhou Bay. J. Shandong Coll. Oceanol., 13(1): 39-56.
(in Chinese with English abstract) |
Rubal M, Veiga P, Cacabelos E, Moreira J, Sousa-Pinto I. 2013. Increasing sea surface temperature and range shifts of intertidal gastropods along the Iberian Peninsula. J. Sea Res., 77: 1-10.
DOI:10.1016/j.seares.2012.12.003 |
Schmid A M M. 2007. The "paradox" diatom Bacillaria paxillifer (Bacillariophyta) revisited. J. Phycol., 43(1): 139-155.
DOI:10.1111/j.1529-8817.2006.00299.x |
Shannon C E, Weaver W. 1949. The Mathematical Theory of Communication. University of Illinois Press, Urbana, Ill. p.45-47.
|
Shen Z L. 2001. Historical changes in nutrient structure and its influences on phytoplantkon composition in Jiaozhou Bay. Estuar. Coast. Shelf Sci., 52(2): 211-224.
DOI:10.1006/ecss.2000.0736 |
Sheppard C, Loughland R. 2002. Coral mortality and recovery in response to increasing temperature in the southern Arabian Gulf. Aquat. Ecosyst. Health. Manage., 5(4): 395-402.
DOI:10.1080/14634980290002020 |
Smayda T J. 1997. Harmful algal blooms:their ecophysiology and general relevance to phytoplankton blooms in the sea. Limnol. Oceanogr., 42(5): 1 137-1 153.
|
Smayda T J, Reynolds C S. 2003. Strategies of marine dinoflagellate survival and some rules of assembly. J. Sea Res., 49(2): 95-106.
DOI:10.1016/S1385-1101(02)00219-8 |
Stoecker D K. 1999. Mixotrophy among dinoflagellates. J.Eukaryot. Microbiol., 46(4): 397-401.
DOI:10.1111/j.1550-7408.1999.tb04619.x |
Sullivan J M, Swift E, Donaghay P L, Rines J E B. 2003. Small-scale turbulence affects the division rate and morphology of two red-tide dinoflagellates. Harmful Algae, 2(3): 183-199.
DOI:10.1016/S1568-9883(03)00039-8 |
Sun J, Liu D Y. 2004. The application of diversity indices in marine phytoplankton studies. Acta Oceanol. Sin., 26(1): 62-75.
(in Chinese with English abstract) |
Sun S, Sun X X, Zhang G T, Tang H B, Liu Q, Li G M. 2011a. Long-term changes in major meteorological and hydrological factors in the Jiaozhou Bay. Oceanol. Limnol.Sin., 42(5): 632-638.
(in Chinese with English abstract) |
Sun X H, Sun S, Li C L, Zhang G T. 2011b. Seasonal and spatial variability in egg production, abundance and production of small copepods in and near Jiaozhou Bay, China. J. Plankton Res., 33(5): 741-750.
DOI:10.1093/plankt/fbq135 |
Sun X X, Sun S, Wu Y L, Zhang Y S, Zheng S. 2011c. Longterm changes of phytoplankton community structure in the Jiaozhou Bay. Oceanol. Limnol. Sin., 42(5): 639-646.
(in Chinese with English abstract) |
Thomas W H, Gibson C H. 1990. Quantified small-scale turbulence inhibits a red tide dinoflagellate, Gonyaulax polyedra Stein. Deep Sea Res. Part A, 37(10): 1 583-1 593.
DOI:10.1016/0198-0149(90)90063-2 |
Tomas C R, Hasle G R. 1997. Identifying Marine Phytoplankton. Academic Press, San Diego, CA. 858p.
|
Turner J T. 2015. Zooplankton fecal pellets, marine snow, phytodetritus and the ocean's biological pump. Prog. Oceanogr., 130: 205-248.
DOI:10.1016/j.pocean.2014.08.005 |
Turner R E, Rabalais N N, Alexander R B, McIsaac G, Howarth R W. 2007. Characterization of nutrient, organic carbon, and sediment loads and concentrations from the Mississippi River into the Northern Gulf of Mexico. Estuar. Coasts, 30(5): 773-790.
DOI:10.1007/BF02841333 |
Wang X L, Li K Q. 2006. Phytoplankton biomass and its community structure in the Jiaozhou Bay. In: Wang X L, Li K Q, Shi X Y eds. Environmental Capacity of Major Chemical Pollutants in the Jiaozhou Bay. Science Press, Beijing, China. p.48-62.
|
Ward B B, Rees A P, Somerfield P J, Joint I. 2011. Linking phytoplankton community composition to seasonal changes in f-ratio. ISME J., 5(11): 1 759-1 770.
DOI:10.1038/ismej.2011.50 |
Welschmeyer N A. 1994. Fluorometric analysis of chlorophyll a in the presence of chlorophyll b and pheopigments. Limnol. Oceanogr., 39(8): 1 985-1 992.
DOI:10.4319/lo.1994.39.8.1985 |
Wu Y L, Sun S, Zhang Y S, Zhang F. 2004. Quantitative study on long-term variation of phytoplankton in Jiaozhou Bay. Oceanol. Limnol. Sin., 35(6): 518-523.
(in Chinese with English abstract) |
Wu Y L, Sun S, Zhang Y S. 2005. Long-term change of environment and it's influence on phytoplankton community structure in Jiaozhou Bay. Oceanol. Limnol.Sin., 36(6): 487-498.
(in Chinese with English abstract) |
Xu Z L. 2009. The inter-annual variations in Noctiluca scintillans abundance and eutrophication in Changjiang estuary. Oceanol. Limnol. Sin., 40(6): 793-798.
(in Chinese with English abstract) |
Yang S M, Wang L S, Shi X Y. 2014. Phytoplankton community of the Jiaozhou Bay in spring 2009. Oceanol. Limnol.Sin., 45(6): 1 234-1 240.
(in Chinese with English abstract) |
Zheng S, Sun X X, Zhao Y F, Sun S. 2014. Annual variation of species composition and abundance distri-bution of phytoplankton in 2010 in the Jiaozhou Bay. Mar. Sci., 38(11): 1-6.
(in Chinese with English abstract) |
Zhong X, Gong X Z, Ma W, Zhang W J. 2013. The characteristics of phytoplankton community structure in the northern Jiaozhou Bay. Trans. Oceanol. Limnol., (4): 11-119.
(in Chinese with English abstract) |
 2019, Vol. 37
2019, Vol. 37




