Institute of Oceanology, Chinese Academy of Sciences
Article Information
- LUO Xiaoxia, LI Changling, HUANG Xianghu
- Effect of diet on the development, survival, and reproduction of the calanoid copepod Pseudodiaptomus dubia
- Journal of Oceanology and Limnology, 37(5): 1756-1767
- http://dx.doi.org/10.1007/s00343-019-8214-x
Article History
- Received Aug. 29, 2018
- accepted in principle Oct. 16, 2018
- accepted for publication Jan. 29, 2019
2 Engineering Technology Research Center for Algae Breeding and Application, Zhanjiang 524088, China;
3 Shenzhen Research Institute of Guangdong Ocean University, Shenzhen 518108, China
Pseudodiaptomus dubia (formerly Schmackeria dubia), is a calanoid copepod widely distributed in the estuarine-coastal waters of Asia; this species is the most abundant copepod in the mariculture ponds of southern China (Li et al., 2008a, 2009). P. dubia not only serves as an excellent food source for aquatic animals such as fish, shrimp, and shellfish, but is also the main consumer of microalgae in breeding ponds (Luo et al., 2008). This species, therefore, plays an extremely important role in the regulation of aquaculture water quality (Li et al., 2008a).
To date, most studies of P. dubia have focused on the adults, and only rarely on the larvae (Huang and Luo, 1980; Shang et al., 2005; Li et al., 2008a). However, the behavior of the latter is of great significance when studying copepod population dynamics, especially for the establishment of dynamic population models (Li et al., 2008a). Previously, Li et al. (2009) studied the effect of temperature on the entire life cycle of P. dubia, and showed that higher temperatures increased egg production and growth rate, while shortening the reproductive cycle and hatching time. However, diet also strongly influences the growth and reproduction of copepods (Ban, 1994; Koski and Kuosa, 1999; Carotenuto et al., 2002; Murray and Marcus, 2002; Yu et al., 2017), as food quality directly affects development, survival, and reproduction (Harrison, 1990; Taipale et al., 2014).
We therefore aimed to investigate the effects of different foods on the entire life cycle of P. dubia, including development, survival, reproduction, and longevity. We aimed to use various microalgal species commonly present in the mariculture ponds of southern China as food for P. dubia: Chlorella saccharophila and Platymonas subcordiformis (phylum Chlorophyta); Isochrysis zhanjiangensis (phylum Chrysophyta); and Chaetoceros muelleri and Cyclotella meneghiniana (phylum Bacillariophyta). We then aimed to compare the intrinsic growth rate, net reproductive rate, and generation time among groups of P. dubia fed different diets. With these results, we aimed to provide a framework for the mass culture of P. dubia, and improve our understanding of P. dubia population dynamics in aquaculture ecosystems.
2 MATERIAL AND METHOD 2.1 Larval production and microalgae usedAll seawater used in this study was first filtered through a plankton net (mesh size: 39 μm), and then boiled to kill any remaining living organisms. P. dubia were collected from several shrimp ponds in Zhanjiang, China. We acclimated the copepods to laboratory conditions (temperature: 28℃; salinity: 27; irradiance: 700–1 200 lux) for three months. During the acclimation period, copepods were fed diatoms (C. muelleri).
After three months, 250 healthy and energetic females carrying mature egg sacs were identified under a microscope (Nikon, Japan). Each eggcarrying female was carefully transferred with a pipette dropper to a different 20 mL glass tube containing 10 mL clean seawater. Every 2 h, newly hatched nauplii were collected in a 1 000-mL glass beaker filled with clean seawater. All nauplii collected at the same time were considered a cohort and were collected in the same beaker. At the end of the hatching period, the largest cohort was used for all experiments.
All microalgae used in this study were provided by the Algal Culture Research Laboratory of Guangdong Ocean University (Zhanjiang, China). Five species of microalgae were tested as food for P. dubia: C. saccharophila and P. subcordiformis (phylum Chlorophyta); I. zhanjiangensis (phylum Chrysophyta); and C. muelleri and C. meneghiniana (phylum Bacillariophyta). All microalgae were fed to P. dubia such that the amount of carbon provided was equivalent (1.5 mg C/L; see Table 1 for the details of the individual microalgal species); all food carbon concentrations were estimated based on the photometric light extinction (682 nm) and the carbonextinction regressions determined in preliminary experiments. The microalgae were cultured in 1-L Erlenmeyer flasks using Zhanshui 107 medium (Chen, 1995), with silicates added for diatoms. Cultures were maintained at 28℃, with irradiance of 1 000 lux and a 12-h light:12-h dark photoperiod. Microalgae were maintained in the logarithmic growth phase by diluting each culture two-fold with fresh medium every 10 days. All cultures were in the exponential growth stage when fed to copepods.

|
The single nauplii cohort was divided into 75 125- mL glass bottles, each bottle containing 100 mL of microalgal suspension and 10 nauplii. Fifteen bottles were used per microalgae species; these 15 bottles were divided into five groups (A–E), each group comprised of three replicate bottles. In group A bottles, we measured the effects of diet on nauplius and copepodite development. We used groups B and C to determine the effects of diet on the survival of nauplii and copepodites, respectively. We used groups D and E to investigate the effects of diet on reproduction. For the purposes of our reproduction experiment, and to ensure a sufficient reproductive population, groups D and E were treated identically and considered a single group. All glass bottles were kept in an incubator (PGX-280A-3H; Lai Fu, China) at 28℃, with light intensity of 1 200 lux, and a 12-h light:12-h dark photoperiod. Seawater in each bottle was refreshed every 2 days. All the feeding experiments were conducted simultaneously, and all experimental animals were considered a single cohort.
2.3 Effect of diet on nauplius and copepodite developmentThe life cycle of P. dubia consists of 12 stages: six nauplius stages (NI, NII, NIII, NIV, NV, and NVI), five copepodite stages (CI, CII, CIII, CIV, and CV), and the adult stage (Li et al., 2009). Because P. dubia is an egg-carrying invertebrate, eggs develop into NI within the egg sac, before the sac detaches from the female and the NI nauplii hatch, and NI nauplii transition to the NII stage occurs within 2–4 min of hatching (Li et al., 2009). Therefore, our observations of larval development began at stage NII.
Larval development in group A was monitored every 6 h by randomly selecting three to four larvae, quickly photographing them using a microscope (Nikon, Japan) equipped with an ocular micrometer (NE1, China), and immediately returning them to the group A bottle. We measured the prosome length of all collected larvae, and determined the developmental stage based on Li et al. (2009). Each stage was considered complete when 50% of the cohort had moulted. The weight (in μg carbon) of each P. dubia nauplius and copepodite was calculated as
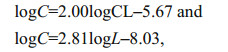
respectively, where CL was the prosome length (in μm) of the nauplius, and L was the prosome lengths (in μm) of the copepodite (Uye et al., 1983; Li et al., 2009). We then calculated the average larval growth rate G (/d) as
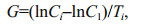
where Ci was the weight (in μg carbon) of the larvae at stage i, C1 was the weight (in μg carbon) at the beginning of the experiment, and Ti was the duration (in days) of stage i (Martin-Creuzburg et al., 2005).
2.4 Effect of diet on nauplius and copepodite survivalAs soon as the larvae in group A reached the nauplius stage NVI, the live larvae in Group B were fixed with Lugol's iodine. As soon as the larvae in group A reached the copepodite stage CV, we fixed all remaining live larvae in Group C. We counted these larvae under a stereomicroscope (SZMN645-B4, China), and calculated separate survival rates (SR) for the nauplii and copepodites as follows:
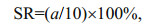
where a was the number of live larvae at stage NVI in group B and at stage CV in group C.
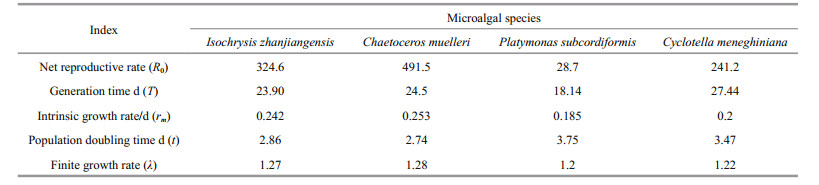
2.5 Effect of diet on P. dubia reproductionAs soon as the larvae in groups D and E reached the adult stage and the females were observed to carry eggs, females and males at a ratio of 1:2 were incubated in ten 20 mL glass tubes, each containing 10 mL of a different microalgal suspension. Nauplius production was recorded, and microalgal suspensions were renewed every 1–2 days. The experiment was not terminated until all adult females had died. We then calculated several life-history parameters, including net reproductive rate (R0), generation time (T), intrinsic rate of population growth (rm), finite rate of growth (λ), and population doubling time (t) as
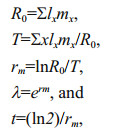
where x was age or time interval, lx was survival rate at time x, and mx was the birth rate at time x (Meyer et al., 1986; Shen and Shi, 2002; Tang et al., 2005; Li et al., 2009).
2.6 Data analysesAll statistics were calculated in SPSS v17.0 (IBM, USA). All significant results were analyzed for homogeneity of variance with Levene's test and then with Duncan's multiple range tests. Results were considered significant at P=0.05.
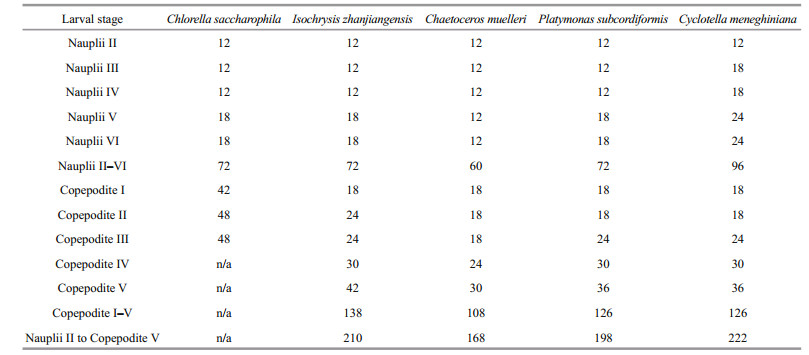
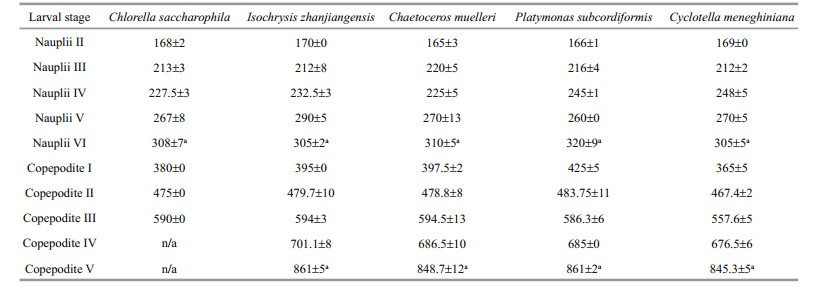
3 RESULT 3.1 Effect of diet on P. dubia larval development time and body lengthThe larvae fed different microalgae had different development times (Table 2). Nauplii developed most quickly when fed C. muelleri, taking only 60 h to progress from stage NII to stage NVI; nauplii fed C. saccharophila, I. zhanjiangensis, and P. subcordiformis took 72 h, and nauplii fed C. meneghiniana took 96 h (Table 2). Similarly, copepodites developed most quickly when fed C. muelleri, taking only 108 h to progress from stage CI to stage CV; copepodites fed P. subcordiformis and C. meneghiniana took 126 h, and copepodites fed I. zhanjiangensis took 138 h. All copepodites fed C. saccharophila died during stage CIII. Therefore, C. saccharophila as food was clearly detrimental to copepodite development. The species of microalgae consumed had no obvious effects on body length (Table 3).
|
|
The species of microalgae consumed significantly affected the growth rate of P. dubia larvae (ANOVA followed by Duncan's multiple range tests, P < 0.05; Fig. 1). At stage NVI, the growth rate of nauplii fed C. muelleri or P. subcordiformis was > 0.44/d, significantly greater than that of nauplii fed I. zhanjiangensis (0.389/d; P < 0.05), C. saccharophila (0.405/d; P < 0.05), and C. meneghiniana (0.295/d; P < 0.05; Fig. 1). There were no significant differences in growth rates between nauplii fed C. saccharophila and I. zhanjiangensis (Fig. 1), but the growth rate of nauplii fed C. meneghiniana was significantly lower than that of all other microalgal diets (P < 0.05 for all comparisons; Fig. 1).
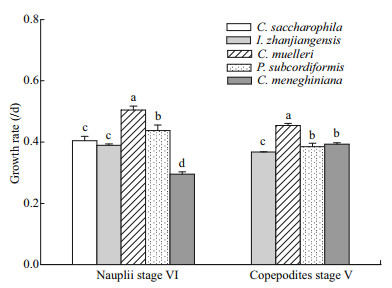
|
| Fig.1 Growth rate of larval Pseudodiaptomus dubia when fed different species of microalgae Bars represent means±standard deviation of three replicates per microalgal species. Bars labeled different lowercase letters are significantly different (analysis of variance (ANOVA) and Duncan's multiple range tests; P < 0.05). For details of larval growth stages, please see the text |
At stage CV, the growth rate of copepodites fed C. muelleri (0.454/d) was significantly higher than that of copepodites fed all other algal species (0.368–0.393/d; P < 0.05; Fig. 1). Indeed, copepodites fed C. muelleri grew 15%–23% faster than those fed I. zhanjiangensis, P. subcordiformis, or C. meneghiniana. The growth rate of copepodites fed P. subcordiformis was significantly greater than that of copepodites fed I. zhanjiangensis, but there were no significant differences in growth rate between copepodites fed C. meneghiniana and P. subcordiformis (Fig. 1).
3.3 Effect of diet on survival of P. dubiaThe microalgal species consumed had a significant effect on the survival rate of larval P. dubia (ANOVA followed by Duncan's multiple range tests, P < 0.05; Fig. 2). Significantly more nauplii and copepodites survived when fed C. muelleri, I. zhanjiangensis, or P. subcordiformis (range: 85%–95%), as compared to those fed C. saccharophila or C. meneghiniana (< 75%; Fig. 2).

|
| Fig.2 Survival rate of larval Pseudodiaptomus dubia when fed different species of microalgae Bars represent means±standard deviation of three replicates per microalgae. Bars labeled different lowercase letters are significantly different (analysis of variance (ANOVA) and Duncan's multiple range tests; P < 0.05). |
The survival rates of P. dubia varied tremendously depending on the microalgal species consumed (Fig. 3). Throughout the entire lifecycle, copepods fed C. muelleri had the highest survival rate, remaining above 87% for 33 days; 30% of the copepods fed C. muelleri remained alive after 57 days (Fig. 3). The survival curve of the copepods fed I. zhanjiangensis was similar, but with a lower rate of survival throughout (Fig. 3). Copepods fed C. meneghiniana had a low survival rate at the larval stage (< 10 days), but had little mortality as adults until ~50 days, when the survival rate dropped sharply (Fig. 3). Copepods fed either P. subcordiformis or C. saccharophila had poor survival rates curves, with all individuals dead by day 37 and day 8, respectively (Fig. 3).

|
| Fig.3 Survival curve for different developmental stages of Pseudodiaptomus dubia fed different microalgal species |
The average longevity of copepod adults fed C. muelleri, I. zhanjiangensis, or C. meneghiniana was 47–55 d (Fig. 4), significantly longer than the longevity of copepods fed P. subcordiformis (32 d; P < 0.05; Fig. 4). There were no significant differences in average longevity between copepods fed I. zhanjiangensis and those fed C. meneghiniana, but the average longevity of adults fed I. zhanjiangensis was significantly lower than that of copepods fed C. muelleri (P < 0.05; Fig. 4).
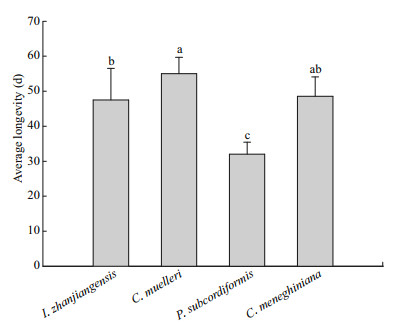
|
| Fig.4 Average longevity of Pseudodiaptomus dubia fed different microalgal species Bars represent means±standard deviation of six replicates per microalgal species. Bars labeled different lowercase letters are significantly different (analysis of variance (ANOVA) and Duncan's multiple range tests; P < 0.05). |
As all copepods fed C. saccharophila died before reaching reproductive maturity, we were only able to measure the reproduction of P. dubia fed the other four microalgal species. Copepods fed C. muelleri, I. zhanjiangensis, and C. meneghiniana had long reproductive periods (averaging 46.2, 35, and 33 days, respectively), while those fed P. subcordiformis reproduced for an average of only 19.1 days (Table 4). Nauplii production did not differ significantly among copepods fed C. muelleri, I. zhanjiangensis, and C. meneghiniana: all females produced > 430 nauplii each (Table 4). However, the production of nauplii by females fed P. subcordiformis was significantly lower: approximately 58 nauplii/female. Copepods fedC.muelleri, I.zhanjiangensis, andC.meneghiniana had similar levels of reproductive frequency (~26– 29 times throughout the experiment) and average clutch size (15.7–19.5 eggs); these metrics were noticeably lower in copepods fed P. subcordiformis (reproduction frequency: ~12 times; average clutch size: 4.8 eggs; Table 4).
|
Pseudodiaptomus dubia fed C. muelleri had the highest net reproductive rate (491.5; Tables 5 & 6), finite growth rate (1.28), and intrinsic growth rate (0.253/d), as well as the fastest population doubling time (2.74 d; Table 6). In contrast, P. dubia fed P. subcordiformis had the lowest net reproductive rate (28.7), finite growth rate (1.2), and intrinsic growth rate (0.185/d), as well as the slowest population doubling time (3.75 d; Table 6). The life-history parameters for P. dubia fed C. meneghiniana and I. zhanjiangensis were intermediate between these two extremes (Table 6).
Diet strongly affects growth and reproduction in copepods (Carotenuto et al., 2002; Murray and Marcus, 2002; Yu et al., 2017). Appropriate foods improve larval survival and increase the speed of larval development, while inappropriate foods may lead to larval death or retard larval development. Inappropriate foods include those that provide inadequate nutrition (Jones and Flynn, 2005), are inappropriately sized (Li et al., 2008b), contain biotoxins (Yu et al., 2017) or produce special chemical compounds (Wolfe et al., 1997; Pohnert et al., 2007; Yu et al., 2017).
It has been suggested that diatoms have deleterious effects on nauplius development (Carotenuto et al., 2002; Ianora et al., 2004; Koski et al., 2008). For example, larvae of the copepod Temora stylifera had high mortality rates and did not develop into adults when fed diatoms such as Thalassiosira, Skeletonema costatum, and Phaeodactylum tricornutum, possibly because these diatoms produce aldehydes that block copepod development (Carotenuto et al., 2002). The development of a closely related species, T. longicornis, was also retarded, with high larval mortality, when fed diatoms (Koski et al., 2008). However, other studies have shown that copepods favored diatoms, and that diatoms sustain copepod development form hatching to adulthood (Vidal, 1980; Koski, 2007). The nutritional status of a given diatom species affects its utility as copepod prey (Jones and Flynn, 2005). Here, P. dubia larvae thrived when fed the diatom species C. muelleri and, to a lesser extent, C. meneghiniana. The rates of larval development, growth, and survival were all significantly greater in larvae fed C. muelleri, as compared to larvae fed chlorophytes (C. saccharophila and P. subcordiformis) or chrysophyte (I. zhanjiangensis). Lora-Vilchis et al. (2004) found that juveniles of the mollusc Atrina maura fed C. muelleri had a higher growth rate than these fed Isochrysis sp., even though Isochrysis sp. was higher in proteins, carbohydrates, and lipids. It may be that the metabolites (e.g., polyunsaturated fatty acids and cholesterol) from the diatom C. muelleri increase the ingestion and growth rates of various mollusk species (Ward et al., 1992; Lora-Vilchis et al., 2004; Chen et al., 2013). Polyunsaturated fatty acids and cholesterol also play a critical role in zooplankton growth (Harrison, 1990; Martin-Creuzburg et al., 2009; Taipale et al., 2014). Thus, our results indicated that C. muelleri was a high-quality diet that improved the growth rate of P. dubia larval. Interestingly, a C. meneghiniana diet had different effects on P. dubia larvae at different developmental stages. During the nauplii stage, larvae fed C. meneghiniana had a significantly lower growth rate than the larvae fed other microalgae (P < 0.05). However, copepodites fed C. meneghiniana had significantly greater growth rates than larvae fed I. zhanjiangensis and C. saccharophila, similar to the growth rates of larvae fed P. subcordiformis. This suggested that C. meneghiniana was more suitable as food for copepodites rather than for nauplii, probably because C. meneghiniana has a hard siliceous shell that is difficult for nauplii to digest and absorb. Therefore, the effects of diatom prey on copepod growth may vary greatly due to species-specific differences.
Platymonas subcordiformis is a suitable food for Acartia bifilosa larvae (Li et al., 2008b) as well as for adult P. dubia (Luo et al., 2008). I. zhanjiangensis is also considered an excellent food for zooplankton (Zhou et al., 2007; Huang et al., 2008), as this species has no cell walls and is easily digestible (Chen et al., 2013). Consistent with these studies, diets of P. subcordiformis and I. zhanjiangensis led to high survival rates for P. dubia larvae.
Copepod fed on C. saccharophila did not exhibit good growth performance; indeed all larvae fed C. saccharophila died before reaching adulthood. Chlorella sp. have low total lipids (Chen et al., 2013), as well thick fibrous cell walls that make this species difficult to digest (Deng et al., 2016). In addition, several researchers have suggested that copepods favor large motile prey (Hargrave and Geen, 1970; Frost, 1977; Calbet et al., 2007). The nauplii of small copepods can ingest foods with a minimum particle size of 3–5 μm (Hargrave and Geen, 1970; Li et al., 2008b). However, the average size of C. saccharophila, as measured here, was 1.8 μm (Table 1). Thus, C. saccharophila particles were too small to be retained by the copepodite of even small copepods. Indeed, the copepodite survival rate decreased dramatically because C. saccharophila particles were too small to be retained by the larger larvae, leading to starvation and death. Similarly, when the microalga Nannochloropsis oculata (maximum particle size: 3.1 μm) were supplied as food to larval Acartia bifilosa, all larvae died upon reaching stage CI, due to the small size of the microalgal particles (Li et al., 2008b). The mass mortality of copepods fed C. saccharophila, may also have been caused by the chemical compounds produced by these microalgae. Unicellular marine algae produce a variety of chemicals as defenses against predators (Wolfe and Steinke, 1996; Wolfe et al., 1997), including toxins and/or intracellular inhibitors such as dimethylsulfoniopropionate (DMSP) (Wolfe and Steinke, 1996; Wolfe et al., 1997). DMSP, which is segregated within microalgal cells and which is only activated during microzooplankton grazing (Wolfe and Steinke, 1996), has deleterious effects on zooplankton ingestion and survival (Wolfe et al., 1997). Li et al., (2010) found that Chllorella (Chlorophyta) produced DMSP, and that DMSP concentrations peaked on days 6–10 of the culture period. This was consistent with our results, as all larvae fed C. saccharophila died on days 8–9 of our feeding experiment (Table 1). Therefore, it is clear that a diet of C. saccharophila is deleterious to the copepod growth probably because this microalga provides inadequate nutrition, is difficult to digest, is too small to retain, and produces biotoxins.
4.2 Effect of diet on reproductionThe amount of polyunsaturated fatty acids (PUFAs), especially docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), in food directly influences the reproduction, development, and survival of copepods (Harrison, 1990; Taipale et al., 2014). Foods containing high levels of PUFAs may increase copepod fertility (Jónasdóttir, 1994; Dam and Lopes, 2003; Yu et al., 2017). Microalgae are one of the few taxa that synthesize EPA and DHA (Brett and Müller-Navarra, 1997; Brett et al., 2009). As different species of microalgae contain different types and levels of nutrients, the fertility, larval development rate, and survival rate of a single species of copepod can vary significantly depending on the microalgal species upon which it feeds (Murray and Marcus, 2002). Here, P. dubia adults fed C. muelleri, I. zhanjiangensis, and C. meneghiniana had significantly higher survival rates, increased nauplii production per female, and longer reproductive periods, as well as more frequent breeding periods as compared to those fed P. subcordiformis. This difference was likely due to the differences in nutritional composition among the microalgal species.
Copepods carry oil sacs that are rich in unsaturated fatty acids; these fatty acids primarily originate from its diet (Brett and Müller-Navarra, 1997; Graeve et al., 2005). The types of foods consumed have been shown to affect lipid accumulation in the copepod Calanus sinicus (Zhou and Sun, 2016). During larval development, we observed that the larvae fed different microalgae had oil sacs of different sizes. Indeed, copepodites fed I. zhanjiangensis, C. muelleri and C. meneghiniana had large oil sacs, while those fed P. subcordiformis and C. saccharophila had no oil sacs. This may have been due to differences in nutritional composition among the microalgae. Several studies have shown that diatoms contain EPA and sterol (Renaud et al., 1995; Lin and Li, 1999; Jiang and Zheng, 2003; Chen et al., 2013), up to 21.45% of the total fatty acids (Lu and Lin, 2001). In addition, I. zhanjiangensis contains high levels of DHA (Chai et al., 2009). However, P. subcordiformis and C. saccharophila contain little or no EPA and DHA (Lu and Lin, 2001; Jiang and Zheng, 2003; Chai et al., 2009; He et al., 2014). This is consistent with our results, as larvae fed C. muelleri, C. meneghiniana and I. zhanjiangensis had good longevity and produced many nauplii, while larvae fed C. saccharophila died at stage CIII stage and larvae fed P. subcordiformis treatment had a relatively low nauplii production per female and poor longevity. Thus, the good performance of P. dubia larvae fed C. muelleri, C. meneghiniana, and I. zhanjiangensis might have been due in part to the high levels of EPA or DHA contained in these microalgae. These results further indicated that C. muelleri, C. meneghiniana, and I. zhanjiangensis were suitable foods for P. dubia.
Copepods have different nutritional needs and restrictions at different life stages (Li et al., 2008b; Yu et al., 2017). Copepod larvae mainly require proteins and carbohydrates for physical development, while female adults need more lipids during the reproductive process (Murray and Marcus, 2002). Indeed, some foods suitable for copepod development are not necessarily suitable for copepod reproduction, while those suitable for copepod reproduction might not be suitable for copepod development and survival (Murray and Marcus, 2002). In addition, the food species which induce the shortest development times do not necessarily result in the highest nauplii production rates (Bonnet and Carlotti, 2001). Consistent with this, although larvae fed P. subcordiformis developed more quickly with a better survival rate at larval stage (Figs. 1, 2), nauplii production was low and longevity was short in adults (Fig. 3; Tables 3 & 4). This indicated that P. subcordiformis was only suitable as feed for developing larvae, and not for reproducing adults. It is also noteworthy that the development and survival rates of larvae fed C. meneghiniana were significantly lower than those of larvae fed other microalgae, but nauplii production by adult females fed C. meneghiniana (440 hatched nauplii/female) was not significantly different from nauplii production of adult females fed the more high-quality foods C. muelleri (566.6 hatched nauplii/female) and I. zhanjiangensis (430 hatched nauplii/female). In addition, a diet of C. meneghiniana led to a long period of vigorous breeding and a high adult survival rate. Thus, C. meneghiniana was a better food for reproductively-active adult P. dubia than for larvae. Some previous studies have shown that mixtures of foods were more favorable for copepod development and reproduction than single foods (Bonnet and Carlotti, 2001; Colin and Dam, 2002). Therefore, a mixture of P. subcordiformis and C. meneghiniana might benefit P. dubia, as these microalgae are nutritionally complementary.
Providing an appropriate diet at each stage of copepod development can significantly improves copepod development, survival, and reproduction (Murray and Marcus, 2002). Our results suggested that foods with a larger particle size should be given at the copepodite stage, such as C. meneghiniana, P. subcordiformis, C. muelleri and I. zhanjiangensis. At the reproductive stage, P. dubia should be fed nutritious foods containing high levels of EPA and DHA, such as C. muelleri, I. zhanjiangensis, and C. meneghiniana. C. muelleri and I. zhanjiangensis were excellent foods for P. dubia throughout its entire lifecycle.
4.3 Effects of diet on population growthThe parameters of population growth (including intrinsic growth rate, net reproduction rate, and finite growth rate) calculated for P. dubia were most optimal in copepods fed C. muelleri. Therefore, C. muelleri was the best food for the support of P. dubia population growth, followed by I. zhanjiangensis and C. meneghiniana. A diet of P. subcordiformis led to slow copepod population growth.
With adequate food, the copepod Euterpina acutifrons had a net reproductive rate of 70.89 and an intrinsic growth rate of 0.161/d (Zurlini et al., 1978). Here, the net reproductive rate of P. dubia fed C. muelleri was 491.5 and the intrinsic growth rate was 0.253/d. Both of these factors were substantially higher in P. dubia, suggesting that P. dubia had a better capacity for population growth than E. acutifrons. Indeed, excluding external factors, populations of P. dubia fed C. muelleri would be expected to double every 2.74 days (Table 6).
5 CONCLUSIONOur results provided a framework for the optimal feeding of P. dubia, based on the five microalgal species most commonly available as live food for P. dubia in the aquaculture ponds of southern China. Our results indicated that C. saccharophila was not a suitable food for P. dubia larvae, as all larvae fed this microalga died at copepodite stage Ⅲ. Both C. muelleri and I. zhanjiangensis were excellent foods for P. dubia throughout its entire life cycle. P. subcordiformis was suitable for developing larvae but not for breeding adults, while C. meneghiniana was suitable for breeding adults but not for developing larvae. Therefore, our results suggested that P. dubia has different nutritional needs and food preferences at different life stages.
6 DATA AVAILABILITY STATEMENTThe datasets generated during and/or analyzed in this study are available from the corresponding author upon reasonable request.
7 ACKNOWLEDGMENTWe thank CHEN Wenjie, ZHANG Chong and ZHANG Bingren for their help with sampling. Sincere thanks go to WEI Dong and KE Sheng for their assistance in P. dubia cultivation.
Ban S. 1994. Effect of temperature and food concentration on post-embryonic development, egg production and adult body size of calanoid copepod Eurytemora affinis. Journal of Plankton Research, 16(6): 721-735.
DOI:10.1093/plankt/16.6.721 |
Bonnet D, Carlotti F. 2001. Development and egg production in Centropages typicus (Copepoda: Calanoida) fed different food types: a laboratory study. Marine Ecology Progress Series, 224: 133-148.
DOI:10.3354/meps224133 |
Brett M T, Kainz M J, Taipale S J, Seshan H. 2009. Phytoplankton, not allochthonous carbon, sustains herbivorous zooplankton production. Proceedings of the National Academy of Sciences of the United States of America, 106(50): 21 197-21 201.
DOI:10.1073/pnas.0904129106 |
Brett M, Müller-Navarra D. 1997. The role of highly unsaturated fatty acids in aquatic foodweb processes. Freshwater Biology, 38(3): 483-499.
DOI:10.1046/j.1365-2427.1997.00220.x |
Calbet A, Carlotti F, Gaudy R. 2007. The feeding ecology of the copepod Centropages typicus (Kröyer). Progress in Oceanography, 72(2-3): 137-150.
DOI:10.1016/j.pocean.2007.01.003 |
Carotenuto Y, Ianora A, Buttino I, Romano G, Miralto A. 2002. Is postembryonic development in the copepod Temora stylifera negatively affected by diatom diets?. Journal of Experimental Marine Biology and Ecology, 276(1-2): 49-66.
DOI:10.1016/S0022-0981(02)00237-X |
Chai Y, Wu Y, Zhao H H, Yu D. 2009. Effects of light qualities on growth and fatty acid composition of Isochrysis zhanjiangensis Hu & Liu. Plant Physiology Communications, 45(6): 571-574.
(in Chinese with English abstract) |
Chen M Y. 1995. Culture of Feed Organisms. China Agriculture Press, Beijing, China.
(in Chinese)
|
Chen Z Q, Shou L, Liao Y B, Zeng J N. 2013. Advance in the effect of Microalgal diets and Nutritional value on the growth of early life stages of bivalves. Bulletin of Science and Technology, 29(7): 46-55, 67.
(in Chinese with English abstract) |
Colin S P, Dam H G. 2002. Testing for toxic effects of prey on zooplankton using sole versus mixed diets. Limnology and Oceanography, 47(5): 1 430-1 437.
DOI:10.4319/lo.2002.47.5.1430 |
Dam H G, Lopes R M. 2003. Omnivory in the calanoid copepod Temora longicornis: feeding, egg production and egg hatching rates. Journal of Experimental Marine Biology and Ecology, 292(2): 119-137.
DOI:10.1016/S0022-0981(03)00162-X |
Deng Z H, Jiang S, Zhang B, Liu B S, Huang G J, Yu D H. 2016. Ingestion and digestion of pearl oyster (Pinctada fucata) on microalgae of different types and concentrations. South China Fisheries Science, 12(3): 112-118.
(in Chinese with English abstract) |
Frost B W. 1977. Feeding behavior of Calanus pacificus in mixtures of food particles. Limnology and Oceanography, 22(3): 472-491.
DOI:10.4319/lo.1977.22.3.0472 |
Graeve M, Albers C, Kattner G. 2005. Assimilation and biosynthesis of lipids in Arctic Calanus species based on feeding experiments with a 13C labelled diatom. Journal of Experimental Marine Biology and Ecology, 317(1): 109-125.
DOI:10.1016/j.jembe.2004.11.016 |
Hargrave B T, Geen G H. 1970. Effects of copepod grazing on two natural phytoplankton populations. Journal of the Fisheries Research Board of Canada, 27(8): 1 395-1 403.
DOI:10.1139/f70-165 |
Harrison N M. 1990. Gelatinous zooplankton in the diet of the parakeet auklet: comparisons with other auklets. Studies in Avian Biology, 14: 114-124.
|
He R, Xu N, Duan S S. 2014. Total lipid content and fatty acid composition of 9 strains of marine microalgae. Eco log ical Science, 33(1): 93-98.
(in Chinese with English abstract) |
Huang H L, Deng C M, Fu S. 2008. Study on the food for the larvae of Pentad margaritifera Linnaeus. Journal of A quaculture, 29(1): 1-4.
(in Chinese with English abstract) |
Huang H Z, Luo H M. 1980. Preliminary investigation on the diet ingestion and absorption of Schmackeria dubia and Artemia salina. Journal of Xiamen University (Natural Science), 19(3): 81-90.
(in Chinese with English abstract) |
Ianora A, Miralto A, Poulet S A, Carotenuto Y, Buttino I, Romano G, Casotti R, Pohnert G, Wichard T, ColucciD'Amato L, Terrazzano G, Smetacek V. 2004. Aldehyde suppression of copepod recruitment in blooms of a ubiquitous planktonic diatom. Nature, 429(6990): 403-407.
DOI:10.1038/nature02526 |
Jiang X M, Zheng Y Z. 2003. Total lipid and fatty acid composition of 14 species of mircoalgae. Acta Hydrobiologica Sinica, 27(3): 243-247.
(in Chinese with English abstract) |
Jónasdóttir S. 1994. Effects of food quality on the reproductive success of Acartia tonsa and Acartia hudsonica: laboratory observations. Marine Biology, 121(1): 67-81.
DOI:10.1007/BF00349475 |
Jones R H, Flynn K J. 2005. Nutritional status and diet composition affect the value of diatoms as copepod prey. Science, 307(5714): 1 457-1 459.
DOI:10.1126/science.1107767 |
Koski M, Kuosa H. 1999. The effect of temperature, food concentration and female size on the egg production of the planktonic copepod Acartia bifilosa. Journal of Plankton Research, 21(9): 1 779-1 789.
DOI:10.1093/plankt/21.9.1779 |
Koski M, Wichard T, Jónasdóttir S H. 2008. "Good" and "bad" diatoms: development, growth and juvenile mortality of the copepod Temora longicornis on diatom diets. Marine Biology, 154(4): 719-734.
DOI:10.1007/s00227-008-0965-4 |
Koski M. 2007. High reproduction of Calanus finmarchicus during a diatom-dominated spring bloom. Marine Biology, 151(5): 1 785-1 798.
DOI:10.1007/s00227-007-0615-2 |
Li C L, Luo X X, Huang X H, Gu B H. 2008a. Effects of temperature, salinity, pH, and light on filtering and grazing rates of a calanoid copepod (Schmackeria dubia). The Scientific World Journal, 8: 1 219-1 227.
DOI:10.1100/tsw.2008.153 |
Li C L, Luo X X, Huang X H, Gu B H. 2009. Influences of temperature on development and survival, reproduction and growth of a calanoid copepod (Pseudodiaptomus dubia). The Scientific World Journal, 9: 866-879.
DOI:10.1100/tsw.2009.96 |
Li C X, Yang G P, Pan J F, Zhang H H. 2010. Experimental studies on dimethylsulfide (DMS) and dimethylsulfoniopropionate (DMSP) production by four marine microalgae. Acta Oceano log ica Sinica, 29(4): 78-87.
DOI:10.1007/s13131-010-0052-9 |
Li J, Sun S, Li C L, Zhang Z, Pu X M. 2008b. Effects of different diets on the reproduction and naupliar development of the copepod Acartia bifilosa. Journal of Experimental Marine Biology and Ecology, 355(2): 95-102.
DOI:10.1016/j.jembe.2007.12.005 |
Lin X Z, Li G Y. 1999. Effects of enviomental factors on microalgal lipids. Journal of Oceano Graphy of Huanghai & Bohai Seas, 17(4): 53-59.
(in Chinese with English abstract) |
Lora-Vilchis M C, Ruiz-Velasco-Cruz E, Reynoso-Granados T, Voltolina D. 2004. Evaluation of five microalgae diets for juvenile pen shells Atrina maura. Journal of the World Aquaculture Society, 35(2): 232-236.
DOI:10.1111/j.1749-7345.2004.tb01079.x |
Lu K H, Lin X. 2001. Screening of fatty acid composition of the 13 microalgae and their application in artificial breeding of Mitten Crab. Journal of Ningbo University (NSEE), 14(3): 27-32.
(in Chinese with English abstract) |
Luo X X, Huang X H, Hong T. 2008. Effect of type and density of feed on ingestion characteristics of Schmackeria dubin. Journal of Guangdong Ocean University, 28(3): 39-44.
(in Chinese with English abstract) |
Martin-Creuzburg D, Sperfeld E, Wacker A. 2009. Colimitation of a freshwater herbivore by sterols and polyunsaturated fatty acids. Proceedings of the Royal Society of London B: Biological Sciences, 276(1663): 1 805-1 814.
DOI:10.1098/rspb.2008.1540 |
Martin-Creuzburg D, Wacker A, Von Elert E. 2005. Life history consequences of sterol availability in the aquatic keystone species Daphnia. Oeco log ia, 144(3): 362-372.
|
Meyer J S, Ingersoll C G, McDonald L L, Boyce M S. 1986. Estimating uncertainty in population growth rates: jackknife vs. bootstrap techniques. Ecology, 67(5): 1 156-1 166.
DOI:10.2307/1938671 |
Murray M M, Marcus N H. 2002. Survival and diapause egg production of the copepod Centropages hamatus raised on dinoflagellate diets. Journal of Experimental Marine Biology and Ecology, 270(1): 39-56.
DOI:10.1016/S0022-0981(02)00016-3 |
Pohnert G, Steinke M, Tollrian R. 2007. Chemical cues, defence metabolites and the shaping of pelagic interspecific interactions. Trends in Ecology & Evolution, 22(4): 198-204.
|
Renaud S M, Zhou H C, Parry D L, Thinh L V, Woo K C. 1995. Effect of temperature on the growth, total lipid content and fatty acid composition of recently isolated tropical microalgae Isochrysis sp., Nitzschia closterium, Nitzschia paleacea, and commercial species Isochrysis sp. (clone T. ISO). Journal of Applied Phycology, 7(6): 595-602.
DOI:10.1007/BF00003948 |
Shang X, Wang G Z, Li S J. 2005. Comparative studies on the group increasing of egg-carrying and free-spawning copepods. Fujian Journal of Agricultural Sciences, 20(4): 251-256.
(in Chinese with English abstract) |
Shen G Y, Shi B Z. 2002. Marine Ecology. Science Press, Beijing, China. p.104-108.
(in Chinese)
|
Taipale S J, Brett M T, Hahn M W, Martin-Creuzburg D, Yeung S, Hiltunen M, Strandberg U, Kankaala P. 2014. Differing Daphnia magna assimilation efficiencies for terrestrial, bacterial, and algal carbon and fatty acids. Ecology, 95(2): 563-576.
DOI:10.1890/13-0650.1 |
Tang B, Zhang F, Hu Z Y, Xiong J W, Geng X L. 2005. The comparison of development and life table of population between Tetranychus cinnabarinus (Boisduval) and T. urticae (Koch). Journal of Mountain Agriculture and Biology, 24(1): 42-47.
(in Chinese with English abstract) |
Uye S, Iwai Y, Kasahara S. 1983. Growth and production of the inshore marine copepod Pseudodiaptomus marinus in the central part of the Inland Sea of Japan. Marine Biology, 73(1): 91-98.
DOI:10.1007/BF00396289 |
Vidal J. 1980. Physioecology of zooplankton. I. Effects of phytoplankton concentration, temperature, and body size on the growth rate of Calanus pacificus and Pseudocalanus sp. Marine Biology, 56(2): 111-134.
DOI:10.1007/BF00397129 |
Ward J E, Cassell H K, MacDonald B A. 1992. Chemoreception in the sea scallop Placopecten magellanicus (Gmelin). I. Stimulatory effects of phytoplankton metabolites on clearance and ingestion rates. Journal of Experimental Marine Biology and Ecology, 163(2): 235-250.
DOI:10.1016/0022-0981(92)90052-C |
Wolfe G V, Steinke M, Kirst G O. 1997. Grazing-activated chemical defence in a unicellular marine alga. Nature, 387(6636): 894-897.
DOI:10.1038/43168 |
Wolfe G V, Steinke M. 1996. Grazing-activated production of dimethyl sulfide (DMS) by two clones of Emiliania huxleyi. Limnology and Oceanography, 41(6): 1 151-1 160.
DOI:10.4319/lo.1996.41.6.1151 |
Yu J, Tian J Y, Yang G P. 2017. Ingestion, fecundity and population growth of Harpacticus sp. (Harpacticoida, copepod) fed on five species of algae. Aquaculture Research, 48(5): 2 209-2 220.
DOI:10.1111/are.13057 |
Zhou K S, Sun S. 2016. Effect of temperature and food on lipid accumulation in Calanus sinicus (copepoda: calanoida). Oceano log ia et Limno log ia Sinica, 47(4): 787-794.
(in Chinese with English abstract) |
Zhou Y H, Huang H L, Deng C M, Fu S. 2007. Effect of microalgae on the growth and survival of Pinctada margaritifera veligers. Journal of Oceanography in Taiwan Strait, 26(2): 249-255.
(in Chinese with English abstract) |
Zurlini G, Ferrari I, Nassogne A. 1978. Reproduction and growth of Euterpina acutifrons (Copepoda: Harpacticoida) under experimental conditions. Marine Biology, 46(1): 59-64.
DOI:10.1007/BF00393821 |
 2019, Vol. 37
2019, Vol. 37