Institute of Oceanology, Chinese Academy of Sciences
Article Information
- WANG Shanyue, CUI Xueping, XU Ruyi, GAO Meirong, SUI Liying
- Effect of carbon and nitrogen ratio control on Artemia growth, water quality, biofloc microbial diversity under high salinity and zero-water exchange culture condition
- Journal of Oceanology and Limnology, 37(5): 1768-1776
- http://dx.doi.org/10.1007/s00343-019-8288-5
Article History
- Received Oct. 5, 2018
- accepted in principle Nov. 15, 2018
- accepted for publication Feb. 13, 2019
Biofloc technology (BFT) has been successfully applied in some aquaculture species. Through adding extra carbon source to the culture water or supplying the formulated feed with high carbon content, the inorganic nitrogen assimilation can be facilitated by heterotrophic microorganisms, which consequently improve the water quality and provide the additional microbial protein and other nutrients to the aquatic animals. It has been reported that application of BFT in the intensive farming systems with minor or no water exchange significantly increased the yield and reduced feed cost in the culture of tilapia Oreochromis niloticus (Crab et al., 2009), white shrimp Litopenaeus vannamei (Schveitzer et al., 2013), Japanese prawn Marsupenaeus japonicas (Zhao et al., 2012) and pink shrimp Farfantepenaeus brasiliensis (Emerenciano et al., 2012).
Maintaining C/N ratio in a proper range is one of the key elements in successful BFT application (Avnimelech, 2006). Previous studies have indicated that higher C/N ratio (10–20) promoted biofloc formation (Hargreaves, 2006; Asaduzzaman et al., 2008; Ballester et al., 2010). Moreover, increasing C/N ratio from 10 to 20 significantly reduced total ammonia nitrogen (TAN), nitrite nitrogen (NO2-N) and nitrate nitrogen (NO3-N) in the water column and total nitrogen (TN), but increased total heterotrophic bacteria in the sediment (Asaduzzaman et al., 2008). Supplementing starch in the feed of tilapia Oreochromis mossambicus at a C/N ratio of 20 resulted in a reduced daily water changing rate from 24% to 10% at a maximum stocking density of 20 kg/m3 (Crab et al., 2009). Wang et al. (2015) demonstrated that BFT could effectively reduce TAN, NO2-N and NO3-N concentration in crucian carp Carassius auratus culture at C/N ratio > 15. Xu et al. (2016) showed better growth, significantly higher yield and lower feed conversion ratio in white shrimp L. vannamei juvenile culture at lower C/N ratios (9 and 12) than the higher ones (15 and 18).
Brine shrimp Artemia is widely distributed in inland salt lakes and coastal solar saltworks. As a nonselective filter feeder, Artemia plays an important role in the aquatic food chain. On the other hand, Artemia nauplii are the most commonly used live food in marine fish and crustacean larviculture (Sorgeloos et al., 2001). The market price of Artemia cysts varies with its annual harvest and aquaculture demand and depends on the hatching performance and nutritional quality of different Artemia strains and batches. Thus, there is a need for the controlled pond production of specific Artemia strains (e.g. smaller size and higher nutrition value) for a niche market. Several studies have proved that BFT could be used for high-density Artemia culture under high salinity conditions. Sui et al. (2013) and Ronald et al. (2014) reported that supplementation of tapioca and chicken manure at C/N ratio of 10 and 7.4, respectively, significantly increased the production of Artemia biomass in the salt ponds. Toi et al. (2013) showed that in algaelimited condition, the addition of sucrose and soluble starch at C/N ratio of 10 and 50 significantly reduced the amount of algae provision, and the added carbon could be indirectly assimilated by Artemia through feeding the isotope-labeled bacteria. Gao et al. (2017) indicated that supplementation of carbon sources (e. g. molasses, glucose, sucrose, and corn flour) at C/N ratio of 20 improved Artemia growth at a salinity of 80.
It has been recognized that the growth of microorganisms at higher salinity is usually slower than that in the fresh and marine environment. Although the above studies have indicated that carbon supplementation could improve Artemia growth, Artemia biomass and cysts production, the microbial development and management of BFT under high salinity condition is still less concerned. The aim of this study is to investigate the effect of C/N ratio management on Artemia growth, water quality, and biofloc microbial diversity and composition in Artemia culture at salinity 100, which should provide information for studying the mechanism of BFT and its application in high salinity culture conditions.
2 MATERIAL AND METHOD 2.1 Artemia hatching and cultureThe Great Salt Lake Artemia franciscana cysts (INVE, Belgium) were hatched following the procedure proposed by Van Stappen (1996). After 24 h hatching, Artemia nauplii were collected and transferred into a conical tank containing diluted brine (salinity 50) at a density of 4 ind./mL. Artemia were cultured at room temperature (22–26℃), with continuous aeration and light regime of 12 h D:12 h L. During 4-d acclimation, the water salinity was gradually increased from 50 to 100 by adding concentrated brine. Artemia were fed formulated feed (Selco-Rotifer, INVE, Belgium) which has been modified according to the recommended feeding regime. The average feeding amount was 0.025 mg/d/Artemia.
Four days later, Artemia were randomly distributed in the plastic cones containing 800 mL diluted brine (salinity 100) at an initial density of 1.5 ind. /mL. The culture condition was kept the same as the acclimation culture. No water renew were conducted during 24-d culture period, except for adding the deionized water to the cones every day (until reaching the 800-mL marked level) to maintain the salinity.
2.2 Experimental designThe experiment was conducted for 24 d. In period of the first 18-d culture, the amount of carbon in culture water were manipulated by adding sucrose at a C/N ratio of 5 (Su5), 15 (Su15), and 30 (Su30), respectively. The culture without sucrose supplementation was considered as the control. Each treatment contained three replicates. No feed and sucrose were added for the last 6 d of the experiment.
The feeding ration of the formulated diet was 60% of the modified feeding level. Every day, 81-, 243-, and 486-mg of sucrose were added into each cone for group of Su5, Su15, and Su30, respectively. The supplementation of sucrose was calculated according to Avnimelech (1999):
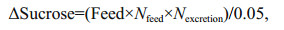
Nfeed is the nitrogen content of the formulated feed (taking the protein concentrate of 36% and a conversion factor to nitrogen of 6.25); Nexcretion is the ratio of the nitrogen excretion by Artemia (generally 50% of the nitrogen content in the feed); 0.05 is a constant.
2.3 Parameter analysis 2.3.1 S urvival rateAt the end of the experiment, the survival rate of Artemia was determined. Fifty-milliliter culture water was randomly sampled from each cone, and four sampling were executed for each cone. The survival rate of Artemia was calculated by dividing the average number of survived Artemia (on base of 800 mL) by the initial stocking number of Artemia (1 500 ind.).
2.3.2 Water qualityThe water temperature, pH (Mettler Toledo), and dissolved oxygen (DO, WTW) were measured daily. Every 3 or 7 d, 50-mL culture water was sampled from each replicate and filtered through 0.22-μm microporous membrane. The dissolved organic carbon (DOC), total phosphorus (TP), TN, TAN, and NO2-N analysis accordingly. DOC was measured by a total organic carbon automatic analyzer (Shimadzu). The content of TN, TP, NO2-N, and TAN were analyzed using spectrophotometer following the standard methods (SOA, 2008).
2.3.3 Biofloc volume and microbial communityAt the end of the experiment, the biofloc volume (BFV) was measured after settling the culture water for 24 h in an Imhoff tube. The flocs were collected and stored at -80℃ for later analysis. The biofloc microbial diversity and composition in control and Su15 group were analyzed.
The genomic DNA was extracted using a Solarbio Genomic DNA kit (Beijing, China) and were sent to Novogene (Beijing, China) for high-throughput sequencing. 16S rRNA gene fragments were amplified with the primers 515F (5′-barcode-GTGCCAGCMGCCGCGGTAA-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′). Amplification condition were: 94℃ for 5 min, 29 cycles with 94℃ for 50 s, 47℃ for 30 s, 72℃ for 30 s and a final elongation step of 72℃ for 5 min. All PCR reactions were carried out with Phusion® HighFidelity PCR Master Mix (New England Biolabs). Sequencing libraries were generated using TruSeq® DNA PCR-Free. The library quality was assessed on the Qubit@ 2.0 Fluorometer (Thermo Scientific) and Agilent Bioanalyzer 2100 system. The library was sequenced on an Illumina HiSeq 2500 platform and 250 bp paired-end reads were generated.
After Illumina sequencing, qualities filtering on the raw tags were performed under specific filtering conditions to get high-quality clean tags according to the QIIME quality controlled process (Caporaso et al., 2010). Sequences identified as chimeras by UCIME (Edgar et al., 2011) were removed (Haas et al., 2011) before the RDP Classifier analysis at confidence threshold of 80% (Wang et al., 2007). OTUs clustering was performed by 97% similarity using Uparse software (Edgar et al., 2013). The richness and diversity index including Chao1, Shannon, Simpson, ACE, and Good-coverage were analyzed in the Mothur (Schloss et al., 2009).
2.4 Statistical analysisData of Artemia growth, BFV, and water quality were presented as means±standard deviation and analyzed by one-way ANOVA, followed by Ducan's test to compare the significant difference among the treatments at P < 0.05 (SPSS Statistics, Version 19). Homogeneity of variance was tested by Levene's test, using arcsine-square root or logarithmic transformation when necessary.
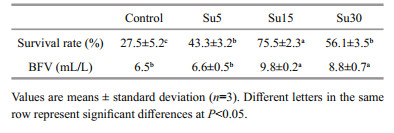
3 RESULT 3.1 Survival rateCompared to the control, the addition of sucrose significantly increased Artemia survival rate, and the highest survival rate (75.5%) was obtained at C/N ratio of 15 (P < 0.05) (Table 1). The control and Su5 group had similar BFV values (P > 0.05), while the addition of sucrose at C/N ratio of 15 and 30 significantly increased BFV (P < 0.05).
|
Over the culture period, the water temperature ranged 23–25℃, the dissolved oxygen ranged 4.6– 5.0 mg/L and pH ranged 7.8–8.0. There were no significantly different for TP, TN, and DOC content between the control and Su5 group (P > 0.05). Sucrose addition at C/N ratio of 15 and 30 significantly reduced TP content but increased DOC content (P < 0.05), whilst C/N ratio of 30 resulted in a significantly lower TN content (P < 0.05) at the end of the experiment (Day 24) (Table 2).

|
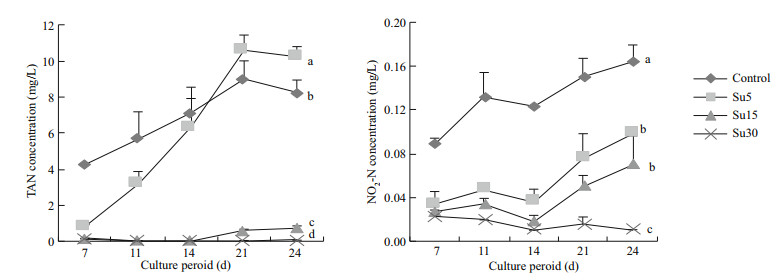
In the culture period, the TAN content in the control and Su5 group increased substantially with a slight declination observed on Day 24 (Fig. 1). However, the TAN content in Su15 and Su30 group remained at a much lower level (< 1 mg/L) compared to the control and Su5 group (P < 0.05). The NO2-N in all treatments remained at a low level (< 0.16 mg/L), among which the control had the highest value and Su30 group had the lowest value (P < 0.05). Increasing C/N ratio remarkably reduced the NO2-N content.
|
| Fig.1 TAN (left) and NO2-N (right) concentration in the culture medium when supplementing sucrose at different C/N ratios |
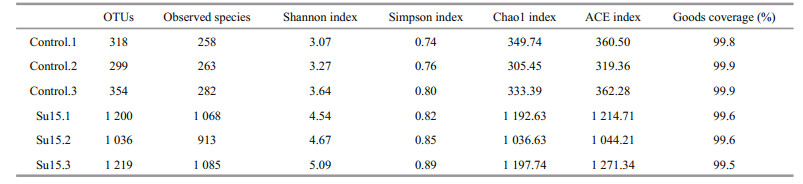
The number of OTUs ranged from 299 to 318 for the control, and from 1 036 to 1 219 for the Su15 group (Table 3). To compare the microbial diversity and richness in each replicate, the diversity (Shannon and Simpson) and richness (Chao1 and ACE) indices were calculated from OTUs of each library. All above indices were higher for the Su15 group than the control, suggesting that supplementation of sucrose resulted in higher biodiversity and richness. Moreover, all the coverage rates were more than 99%, indicating that the sample library coverage was sufficient to represent the microbial communities of the samples.
|
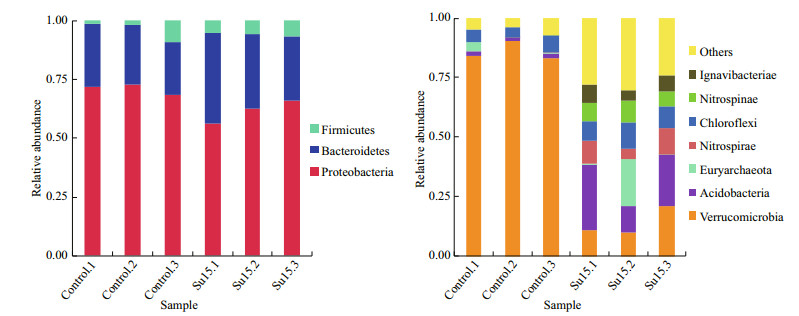
In total, 40 phyla were found in average value of 19 in the control and 35 for Su15 group, respectively. Among them, Proteobacteria (> 50% of the total sequences) dominated in all samples, followed by Bacteroides and Firmicutes, no obvious difference could be observed between two groups (Fig. 2, left). However, when considering the phylum rank 4–10, remarkable differences were observed between two groups (Fig. 2, right). Phylum Verrucomicrobia was the majority in the control; the replicate Su15.2 had high proportion of the phylum Euryarchaeota.
|
| Fig.2 Relative abundance of top 3 phylum (left) and rank 4–10 phylum (right) were analyzed from sequencing libraries of the control and Su15 group |
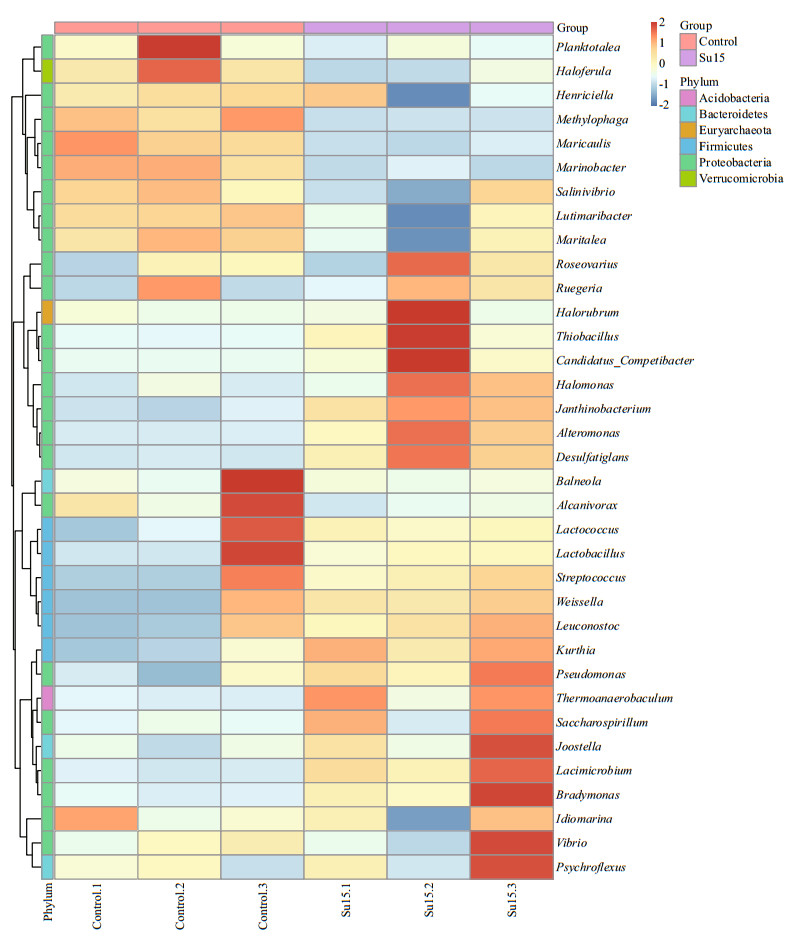
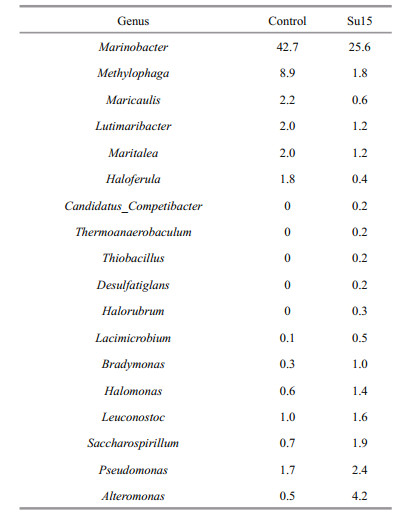
Microbial diversity and abundance were analyzed at the genus level according to the annotation and abundance information. The abundance of ranking top 35 genera is shown in Fig. 3. Cluster analysis showed that the bacterial community structures of the bioflocs were different between two groups and sucrose supplementation resulted generally in higher content in the most genus. The predominant genera (> 1% of the total observed genus) in the control included Mariobacter, Methylophaga, Lutimaribacter, Leuconostoc, Pseudomonas, and Maritalea (Table 4). The addition of sucrose reduced the abundance of Maricaulis, Mariobacter, Methylophaga, and Haloferula but increased that of Halomonas, Bradymonas, Saccharospirillum, and Alteromonas. Genera Candidatus_Competibacter, Thermoanaerobaculum, Thiobacillus, and Desulfatiglans were hardly found in the control.
|
| Fig.3 Heat map illustrating the abundance of the top 35 genera The color intensity (log scale) in each panel indicates the relative abundance of the genus in the control and Su15 group. |
|
Usually, the microbial growth rate is relatively lower in higher salinity conditions; therefore, a broader range of C/N ratios (5, 15, and 30) was chosen to identify the proper C/N ratio in this study. An improved Artemia survival and BFV were reached by supplementing sucrose, suggesting that microorganisms grown on sucrose contributed as a food source for Artemia. The C/N ratio of 15 had the highest survival and BFV, and insufficient (Su5) or excessive addition (Su30) of carbon may result in an inferior survival of Artemia due to a less food supply and detrimental water quality, thus proper C/N ratio should be controlled for carbon supplementation.
4.2 Water qualityAmmonia nitrogen and nitrite nitrogen are major stress factors in an intensive aquaculture (Tovar et al., 2000). Ammonia nitrogen can be converted into nitrite and nitrate via autotrophic nitrifying bacteria (Ray and Lotz, 2014), or directly assimilated by heterotrophic bacteria in presence of extra carbon source referring BFT. It has been reported that microorganisms that attached to the bioflocs are able to assimilate 0.2 g nitrogen per square meter per day in condition of C/N > 10 (Azim et al., 2008), and 10 mg/L TAN could be completely converted by microorganisms within 5 h without NO2-N and NO3-N accumulation at C/N ratio of 20 (Asaduzzaman et al., 2008). In our study, the TAN and NO2-N content in Su5 group increased with the culture period, indicating the sub-optimal growth of heterotrophic microorganisms under low C/N ratio conditions. On the other hand, although there was a slight increment over the culture period, the TAN and NO2-N contents were effectively reduced at C/N ratio of 15 and 30. Together with the better Artemia survival and BFV value, we assumed that higher C/N ratio could offer better growth conditions for Artemia through efficient growth and assimilation of heterotrophic microorganisms.
High DOC content in the water could result in the extra cost and increase the osmotic pressure, particularly with sucrose, which has been reported to inhibit the growth of some microorganisms (Chirife et al., 1983). It should be mentioned that, although the lowest nitrogenous and TP contents were obtained at C/N ratio of 30, the Artemia survival rate was significantly lower than Su15 group (P < 0.05) in this study. This may be associated with the high level of DOC remained in the water.
In this study, the TN and TP values remained at similar levels except for group Su30. Total nitrogen and total phosphorus in the water were resulted from Artemia metabolism, decay of dead Artemia, and uneaten feed, etc., which contain different types of phosphorus and nitrogen and may not be assimilated by microorganism efficiently at lower C/N ratio.
4.3 Microbial communityMicroorganisms that developed in the BFT system play an important role in the provision of essential nutrients, enzymes, and immune stimulants (Loreau et al., 2001; Toi et al., 2013; Wei et al., 2016). We demonstrated that sucrose supplementation (in Group Su15) could improve significantly the bacterial microbial diversity and shape the microbial composition of the bioflocs compared to the control. The high degree of bacterial microbial diversity in biofloc system could facilitate the nitrogen removal and microbiota stability (Briones and Raskin, 2003; Ebeling et al., 2006), and thus enhance the survival and water quality in Artemia culture.
In this study, Proteobacteria and Bacteroidetes are the dominant bacteria phyla in each sample. Similar results have been reported when adding different carbon sources into the biofloc systems (Wei et al., 2016; Deng et al., 2017). Proteobacteria is widely dispersed in aquaculture or natural environment, which plays an important role in the nutrient cycling and mineralization of the organic compounds (Kersters et al., 2006; Cardona et al., 2016). Moreover, sucrose supplementation changed the diversity and composition of microbes at genus level; for example, genera Saccharospirillum (Labrenz et al., 2003), Leuconostoc (Santos et al., 2000), and Alteromonas (Bowman and McMeekin, 2015) that are able to use carbohydrates reached much higher abundance in group Su15.
In the nature, the optimal salinity for Artemia growth and reproduction ranges approximately 80– 150 (Van Stappen, 2002), in which halophilic bacteria and archaea coexist with Artemia, or they might form a part of the food chain of Artemia. Bellisario et al. (2013) identified seven halophilic bacteria and five archaea species in adult Artemia using molecular tools. Rahmani et al. (2016) stated that archaea Halorubrum existed in both Urmia Salt Lake and the adult Artemia. Lopes-dos-Santos et al. (2019) confirmed that several halophilic bacteria species could be the food source for Artemia. In our study, the high throughput sequencing analysis showed a high microbial biodiversity; most of them belonged to bacteria and few to archaea (e.g. Halorubrum), the third domain of life. Although 16S rRNA gene of Halorubrum could be PCR amplified with a universal bacterial primer, the preferences of the primer could result in less determined richness of other archaea.
5 CONCLUSIONUnder a high salinity culture condition, a supplementation of sucrose at C/N ratios of 15 and 30 could much stimulate the biofloc development, enhance the water quality, and improve the Artemia survival. The addition of sucrose at C/N ratio of 15 in the water could improve the biodiversity and richness of the microbial community of the bioflocs. In the future study, a biofloc technology relating microbial management shall focus on halophilic archaea, to test whether these microorganisms can be integrated in the bioflocs and whether they can be used as a food item for the aquatic animals in a hypersaline environment.
6 ETHICAL STATEMENTAll experimental and analytical procedures were approved by the Ethics Committee at Tianjin University of Science and Technology.
7 DATA AVAILABILITY STATEMENTThe data used to support the findings of this study are available from the corresponding author upon request.
Asaduzzaman M, Wahab M A, Verdegem M C J, Huque S, Salam M A, Azim M E. 2008. C/N ratio control and substrate addition for periphyton development jointly enhance freshwater prawn Macrobrachium rosenbergii production in ponds. Aquaculture, 280(1-4): 117-123.
DOI:10.1016/j.aquaculture.2008.04.019 |
Avnimelech Y. 1999. Carbon/nitrogen ratio as a control element in aquaculture systems. Aquaculture, 176(3-4): 227-235.
DOI:10.1016/S0044-8486(99)00085-X |
Avnimelech Y. 2006. Bio-filters: the need for an new comprehensive approach. Aquacultural Engineering, 34(3): 172-178.
DOI:10.1016/j.aquaeng.2005.04.001 |
Azim M E, Little D C, Bron J E. 2008. Microbial protein production in activated suspension tanks manipulating C:N ratio in feed and the implications for fish culture. Bioresource Technology, 99(9): 3 590-3 599.
DOI:10.1016/j.biortech.2007.07.063 |
Ballester E L C, Abreu P C, Cavalli R O, Emerenciano M, de Abreu L, Wasielesky JR W. 2010. Effect of practical diets with different protein levels on the performance of Farfantepenaeus paulensis juveniles nursed in a zero exchange suspended microbial flocs intensive system. Aquaculture Nutrition, 16(2): 163-172.
DOI:10.1111/j.1365-2095.2009.00648.x |
Bellisario B, Carere C, Cerfolli F, Angeletti D, Nascetti G, Cimmaruta R. 2013. Infaunal macrobenthic community dynamics in a manipulated hyperhaline ecosystem: a long-term study. Aquatic Biosystems, 9: 20.
DOI:10.1186/2046-9063-9-20 |
Bowman J P, McMeekin T A. 2015. Alteromonas. In: Whitman W B ed. Bergey's Manual of Systematics of Archaea and Bacteria. John Wiley & Sons, Ltd., New York.
|
Briones A, Raskin L. 2003. Diversity and dynamics of microbial communities in engineered environments and their implications for process stability. Current Opinion in Biotechnology, 14(3): 270-276.
DOI:10.1016/S0958-1669(03)00065-X |
Caporaso J G, Kuczynski J, Stombaugh J, Bittinger K, Bushman F D, Costello E K, Fierer N, Peña A G, Goodrich J K, Gordon J I, Huttley G A, Kelley S K, Knights D, Koenig J E, Ley R E, Lozupone C A, McDonald D, Muegge D B, Pirrung M, Reeder J, Sevinsky J R, Turnbaugh P J, Walters W A, Widmann J, Yatsunenko T, Zaneveld J, Knight R. 2010. QIIME allows analysis of high-throughput community sequencing data. Nature Methods, 7(5): 335-336.
DOI:10.1038/nmeth.f.303 |
Cardona E, Gueguen Y, Magré K, Lorgeoux B, Piquemal D, Pierrat F, Noguier F, Saulnier D. 2016. Bacterial community characterization of water and intestine of the shrimp Litopenaeus stylirostris in a biofloc system. BMC Microbiology, 16: 157e.
DOI:10.1186/s12866-016-0770-z |
Chirife J, Herszage L, Joseph A, Kohn E S. 1983. In vitro study of bacterial growth inhibition in concentrated sugar solutions: microbiological basis for the use of sugar in treating infected wounds. Antimicrobial Agents and Chemotherapy, 23(5): 776-773.
DOI:10.1128/AAC.23.5.766 |
Crab R, Kochva M, Verstraete W, Avnimelech Y. 2009. Bioflocs technology application in over-wintering of tilapia. Aquacultural Engineering, 40(3): 105-112.
DOI:10.1016/j.aquaeng.2008.12.004 |
Deng M, Chen J Y, Hou J, Li D P, He X G. 2017. The effect of different carbon sources on water quality, microbial community and structure of biofloc systems. Aquaculture, 482: 103-110.
DOI:10.1016/j.aquaculture.2017.09.030 |
Ebeling J M, Timmons M B, Bisogni J J. 2006. Engineering analysis of the stoichiometry of photoautotrophic, autotrophic, and heterotrophic removal of ammonianitrogen in aquaculture systems. Aquaculture, 257(1-4): 346-358.
DOI:10.1016/j.aquaculture.2006.03.019 |
Edgar R C, Haas B J, Clemente J C, Quince C, Knight R. 2011. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics, 27(16): 2 194-2 200.
DOI:10.1093/bioinformatics/btr381 |
Edgar R C. 2013. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nature Methods, 10(10): 996-998.
DOI:10.1038/nmeth.2604 |
Emerenciano M, Ballester E L C, Cavalli R O, Wasielesky W. 2012. Biofloc technology application as a food source in a limited water exchange nursery system for pink shrimp Farfantepenaeus brasiliensis (Latreille, 1817). Aquaculture Research, 43(3): 447-457.
DOI:10.1007/s10499-010-9408-6 |
Gao M R, Wang J, Ma G N, Van Stappen G, Sui L Y. 2017. Carbon supplementation and microbial management to stimulate Artemia biomass production in hypersaline culture conditions. Aquaculture Research, 48(3): 1 240-1 250.
DOI:10.1111/are.12965 |
Haas B J, Gevers D, Earl A M, Feldgarden M, Ward D V, Giannoukos G, Ciulla D, Tabbaa D, Highlander S K, Sodergren E, Methé B, De Santis T Z, Petrosino J F, Knight R, Birren B W. 2011. Chimeric 16s rRNA sequence formation and detection in Sanger and 454-pyrosequenced PCR amplicons. Genome Research, 21(3): 494-504.
DOI:10.1101/gr.112730.110 |
Hargreaves J A. 2006. Photosynthetic suspended-growth systems in aquaculture. Aquacultural Engineering, 34(3): 344-363.
DOI:10.1016/j.aquaeng.2005.08.009 |
Kersters K, De Vos P, Gillis M, Swings J, Vandamme P, Stackebrand E. 2006. Introduction to the proteobacteria. In: Dworkin M, Falkow S, Rosenberg E, Schleifer K H eds. The Prokaryotes. Springer, New York, NY. p.3-37, https://doi.org/10.1007/0-387-30745-1_1.
|
Labrenz M, Lawson P A, Tindall B J, Collins M D, Hirsch P. 2003. Saccharospirillum impatiens gen. nov., sp. nov., a novel γ-proteobacterium isolated from hypersaline Ekho Lake (East Antarctica). International Journal of Systematic and Evolutionary Microbiology, 53(3): 653-660.
DOI:10.1099/ijs.0.02406-0 |
Lopes-dos-Santos R M A, Groot R, Sui L Y, Bossier P, Van Stappen G. 2019. Halophilic bacteria as a food source for the brine shrimp Artemia. Aquaculture, 500: 631-639.
DOI:10.1016/j.aquaculture.2018.10.068 |
Loreau M, Naeem S, Inchausti P, Bengtsson J, Grime J P, Hector A, Hooper D U, Huston M A, Raffaelli D, Schmid B, Tilman D, Wardle D A. 2001. Biodiversity and ecosystem functioning: current knowledge and future challenges. Science, 294(5543): 804-808.
DOI:10.1126/science.1064088 |
Rahmani R, Zarrini G, Aein F, Hosseingholi E Z. 2016. Identification of extremely halophilic archaea associated with adult Artemia urmiana. Microbiology, 85(3): 386-388.
DOI:10.1134/S0026261716030127 |
Ray A J, Lotz J M. 2014. Comparing a chemoautotrophicbased biofloc system and three heterotrophic-based systems receiving different carbohydrate sources. Aquacultural Engineering, 63: 54-61.
DOI:10.1016/j.aquaeng.2014.10.001 |
Ronald L, Van Stappen G, Van Hoa N, Sorgeloos P. 2014. Effect of carbon/nitrogen ratio manipulation in feed supplements on Artemia production and water quality in solar salt ponds in the Mekong delta, Vietnam. Aquaculture Research, 45(12): 1 906-1 912.
DOI:10.1111/are.12135 |
Santos M, Teixeira J, Rodrigues A. 2000. Production of dextransucrase, dextran and fructose from sucrose using Leuconostoc mesenteroides NRRL B512 (f). Biochemical Engineering Journal, 4(3): 177-188.
DOI:10.1016/S1369-703X(99)00047-9 |
Schloss P D, Westcott S L, Ryabin T, Hall J R, Hartmann M, Hollister E B, Lesniewski R A, Oakley B B, Parks D H, Robinson C J, Sahl J W, Stres B, Thallinger G G, Van Horn D J, Weber C F. 2009. Introducing Mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Applied and Environmental Microbiology, 75(23): 7 537-7 541.
DOI:10.1128/AEM.01541-09 |
Schveitzer R, Arantes R, Costódio P F S, do Espírito Santo C M, Arana L V, Seiffert W Q, Andreatta E R. 2013. Effect of different biofloc levels on microbial activity, water quality and performance of Litopenaeus vannamei in a tank system operated with no water exchange. Aquacultural Engineering, 56: 59-70.
DOI:10.1016/j.aquaeng.2013.04.006 |
SOA. 2008. GB/T 12763.4-2007. Specifications for oceanographic survey, Part 4: survey of chemical parameters in sea water. Beijing: Standards Press of China. 75p. (in Chinese)
|
Sorgeloos P, Dhert P, Candreva P. 2001. Use of the brine shrimp, Artemia spp., in marine fish larviculture. Aquaculture, 200(1-2): 147-159.
DOI:10.1016/S0044-8486(01)00698-6 |
Sui L Y, Wang J, Nguyen V H, Sorgeloos P, Bossier P, van Stappen G. 2013. Increased carbon and nitrogen supplementation in Artemia, culture ponds results in higher cyst yields. Aquaculture International, 21(6): 1 343-1 354.
DOI:10.1007/s10499-013-9637-6 |
Toi H T, Boeckx P, Sorgeloos P, Bossier P, Van Stappen G. 2013. Bacteria contribute to Artemia nutrition in algae-limited conditions: a laboratory study. Aquaculture, 388-391: 1-7.
DOI:10.1016/j.aquaculture.2013.01.005 |
Tovar A, Moreno C, Mánuel-Vez M P, Garcı́a-Vargas M. 2000. Environmental impacts of intensive aquaculture in marine waters. Water Research, 34(1): 334-342.
DOI:10.1016/S0043-1354(99)00102-5 |
Van Stappen G. 1996. Use of cysts. In: Lavens P, Sorgeloos P eds. Manual on the Production and Use of Live Food for Aquaculture. FAO, Rome, Italy. p.107-136.
|
Van Stappen G. 2002. Zoogeography. In: Abatzopoulos T J, Beardmore J A, Clegg J S, Sorgeloos P eds. Artemia: Basic and Applied Biology. Kluwer Academic Publishers, Dordrecht, the Netherlands. p.286.
|
Wang G J, Yu E M, Xie J, Yu D G, Li Z F, Luo W, Qiu L J, Zheng Z L. 2015. Effect of C/N ratio on water quality in zero-water exchange tanks and the biofloc supplementation in feed on the growth performance of crucian carp, Carassius auratus. Aquaculture, 443: 98-104.
DOI:10.1016/j.aquaculture.2015.03.015 |
Wang Q, Garrity G M, Tiedje J M, Cole J R. 2007. Naïve Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Applied and Environmental Microbiology, 73(16): 5 261-5 267.
DOI:10.1128/AEM.00062-07 |
Wei Y F, Liao S A, Wang A L. 2016. The effect of different carbon sources on the nutritional composition, microbial community and structure of bioflocs. Aquaculture, 465: 88-93.
DOI:10.1016/j.aquaculture.2016.08.040 |
Xu W J, Morris T C, Samocha T M. 2016. Effects of C/N ratio on biofloc development, water quality, and performance of Litopenaeus vannamei juveniles in a biofloc-based, high-density, zero-exchange, outdoor tank system. Aquaculture, 453: 169-175.
DOI:10.1016/j.aquaculture.2015.11.021 |
Zhao P, Huang J, Wang X H, Song X L, Yang C H, Zhang X G, Wang G C. 2012. The application of bioflocs technology in high-intensive, zero exchange farming systems of Marsupenaeus japonicus. Aquaculture, 354-355: 97-106.
DOI:10.1016/j.aquaculture.2012.03.034 |
 2019, Vol. 37
2019, Vol. 37


