Institute of Oceanology, Chinese Academy of Sciences
Article Information
- WANG Ruifang, HUANG Xiaorong, WANG Haihua, LU Jianxue, SHI Xiaotao, FENG Guangpeng, ZHUANG Ping
- Effects of salinity on embryonic and larval development of Chinese mitten crab Eriocheir sinensis (Decapoda: Brachyura) and salinity-induced physiological changes
- Journal of Oceanology and Limnology, 37(5): 1777-1788
- http://dx.doi.org/10.1007/s00343-019-8190-1
Article History
- Received Aug. 8, 2018
- accepted in principle Dec. 3, 2018
- accepted for publication Feb. 1, 2019
2 College of Animal Science, Inner Mongolia Agricultural University, Hohhot 010018, China;
3 Engineering Research Center of Eco-environment in Three Gorges Reservoir Region, Ministry of Education, Three Gorges University, Yichang 443002, China
Estuaries are challenging habitats for organisms because of their temporal and spatial variation in environmental conditions (Ehlinger and Tankersley, 2004). Of environmental variables, salinity is of considerable importance in the lives of decapod crustaceans, due to its influence on embryo and larval biology (Anger, 2001, 2003; Ituarte et al., 2005; Samuel and Soundarapandian, 2010). Fertilized eggs of decapod crustaceans are clustered on the pleopod setae beneath the abdomen, and carried by the female until they hatch. Accordingly they would be exposed to intermittent and at times extreme salinity conditions. Although the ontogeny of salinity tolerance and the physiological mechanisms enabling decapods to tolerate wide fluctuations in salinity have been detailed (Charmantier and Aiken, 1987; Charmantier et al., 1998; Cieluch et al., 2007), most investigations have focused on changes during postembryonic larval development and metamorphosis to post-larvae or juveniles; rarely have studies focused on salinity tolerance and the regarding physiological mechanisms in the embryonic stage, particularly, embryonic stages before the appearance/full differentiation, of osmoregulatory organs (Charmantier and Charmantier-Daures, 2001; Susanto and Charmantier, 2001).
Successful embryonic development of shrimps and crabs living in freshwater and estuarine environments, experiencing a wide range of salinities, may depend upon: ⅰ) the low ion permeability of egg membranes, providing embryos with a passive osmotic protection (Charmantier and Aiken, 1987); and ⅱ), the ability to osmoregulate during embryonic development, e.g. the appearance of ion-regulating key enzyme Na+/K+- ATPase (NKA) (Seneviratna, 2003). The role of NKA in osmoregulation is well-established in adult crustaceans (Taylor and Seneviratna, 2005; Serrano et al., 2007; Ituarte, 2008), and increased NKA activity has been found to correlate with increased ontogenetic (embryonic and larval) osmoregulatory ability in shrimps and crabs. Previous studies on adult and juvenile American lobster (Homarus americanus) have shown Na+ levels and osmotic regulation to be neuroendocrinologically controlled (Charmantier et al., 1984; Charmantier-Daures et al., 1994), probably mediated by an isoform of the neuropeptide crustacean hyperglycaemic hormone (CHH), a family of neuroendocrine hormones, synthesized in the eyestalk neuroendocrine centres and released through the sinus glands (Charmantier-Daures et al., 1994). The CHH hormone is involved mainly in regulation of hemolymph glucose levels, and metabolism of carbohydrates and lipids (Lago-Lestón et al., 2007), in addition to being involved in adult osmoregulation (Charmantier-Daures et al., 1994; Spanings-Pierrot et al., 2000; Serrano et al., 2003; Chung and Webster, 2006). However, it is not known if CHH is involved in embryonic osmoregulation, nor is the relationship between it and NKA activity clear.
The Chinese mitten crab (Eriocheir sinensis) is endemic to the Yellow Sea region bordering China and Korea, eastern Asia, in addition to being a globally invasive species in estuarine environments. Its high invasive potential might be in large part due to its euryhaline, catadromous life cycle (Bentley, 2010). Adult crabs spend most of their life in fresh- and brackish waters, but migrate towards the estuary or sea to reproduce (Zhang et al., 2001; Bentley, 2010). Following mating, fertilized eggs are carried by the female, which then moves to relatively higher salinities to overwinter, before eggs develop to zoeal stages, followed by a megalopa, which then molts to the juvenile crab (Herborg et al., 2003). All these early development stages occur within estuaries and along shores where considerable variation in salinity can be experienced. The ontogeneses of salinity tolerance (Anger, 1991; Xu and Jiang, 1996; Zhao et al., 2004) and physiological mechanisms (Cieluch et al., 2007) have been extensively studied in indigenous and/or invasive E. sinensis, but studies have focused on postembryonic larval development, and metamorphosis to post-larval or juvenile stages. It is unclear how developing E. sinensis embryos are protected from salinity fluctuations.
Our objectives were to determine the tolerance and effects of salinity on embryos and zoea, and the molting of megalopa. We also sought to determine the effect of acute hyposaline and hypersaline stress on egg diameter, water content, NKA activity (including in the megalopa stage) and CHH mRNA expression of E. sinensis embryos, to better understand the physiological mechanisms of embryo tolerance to high and low salinity. Results improve our understanding of the embryonic tolerance mechanisms of hyper-regulating crustaceans, and this particularly invasive species, following osmotic stress.
2 MATERIAL AND METHOD 2.1 Origin of materials, larval rearingTwo-year-old ovigerous E. sinensis were provided by the Golden Coast Fisheries Research Institute (Qidong, Jiangsu Province, China). Adult (female and male) specimens were collected during the spawning migration season in the Changjiang (Yangtze) River estuary, and overwintered in separately cement tanks with brackish water (salinity 15), aerated oxygen, and a natural photoperiod at 19±1℃. Crabs were fed clamworms for nutrient enrichment before mating. Females and males were polycultured at a ratio of 2:1 for mating and laying egg on March 2011.
After the appearance of eggs, 30 ovigerous females were separated and maintained in three flow-through cylindrical aquaria (diameter 1.5 m, height 80 cm) containing brackish water (temperature 18±1℃, salinity 15), prepared by adding sea salt (Tropic Fish Sea, China) to tap water. Potassium permanganate and methyl aldehyde were added to prevent bacterial and fungal infections. Frozen snail meat was provided daily as food. Aquaria were examined every morning and eggs were periodically removed from pleopods using forceps to examine developmental stages following Anger (1991) and Wang et al. (2005). For further study we selected representative developmental stages of eggs at gastrula and eyespot stages, and prehatching embryos.
Freshly hatched stage Ⅰ zoea were reared in 60-L aquaria at the same temperature, salinity and light conditions as adult crabs. Stage Ⅰ zoea were fed algae (Chlorella marina) and rotifers (Branchionus plicatilis) from 3 h after hatching, and brine shrimp (Artemia) nauplii after three days. Stage Ⅰ zoea that hatched within 24 h were used in salinity tolerance experiments.
As stage Ⅰ zoea do not molt successfully to a megalopa stage in the present study, salinity tolerance experiments were not performed on later-stage zoea. Megalopa for salinity tolerance tests were provided separately by the Golden Coast Fishery Research Institute. Megalopa that molted on the same day (from 10 ovigerous females) were reared in an aquarium, and acclimated to a salinity of 15. Newly-hatched brine shrimp were supplied to these megalopa every other day. Tank was wrapped on vertical sides with opaque black plastic to minimize disturbance. Cobblestones were placed into the bottom each tank for attachment and refuge. Water temperature was maintained at 18±1℃, and natural light periods were provided.
Chlorella marinas were inoculated into synthetic seawater enriched with ammonium sulphate, super phosphate and urea at a ratio of 10:1:1, under germfree conditions; cultures turned green within 3 to 4 days. Brachionus plicatilis were cultured following Samuel and Soundarapandian (2010). Artemia nauplii were harvested from the resting eggs of brine-shrimp and placed into a conical flask with an appropriate quantity of water at salinity 18.
2.2 Experimental design 2.2.1 Effect of salinity on embryo hatchingEggs at specific developmental stages were detached from the pleopods of 10 ovigerous females and conditioned in 15 saltwater for 2 h. Groups of 25 viable eggs from each female at each of three stages were transferred to petri dishes containing 50 mL of low (1, 5, 10), control (15), and high salinity (20, 25, 30, 35) water. This culture medium was changed every 6 h to limit effects of evaporation. Culture dishes were exchanged and disinfected daily with sodium hypochlorite, and carefully washed before reuse. Eggs were checked beneath a microscope each day for mortality or hatching. Survival of stage Ⅰ zoea at different salinities was monitored. Saltwater media were obtained by adding artificial salts to tap water; salinity was measured using hand refractometer (OxyGuard, Denmark), before being biologically filtered for days to weeks under constant aeration to control ammonium and nitrite levels. Temperature was maintained as 22±1℃ during experimentation.
2.2.2 Effect of salinity on survival of stage Ⅰ zoeaOne-day (24 h) old actively swimming stage Ⅰ zoea were collected by light trap and transferred to beaker (250 mL) containing 150 mL of water (salinity 15), before being slowly acclimated (intervals of 2 h) up or down in increments of 5 salinity units to the desired experimental salinity (1, 5, 10, 20, 25, 30, 35 and 40). Each treatment was replicated three times, with each replicate containing 20 stage Ⅰ zoea. These zoea were fed with rotifers after 3 h and Artemia nauplii after three days of hatching. The culture medium was changed every 24 h to reduce effects of evaporation and diet, with salinity measured before the culture medium was changed. Mortality was monitored daily. Experiments ended on day 8, by which time most zoea in lower and higher, but not 10–20 salinity treatments had died. Death was presumed when larvae were inactive, despite repeat probing with delicate forceps.
2.2.3 Effect of salinity on metamorphosis of megalopaEffects of salinity on the molting frequency of 20 megalopa (15 days post-metamorphosis) was determined in 500 mL glass beakers, replicated three times at each of 1, 5, 10, 15, 20, 25, 30, 35 and 40 salinity. Megalopa were first transferred from holding salinity (15) to the desired salinity by reducing or increasing salinity in steps of 2.5 units every 2 h until the final salinity was reached. Megalopa were fed newly hatched Artemia nauplii, with food residue removed the following morning (the number of nauplii provided was adjusted according to the amount of food residue). One third of the experimental medium was exchanged daily; salinity was measured before water exchange. Molt and survival rates of megalopa were monitored daily. Experimentation was terminated when all megalopa molted to the first instar stage, or had died.
2.2.4 Physiological effects of acute hyper- and hyposaline stress on embryos and megalopaEmbryos at different developmental stage (gastrula, eyespot and pre-hatching stages) from nine, and megalopa from 10 ovigerous females held at a salinity of 15, were transferred to hyposaline (1) and hypersaline (35) media for 24 h. Egg diameter, water content, CHH gene mRNA relative expression, and Na+/K+-ATPase activity in these embryos and megalopa were recorded.
2.3 Experimental methods 2.3.1 Egg diameter and water contentMean egg diameter was determined from four measurements under a stereo microscope (Olympus SZH) equipped with a micrometric eyepiece. Egg water content was measured following Petersen and Anger (1997), with approximately 0.05–0.2 g egg first rinsed with deionized water, blotted on filter paper, placed into preweighed 5 mL tubes for fresh weight measurement to the nearest 0.1 mg, and then their dry weight measured after freeze-drying to constant weight; water content is the difference between fresh and dry weights, with the difference expressed as a percentage.
2.3.2 NKA activity analysesNKA activity was measured in five replicate 0.2 g aliquots pooled from embryos at the same developmental stage and salinity treatment. Eggs were removed from the egg-mass of a single ovigerous female. Embryos were blotted dry, weighed and homogenized in an ice-cold homogenization buffer containing 0.01 mol/L Tris-HCl, 0.001 mol/L EDTANa2, 0.01 mol/L sucrose and 0.8% NaCl, at pH 7.3. Homogenization was performed with a hand-operated ground glass homogenizer (Shengbo, China, 2 mL) until intact embryos were no longer visible. The resulting suspension was centrifuged at 1 000 r/min for 5 min to remove debris. The supernatant was then stored at -80℃ until NKA activity and protein concentration could be measured (within one week).
Supernatant protein concentrations were determined using the Coomassie Brilliant Blue method and a Total Protein Quantification Kit. Homogenate NKA activity was assessed by measuring the rate of inorganic phosphate liberated from the hydrolysis of ATP in the presence of enzyme using an NKA activity Detection Kit and spectrophotometer (UV-2600A, UNICO, USA). The two detection kits were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Ouabain was used to inhibit NKA activity, with enzymatic activity calculated according to the difference between assays with and without it (1.0 mmol/L). Enzymatic activities were expressed as micromoles of Pi liberated per milligram of protein per hour (i.e., μmol Pi/mg protein/h).
2.3.3 RNA extraction and real time RT-PCRAfter 24 h of hypo- and hypersaline stress, samples of 30–40 eggs per replication were removed from the egg-mass of one ovigerous female. Nine replicate samples were performed at each salinity. Eggs were immediately frozen in liquid nitrogen and stored at -80℃. Total RNA was extracted by TRIzol (Invitrogen, Carlsbad, CA, USA) following manufacturer instructions. The quantity of RNA in samples was determined by measuring absorbance at 260 nm, with the quality verified by measuring the 260/280 nm ratio. RNA was also loaded onto 1.5% Agarose gels to verify the 28S and 18S integrity. First-strand cDNA synthesis was performed using a cDNA synthesis kit (TOYOBO, Kyoto, Japan) following manufacturer protocols.
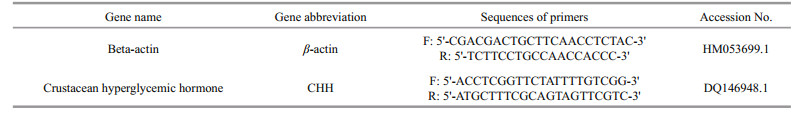
Primers used in real-time PCR were designed by Primer 5 software based on GenBank sequences (Table 1). Expression of crustacean hyperglycemic hormone (CHH) genes was determined using realtime PCR on an ABI-7500 detection system (Applied Biosystems, Foster City, CA, USA). SYBR Green PCR Master Mix (TOYOBO, Kyoto, Japan) was used for real-time PCR analysis, with the β-actin gene used as an internal control gene to normalize expression of CHH. Relative gene expressions were calculated according to: relative expression =2-ΔΔCt. PCR was performed three times in all cases.
Values are presented as means±SE. The effect of salinity and developmental stage on hatching rate, survival rates of freshly hatched stage Ⅰ zoea, and egg diameters, water content, NKA activity and CHH gene mRNA expression, were analyzed using twoway ANOVA. When significant interactions between salinity and developmental stage were observed, the main effects were not further discussed, but multiple comparison tests were performed to determine simple effects. All statistical analyses were performed using SPSS 16.0 for Windows. Normality and homogeneity of variances were previously checked. Differences were considered significant at P < 0.05.
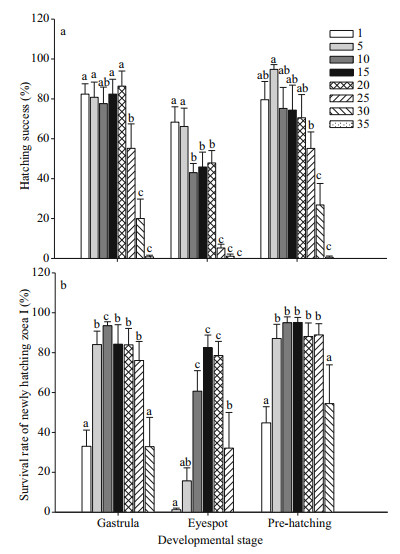
3 RESULT 3.1 Effect of salinity on embryonic hatchingTwo-way ANOVA revealed both developmental stage and salinity had significant effects on hatching success, with the interaction between the two also significant (P < 0.05) (Table 2). Specifically, gastrula and pre-hatching stage embryos in salinities between 1 and 20 had high hatching rates (nearly 80% or higher), with no significant difference between salinities (P > 0.05, Fig. 1a). At salinity ≥25, hatching success significantly decreased (P < 0.05), with almost no stage Ⅰ zoea hatching at salinity 35 (Fig. 1a). For embryos at eyespot stages, 66% and 68% of them hatched at salinities of 1 and 5, respectively, while hatching success decreased to 43%–47% at salinities from 10 to 20, with few stage Ⅰ zoea hatching at salinities ≥25. Optimal ranges for gastrula and prehatching embryonic stages to hatch lies between salinities of 1 and 20, regardless of the survival of freshly hatched stage Ⅰ zoea (Fig. 1a).
|
|
| Fig.1 Hatching success (%) (a), and survival rate (%) (b) of freshly hatched stage Ⅰ E. sinensis zoea following exposure of eggs of three developmental stages (gastrula, eyespot, pre-hatching) to different salinities Letters above bars indicate significant differences between salinity treatments. Data based on 10 replicates per salinity treatment, and 25 embryos per replicate. |
Two-way ANOVA revealed developmental stage and salinity both significantly affected survival of freshly hatched stage Ⅰ zoea, with the interaction between the two also significant (P < 0.05) (Table 2). Freshly hatched stage Ⅰ zoea were less tolerant of low salinities than they were of high salinities (Fig. 1b). When gastrula and pre-hatching stage embryos were exposed to different salinities, survival rates of freshly hatched stage Ⅰ zoea were significantly higher at salinities ranging 5–25 (84%–95%) than those at salinity 1 and 30 (P < 0.05). Survival rates of freshly hatched stage Ⅰ zoea were lower when eyespot stage embryos were exposed to different salinities, with 60%–80% of stage Ⅰ zoea surviving at salinities ranging 10–20, with survival rate significantly decreasing beyond this range (P < 0.05). Accordingly, results of gastrula and pre-hatching stage embryos reveal the optimal salinity range for freshly hatched stage Ⅰ zoea survival to lie between 5 and 25.
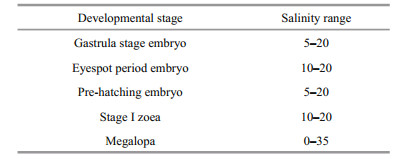
Optimal salinity ranges for embryonic development and hatching lie between 5 and 20 (Table 3).
|
Stage Ⅰ zoea have a higher survival rate until 5 day at salinities ranging 10–20, during which time almost no zoea died. Mortality gradually increased thereafter, with less than 40% of stage Ⅰ zoea surviving after 8 days (Fig. 2). No stage Ⅰ zoea survived at salinities of 1 or 40. Survival rate of stage Ⅰ zoea significantly reduced three days after exposure at a salinity of 5. Stage 1 zoea gradually died at salinities between 25 and 35, with most having died by 8 days. The optimal salinity range for stage Ⅰ zoea survival lies between 10 and 20 (Table 3).
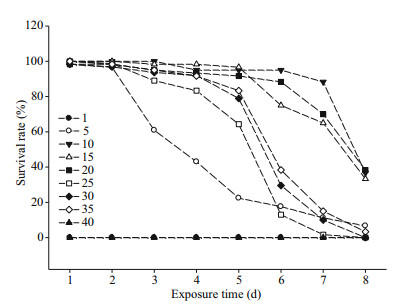
|
| Fig.2 Survival rate (%) of E. sinensis stage Ⅰ zoea at different salinities Data based on three replicates per salinity treatment, and 20 zoea per replicate. |
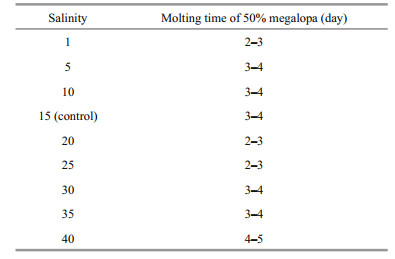
Salinity had no significant effect on molting of megalopa, with between 82% and 96% of all megalopa successfully molting to the first juvenile instar at all salinities (Fig. 3). However, an obvious delay was apparent for megalopa raised at salinity 40 from 3 to 5 days, compared to other treatments. The time needed for 50% of megalopa to molt into the first juvenile instar was between 4 and 5 days at salinity 40, but between 2 and 3 days at salinities of 1, 20 and 25 (Table 4).
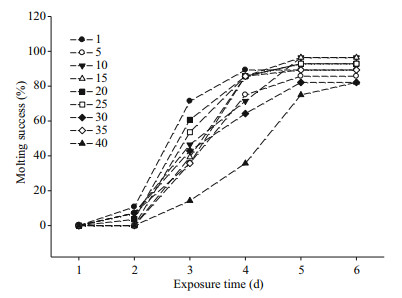
|
| Fig.3 E. sinensis megalopa molting rate (%) to first juvenile instar at different salinities Data based on three replicate treatments per salinity, and 20 megalopa per replicate. |
|
Two-way ANOVA revealed developmental stage and salinity both significantly affect egg diameter and embryo water content (P < 0.001), with a significant interaction between the two (Table 2). During development, egg size increased and reached a maximum prior to hatching that was significantly greater than egg sizes of gastrula and eyespot stages in the control treatment (P < 0.05) (Fig. 4a). Hypersaline stress resulted in a significant reduction in egg diameter of embryos at gastrula and pre-hatching stages (P < 0.05), but egg diameter showed no significant change after hyposaline stress. Water content significantly increased with embryonic development from gastrula to pre-hatching stage (P < 0.05), and variation trend of water content in embryo is consistent with the change of egg diameter after hypo- and hyper-saline stress (Fig. 4b).
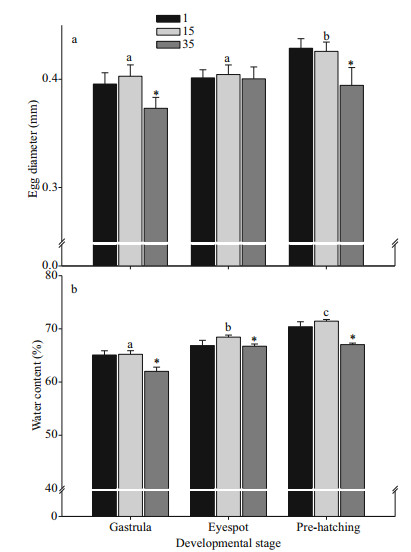
|
| Fig.4 Egg diameter (mm) (a), and water content (%) (b) of Chinese mitten crab embryos at different development stages (gastrula, eyespot, pre-hatching) exposed to hyper (35)- and hypo(1)-saline stress for 24 h Letters above bars indicate significant differences exist between embryos of different developmental stages. An asterisk (*) denotes if post-saline stress egg diameter or embryo water content differed significantly from control treatments. Data based on three replicates per salinity treatment, and 10 of each egg developmental stage per replicate. |
Two-way ANOVA revealed developmental stage and salinity both significantly affected NKA activity and CHH mRNA expression in embryos, with a significant interaction also apparent between the two (P < 0.05) (Table 2). At all salinities, embryonic NKA activity was first detected during gastrulation. Enzyme activity significantly increased (8-fold) between gastrulation and hatching-stage embryos, and further (155-fold) between gastrulation stage embryos and megalopa stages in the control treatment (15) (Fig. 5; gastrulation stage, 0.054 0±0.005 6; eyespot stage, 0.274 6±0.014 7; pre-hatching stage, 0.429 6±0.011 2; megalopa, 8.366 2±1.360 0 μmol Pi/mg protein/ hour). At salinities of 1 and 35, ontogenetic patterns in activity were similar to those observed at salinity 15 (Fig. 5). NKA activity tended to increase in embryos exposed to hypersaline media, with a significant difference apparent at gastrulation and in hatching-stage embryos (P < 0.05). Hyposaline stress reduced NKA activity, with a significant difference apparent at gastrulation and in eyespot-stage embryos (P < 0.05). As for the megalopa stage, hypersaline and hyposaline stress both significantly increased NKA activities (P < 0.05).
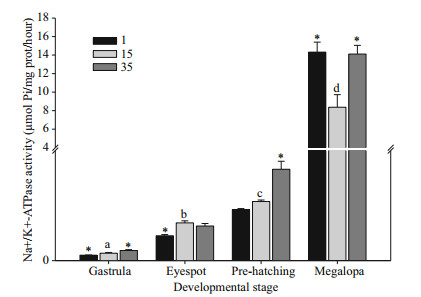
|
| Fig.5 Total Na+/K+-ATPase activity in E. sinensis embryos at different developmental stages (gastrula, eyespot, pre-hatching) and megalopa following hyper (35)- and hypo (1)-saline stress for 24 h Control values (15) with different letters indicate significant differences between developmental stages. An asterisk (*) denotes a significant difference from control treatments. Data based on five replicates per salinity treatment, and 0.2 g embryo or megalopa per replicate. |
mRNA levels, measured by real-time PCR for CHH, are shown in Fig. 6. Low-level expression of CHH was observed during eyespot formation (eyespot stage). Expression increased significantly before hatching (P < 0.05). Hyposaline stress resulted in a significant decrease in CHH mRNA expression in eyespot embryos (P < 0.05), while hypersaline stress resulted in upregulation of CHH mRNA in prehatching stage embryos (P < 0.05).
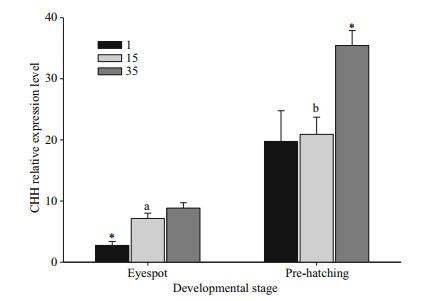
|
| Fig.6 Expression of crustacean hyperglycaemic hormone (CHH) mRNAs during E. sinensis embryonic development (eyespot and pre-hatching stage) and hyper (35)- and hypo-(1) saline stress over 24 h; control values (15) with different letters indicate significant differences between developmental stages An asterisk (*) denotes a significant difference from control treatments after hyper- or hyposaline stress. Data based on nine replicates per salinity, and 30-40 embryos per replicate. |
We demonstrate E. sinensis embryos to be more tolerant of hyposaline than hypersaline conditions. Optimal ranges for embryo hatching and freshly hatched stage Ⅰ zoea survival lie between salinities of 5 and 20. Stage Ⅰ zoea can survive to metamorphosis at salinities ranging 10–20, and megalopa can molt to first juvenile instar stage from almost fresh to saltwater (salinities 1–35). Embryonic salinity tolerance is consistent with earlier reports for this species, for which the optimum salinity for hatching and complete larval development is 20 reported in invasive E. sinensis (Panning, 1939; Montú et al., 1996; Dittel and Epifanio, 2009). Optimal salinity ranges for zoea development in indigenous E. sinensis have been reported as 15–27 (Zang et al., 1999), 13–26 (Xu and He, 1987) or 15–30 (Huang, 1989).
The ontogenetic variation in salinity tolerance we report for E. sinensis is consistent with the thesis that early zoea (concentrated in surface waters) are transported by surface currents from the estuary, for zoeal development to occur in increased salinity in near-shore marine waters, and for megalopa to return in onshore-directed, near-bottom counter currents to estuaries, facilitated by swimming activity (Anger, 1991). Therefore, stage Ⅰ zoea should be more tolerant of low and less tolerant of high salinities, and megalopa should be capable of (at least gradually) adapting to any salinity. We note that the lower salinity tolerance of embryos (less than 5) we report for this species is lower than that of Anger (1991), although this difference between native and invasive species might be a result of their acclimation to different estuary habitats.
Low salinity is the most significant factor limiting hatching of E. sinensis eggs in estuaries (Panning, 1939; Otto and Brandis, 2011), but until now this lower limit was unknown. We report more than 80% of eggs to remain viable and capable of hatching at salinity 1, and hatched stage Ⅰ zoea to survive at salinities ≥5. Field investigations have shown embryos of invasive and indigenous E. sinensis can tolerate low estuarine salinities during reproductive stages, with gravid females collected in the southern Baltic Sea at salinities of 7 (Wójcik and Normant, 2014); we have also found gravid females in Changjiang estuary at 2 (unpublished data). As we reared E. sinensis embryos in vitro, isolated from any potential maternal influence, therefore, successful hatching of embryo in 1 and 5 confirms their strong low salinity tolerance.
4.2 Water balance and egg size regulation after acute hypo- and hyper-saline stressDevelopmental increase in egg volume has been often described for decapods (Wear, 1974; Mashiko, 1983). We report an increase in egg diameter (or volume) and water content during embryonic development for E. sinensis embryos. This increase is probably due to a combined uptake of water from the external medium and an internal production of metabolic water (Anger, 2003; Ituarte et al., 2005). Because no change in embryonic water content was apparent following hyposaline stress, passive osmotic intake ceased, reflected also in no significant change in the size of the perivitelline space. We suggest that the high tolerance to low salinity of E. sinensis embryos is a consequence of reduced ion permeability of the egg envelope.
The osmo-protective role of the egg envelope has been reported for many decapods (Charmantier and Charmantier-Daures, 2001; Susanto and Charmantier, 2001). Despite their higher tolerance to low salinities, E. sinensis embryos were less tolerant of higher salinities. Exposure of E. sinensis gastrulas to salinity 25 significantly reduced hatching success, while exposure to hypersaline (35) conditions led to water loss and egg shrinkage. These results are similar to those of Ehlinger (2002), who reported horseshoe crab (Limulus polyphemus) egg volume to decrease in hyper-osmotic conditions, and to increase in hypoosmotic conditions over a 6-h exposure period, over a salinity range from 5 to 90. As such, the hypersaline effect might be explained by a passive loss of water through the egg membrane. Larvae of the carridean shrimp Palaemonetes argentinus hatched at relatively high salinities retained more yolk, suggesting that hyperosmotic stress interfered with metabolic energy mobilization (Ituarte et al., 2005). Reduced moisture in E. sinensis embryos might also weaken metabolism and decrease metabolic water production; hypersaline stress might even cause irreversible physiological damage to E. sinensis embryos, given no stage Ⅰ zoea hatched successfully at a salinity of 35.
4.3 NKA activity after acute hypo- and hypersaline exposureOur experiments demonstrate the catadromous E. sinensis to have NKA activity that varies ontogenetically and following salinity stress. Activity increased by up to 8-fold from gastrula to prehatching stages in the control group, and almost 155- fold in the megalopa compared to gastrula stages. Increased NKA activity during embryonic development has been reported for several other decapod crustaceans (Wilder et al., 2001; Taylor and Seneviratna, 2005).
A common pattern in euryhaline crabs is for NKA activity to decrease after high salinity stress (McNamara and Lima, 1997; Lucu and Towle, 2003), yet these conditions increased NKA activity in gastrula and pre-hatching embryos, as well as the megalopa stage of E. sinensis in the present study. While decreased NKA activity and its mRNA expression in the posterior gills has been reported for adult male E. sinensis (Long et al., 2017), this activity in embryos of the freshwater P. argentines (Ituarte, 2008), and in the gills of anadromous fish transferred to seawater from freshwater (Deane and Woo, 2004; Bystriansky et al., 2006), also increased in elevated salinities. Adult E. sinensis is a strong hyperosmoregulator in fresh to brackish (salinity 21) waters, and is slightly hypo-regulated at salinities of 35 (Wang et al., 2012). NKA is likely involved in excretion of salts across the gill (Kamemoto, 1991; Lucu and Towle, 2003). Upregulation of NKA activity in embryo and megalopa stages following hypersaline stress is consistent with its ability to hypo-regulate at high salinity. Furthermore, NKA is also involved in intracellular volume regulation (Ituarte, 2008). We report a significant decrease in egg diameter when subject to hypersaline stress, so upregulation of NKA activity in the embryo following hypersaline stress is also associated with regulating cell volume. Hyper-osmotic conditions have previously been reported to not significantly affect the activity of NKA (Wilder et al. 2000). These inconsistent reports suggest the mechanisms of NKA hypo-osmoregulation in crustaceans, particularly during embryonic stages, are not fully understood and warrant further study.
Increased NKA activity has been correlated with an ontogenetic increase of the hyper-regulatory ability of crustaceans in low salinity environments (Serrano et al., 2003; Cieluch et al., 2007). Hyposaline-stressinduced increases in NKA activity involving salt uptake have been extensively reported in adult crabs and their early developmental stages (Lucu and Flik, 1999; Flik and Haond, 2000; Taylor and Seneviratna, 2005). However, rather than an increase in NKA activity in E. sinensis embryos following hyposaline stress, we found it to decrease in gastrula and eyespot embryological stages. Although NKA is thought to be a major driving force in ion transport (Lucu and Towle, 2003), several other transporters are involved in mediating NaCl uptake across the gill also (Towle, 1990). If is not known if down-regulation of NKA activity in E. sinensis embryos following hyposaline stress is compensated by an increase in the expression of other transporters. Modulation of the concentration of free amino acid osmolytes for hyper-osmoregulation has been described for E. sinensis post-metamorphosis to the megalopa stage (Hui et al., 2014; Liu et al., 2018). To determine any change in free amino acid concentrations of embryos following hyposaline stress requires additional study. For developing embryos, the egg envelope may afford great osmotic protection, whereas it is the osmoregulatory tissues of the megalopa, characterized by an increase of NKA activity, that are responsible for effective hypo/hyperosmotic regulation.
4.4 Crustacean hyperglycemic hormone (CHH) involvement in osmoregulationStudies on decapod crustaceans have shown that osmotic and ionic regulation is controlled by neuroendocrine mechanisms (Mantel, 1985; Kamemoto, 1991). The likely involvement of the crustacean hyperglycemic hormone (CHH) in osmoregulation has been extensively proposed in previous studies on Decapoda (Spanings-Pierrot et al., 2000; Serrano et al., 2003; Chung and Webster, 2006). We report a significant increase in CHH mRNA expression in pre-hatching embryos following hypersaline stress, but a significant decrease in CHH mRNA in embryos at an eyespot-developmentalstage following hyposaline stress. This response pattern is consistent with NKA activity in embryos following salinity stress, indicating a neuroendocrine regulation role of CHH in iono-osmoregulation probably occurs during embryonic development. Although many studies have investigated the possible role of CHH in iono-osmoregulation, inconsistent results have been reported (from no change to an increase following hyper-and hypo-osmotic stress) in different taxa (Chang, 2005; Chung and Webster, 2006; Lago-Lestón et al., 2007). An in vivo experiment on the landcrab Discoplax celeste revealed CHH had no significant effect on gill NKA activity, suggesting an unknown mechanism was responsible for this hormone's action on Na+ transport (Turner et al., 2013). The roles of CHH in iono-osmoregulation, whether it directly or indirectly regulates NKA expression, or is involved in regulating ion and water balance during embryonic development, need to be investigated in other decapod crustaceans.
5 CONCLUSIONGiven the limited data on salinity tolerances of developing decapod embryos, and the physiological bases for hyper- and hypo-saline tolerance to date, we report novel and valuable data for a popular model and highly invasive species, the Chinese mitten crab, E. sinensis. We clearly demonstrate embryos of this species to be more tolerant of hyposaline than hypersaline environments. Osmotic protection of egg membrane seems as an important tolerance mechanism under hyposaline, whereas, it couldn't prevent embryo from loss of moist in hypersaline environment. We also report NKA might play an important role in hyporegulation of E. sinensis embryos in hypersaline environments, meanwhile CHH is probably involved in embryo osmoregulation, especially when subject to hypersaline stress.
6 DATA AVAILABILITY STATEMENTAll data generated or analysed during this study are included in this published article (and its supplementary information files).
7 ACKNOWLEDGMENTWe would like to thank Steve O'Shea, PhD, of the Edanz Group (www.edanzediting.com/ac), for editing a draft of this manuscript.
Anger K. 1991. Effects of temperature and salinity on the larval development of the Chinese mitten crab Eriocheir sinensis (Decapoda: Grapsidae). Mar. Ecol. Prog. Ser., 72: 103-110.
DOI:10.3354/meps072103 |
Anger K. 2001. The Biology of Decapod Crustacean Larvae. A.A. Balkema Publishers, Lisse Exton. p.1-420.
|
Anger K. 2003. Salinity as a key parameter in the larval biology of decapod crustaceans. Inv. Repr. Dev., 43(1): 29-45.
DOI:10.1080/07924259.2003.9652520 |
Bentley M G. 2010. The global spread of the Chinese mitten crab Eriocheir sinensis. In: Galil B S, Clark P F, Carlton J T eds. In the Wrong Place-Alien Marine Crustaceans: Distribution, Biology and Impacts. Springer, Dordrecht.
|
Bystriansky J S, Richards J G, Schulte P M, Ballantyne J S. 2006. Reciprocal expression of gill Na+/K+-ATPaseα-subunit isoforms α1a and α1b during seawater acclimation of three salmonid fishes that vary in their salinity tolerance. J. Exp. Biol., 209(10): 1 848-1 858.
DOI:10.1242/jeb.02188 |
Chang E S. 2005. Stressed-out lobsters: crustacean hyperglycemic hormone and stress protein. Integr. Comp. Biol., 45(1): 43-50.
DOI:10.1093/icb/45.1.43 |
Charmantier G, Aiken D E. 1987. Osmotic regulation in late embryos and prelarvae of the American lobster Homarus americanus H. Milne-Edwards 1837 (Crustacea, Decapoda). J. Exp. Mar. Biol. Ecol., 109(2): 101-108.
DOI:10.1016/0022-0981(87)90009-8 |
Charmantier G, Charmantier-Daures M, Aiken D E. 1984. Neuroendocrine control of hydromineral regulation in the American lobster Homarus americanus H. MilneEdwards, 1837 (Crustacea, Decapoda): 1-Juveniles. Gen. Comp. Endocrinol., 54(1): 8-19.
DOI:10.1016/0016-6480(84)90193-X |
Charmantier G, Charmantier-Daures M, Anger K. 1998. Ontogeny of osmoregulation in the grapsid crab Armases miersii (Crustacea, Decapoda). Mar. Ecol. Prog. Ser., 164: 285-292.
DOI:10.3354/meps164285 |
Charmantier G, Charmantier-Daures M. 2001. Ontogeny of osmoregulation in Crustaceans: the embryonic phase. Amer. Zool., 41(5): 1 078-1 089.
|
Charmantier-Daures M, Charmantier G, Janssen K P C, Aiken D E, Van Herp F. 1994. Involvement of eyestalk factors in the neuroendocrine control of Osmoregulation in adult American lobster Homarus americanus. Gen. Comp. Endocrinol., 94(3): 281-293.
DOI:10.1006/gcen.1994.1085 |
Chung J S, Webster S G. 2006. Binding sites of crustacean hyperglycemic hormone and its second messengers on gills and hindgut of the green shore crab, Carcinus maenas: a possible osmoregulatory role. Gen. Comp. Endocrinol., 147(2): 206-213.
DOI:10.1016/j.ygcen.2006.01.002 |
Cieluch U, Anger K, Charmantier-Daures M, Charmantier G. 2007. Osmoregulation and immunolocalization of Na+/ K+-ATPase during the ontogeny of the mitten crab Eriocheir sinensis (Decapoda, Grapsoidea). Mar. Ecol. Prog. Ser., 329: 169-178.
DOI:10.3354/meps329169 |
Deane E E, Woo N Y S. 2004. Differential gene expression associated with euryhalinity in sea bream (Sparus sarba). Am. J. Physiol. Regul. Integr. Comp. Physiol., 287(5): R1 054-R1 063.
DOI:10.1152/ajpregu.00347.2004 |
Dittel A I, Epifanio C E. 2009. Invasion biology of the Chinese mitten crab Eriochier sinensis: a brief review. J. Exp. Mar. Biol. Ecol., 374(2): 79-92.
DOI:10.1016/j.jembe.2009.04.012 |
Ehlinger G S, Tankersley R A. 2004. Survival and development of horseshoe crab (Limulus polyphemus) embryos and larvae in hypersaline conditions. Biol. Bull., 206(2): 87-94.
DOI:10.2307/1543539 |
Ehlinger G S. 2002. Spawning Behavior and Larval Biology of the American Horseshoe Crab, Limulus polyphemus, in A Microtidal Coastal Lagoon. Florida institute of Technology, Melbourne, FL. 133p.
|
Flik G, Haond C. 2000. Na+ and Ca2+ pumps in the gills, epipodites and branchiostegites of the European lobster Homarus gammarus: effects of dilute sea water. J. Exp. Biol., 203: 213-220.
|
Herborg L M, Rushton S P, Clare A S, Bentley M G. 2003. Spread of the Chinese mitten crab (Eriocheir sinensis H. Milne Edwards) in Continental Europe: analysis of a historical data set. Hydrobiologia, 503(1-3): 21-28.
DOI:10.1023/B:HYDR.0000008483.63314.3c |
Huang W. 1989. The artificial breeding of natural marine Chinese mitten crab Eriocheir sinensis. J. Aquac., 5: 2-3.
(in Chinese) |
Hui M, Liu Y, Song C W, Li Y D, Shi G H, Cui Z X. 2014. Transcriptome Changes in Eriocheir sinensis Megalopae after desalination provide insights into osmoregulation and stress adaption in larvae. PLoS One, 9(12): e114187.
DOI:10.1371/journal.pone.0114187 |
Ituarte R B, Spivak E D, Anger K. 2005. Effects of salinity on embryonic development of Palaemonetes argentinus (Crustacea: Decapoda: Palaemonidae) cultured in vitro. Invertebr. Reprod. Dev., 47(3): 213-223.
DOI:10.1080/07924259.2005.9652162 |
Ituarte R B. 2008. Efectos de la Salinidad Sobre la Reproduccióny el Desarrollo del Camarón de Agua Dulce Palaemonetes Argentinus. Universidad Nacional de Mar del Plata, Mar del Plata.
|
Kamemoto F I. 1991. Neuroendocrinology of osmoregulation in crabs. Zool. Sci., 8: 827-833.
|
Lago-Lestón A, Ponce E, Muñoz M E. 2007. Cloning and expression of hyperglycemic (CHH) and molt-inhibiting (MIH) hormones mRNAs from the eyestalk of shrimps of Litopenaeus vannamei grown in different temperature and salinity conditions. Aquaculture, 270(1-4): 343-357.
DOI:10.1016/j.aquaculture.2007.04.014 |
Liu Z Q, Zhou Z, Wang L L, Li M J, Wang W L, Yi Q L, Huang S, Song L S. 2018. Dopamine and serotonin modulate free amino acids production and Na+/K+ pump activity in Chinese mitten crab Eriocheir sinensis Under Acute Salinity Stress. Front. Physiol., 9: 1 080.
DOI:10.3389/fphys.2018.01080 |
Long X W, Wu X G, Zhao L, Ye H H, Cheng Y X, Zeng C S. 2017. Effects of salinity on gonadal development, osmoregulation and metabolism of adult male Chinese mitten crab, Eriocheir sinensis. PLoS One, 12(6): e0179036.
DOI:10.1371/journal.pone.0179036 |
Lucu Č, Flik G. 1999. Na+-K+-ATPase and Na+/Ca2+ exchange activities in gills of hyperregulating Carcinus maenas. Am. J. Physiol., 276(2): R490-R499.
|
Lucu Č, Towle D W. 2003. Na+/K+-ATPase in gills of aquatic crustacea. Comp. Biochem. Physiol A Mol. Integr. Physiol., 135(2): 195-214.
DOI:10.1016/S1095-6433(03)00064-3 |
Mantel L H. 1985. Neurohormonal integration of osmotic and ionic regulation. Integr. Comp. Biol., 25(1): 253-263.
|
Mashiko K. 1983. Differences in the egg and clutch sizes of the prawn Macrobrachium nipponense (de Haan) between brackish and fresh waters of a river. Zool. Mag. Tokyo, 92: 1-9.
|
McNamara J C, Lima A G. 1997. The route of ion and water movements across the gill epithelium of the freshwater shrimp Macrobrachium olfersii (Decapoda, Palaemonidae): evidence from ultrastructural changes induced by acclimation to saline media. Biol. Bull., 192(2): 321-331.
DOI:10.2307/1542725 |
Montú M, Anger K, de Bakker C. 1996. Larval development of the Chinese mitten crab Eriocheir sinensis H. MilneEdwards (Decapoda: Grapsidae) reared in the laboratory. Helgol. Meeresunters., 50(2): 223-252.
DOI:10.1007/BF02367153 |
Otto T, Brandis D. 2011. First evidence of Eriocheir sinensis reproduction from Schleswig-Holstein, Northern Germany, western Baltic Sea. Aquat. Inv., 6 Suppl 1: S65-S69.
|
Panning A. 1939. The Chinese mitten crab. Annu. Rep. Smithson. Inst., 1 938: 361-375.
|
Petersen S, Anger K. 1997. Chemical and physiological changes during the embryonic development of the spider crab, Hyas araneus L. (Decapoda: Majidae). Comp. Biochem. Phys Part B Biochem. Mol. Biol., 117(2): 299-306.
DOI:10.1016/S0305-0491(97)00091-6 |
Samuel N J, Soundarapandian P. 2010. Effect of salinity on the growth, survival and development of the commercially important portunid crab larvae of Portunus sanguinolentus (Herbst). Curr. Res. J. Biol. Sci., 2(4): 286-293.
|
Seneviratna D. 2003. Ontogeny of Osmoregulation of the Embryos of Two Intertidal Crabs Hemigrapsus edwardsii and Hemigrapsus crenulatus. University of Canterbury, New Zealand.
|
Serrano L, Blanvillain G, Soyez D, Charmantier G, Grousset E, Aujoulat F, Spanings-Pierrot C. 2003. Putative involvement of crustacean hyperglycemic hormone isoforms in the neuroendocrine mediation of osmoregulation in the crayfish Astacus leptodactylus. J. Exp. Biol., 206(6): 979-988.
DOI:10.1242/jeb.00178 |
Serrano L, Towle D W, Charmantier G, Spanings-Pierrot C. 2007. Expression of Na+/K+-ATPase α-subunit mRNA during embryonic development of the crayfish Astacus leptodactylus. Comp. Biochem. Physiol Part D Genomics Proteomics, 2(2): 126-134.
DOI:10.1016/j.cbd.2007.01.004 |
Spanings-Pierrot C, Soyez D, van Herp F, Gompel M, Skaret G, Grousset E, Charmantier G. 2000. Involvement of crustacean hyperglycemic hormone in the control of gill ion transport in the crab Pachygrapsus marmoratus. Gen. Comp. Endocrinol., 119(3): 340-350.
DOI:10.1006/gcen.2000.7527 |
Susanto N G, Charmantier G. 2001. Crayfish freshwater adaptation starts in eggs: ontogeny of osmoregulation in embryos of Astacus leptodactylus. J. Exp. Zool., 289(7): 433-440.
DOI:10.1002/jez.1024 |
Taylor H H, Seneviratna D. 2005. Ontogeny of salinity tolerance and hyper-osmoregulation by embryos of the intertidal crabs Hemigrapsus edwardsii and Hemigrapsus crenulatus (Decapoda, Grapsidae): survival of acute hyposaline exposure. Comp. Biochem. Physiol. Part A Mol. Int. Physiol., 140(4): 495-505.
DOI:10.1016/j.cbpb.2005.03.005 |
Towle D W. 1990. Sodium transport systems in gills. In: Kinne R K H ed. Comparative aspects of sodium cotransport systems. Karger Publishing, Basel. p.241-263.
|
Turner L M, Webster S G, Morris S. 2013. Roles of crustacean hyperglycaemic hormone in ionic and metabolic homeostasis in the Christmas Island blue crab, Discoplax celeste. J. Exp. Biol., 216(7): 1 191-1 201.
DOI:10.1242/jeb.078527 |
Wang J Q, Zhang T, Tong Y, Liu J, Luo M, Liu W F, Geng W, Zhang Y. 2005. Effects of embryonic developmental stages in vitro from fertilized eggs in Chinese mitten crab Eriocheir sinensis on larva rearing. J. Dalian Fish. Univ., 20(3): 192-197.
(in Chinese with English abstract) |
Wang R F, Zhuang P, Feng G P, Zhang L Z, Huang X R, Jia X Y. 2012. Osmotic and ionic regulation and Na+/K+-ATPase, carbonic anhydrase activities in mature Chinese mitten crab, Eriocheir sinensis H. Milne Edwards, 1853 (Decapoda, Brachyura) exposed to different salinities. Crustaceana, 85(12-13): 1 431-1 447.
|
Wear R G. 1974. Incubation in British decapod Crustacea, and the effects of temperature on the rate and success of embryonic development. J. Mar. Biol. Assoc. UK, 54(3): 745-762.
DOI:10.1017/S0025315400022918 |
Wilder M N, Huong D T T, Atmomarsono M, Hien T T T, Phu T Q, Yang W J. 2000. Characterization of Na/K-ATPase in Macrobrachium rosenbergii and the effects of changing salinity on enzymatic activity. Comp. Biochem. Physiol Part A Mol. Int. Physiol., 125(3): 377-388.
DOI:10.1016/S1095-6433(00)00162-8 |
Wilder M N, Huong D T T, Okuno A, Atmomarsono M, Yang W J. 2001. Ouabain-sensitive Na/K-ATPase activity increases during embryogenesis in the giant freshwater prawn Macrobrachium rosenbergii. Fish. Sci., 67(1): 182-184.
DOI:10.1046/j.1444-2906.2001.00219.x |
Wójcik D, Normant M. 2014. Gonad maturity in female Chinese mitten crab Eriocheir sinensis from the southern Baltic Sea–the first description of ovigerous females and the embryo developmental stage. Oceanologia, 56(4): 779-787.
DOI:10.5697/oc.56-4.779 |
Xu B S, He L G. 1987. Culture technology of Chinese mitten crab Eriocheir sinensis. Jindun Publishers, Beijing. p.67-68.
|
Xu R W, Jiang J B. 1996. Preliminery study on tolerance of mitten crab larva to salinity variations. Fish. Sci. Technol. Inform., 23(4): 147-150.
(in Chinese with English abstract) |
Zang W L, Jiang M, Dai X L, Geng Y H, Shen L H, Wang J L, Wang J Z, Liu Z K, Zhang S H. 1999. Effects of salinity on larval development of Eriocheir sinensis. J. Shanghai Fish. Univ., 8(2): 174-178.
(in Chinese) |
Zhang T L, Li Z J, Cui Y B. 2001. Survival, growth, sex ratio, and maturity of the Chinese mitten crab (Eriocheir sinensis) reared in a Chinese pond. J. Freshw. Ecol., 16(4): 633-640.
DOI:10.1080/02705060.2001.9663855 |
Zhao L, Chen H L, Sun D X. 2004. Effect of salinity on larval culture in crab. Freshw. Fish., 34(4): 33-35.
(in Chinese) |
 2019, Vol. 37
2019, Vol. 37