Institute of Oceanology, Chinese Academy of Sciences
Article Information
- LI Qiao, ZHANG Fang, WANG Minxiao, LI Mengna, SUN Song
- Effects of hypoxia on survival, behavior, and metabolism of Zhikong scallop Chlamys farreri Jones et Preston 1904
- Journal of Oceanology and Limnology, 38(2): 351-363
- http://dx.doi.org/10.1007/s00343-019-9074-0
Article History
- Received Mar. 19, 2019
- accepted in principle May. 12, 2019
- accepted for publication Jul. 16, 2019
2 University of Chinese Academy of Sciences, Beijing 100049, China
Zhikong scallop Chlamys farreri, a sessile and filter-feeding benthic animal, is widely distributed along the coast of northern China, and constitutes one of the major cultured bivalves due to its high nutritional value (Guo and Luo, 2006). In the past 30 years, both the culture area and density have soared, resulting in the deterioration of the environment. Mass mortalities were recorded several times during summer since 1996 (Zhang and Yang, 1999; Xiao et al., 2005; Wang et al., 2018). In an effort to determine the possible reasons accounting for the increase in mortality rates of scallops, several hypotheses have been proposed. Among these, pathogens including parasitic organisms and viruses are considered crucial factors. Additionally, the rising sea temperature has been proven an impact on immunity of the scallops (Chen et al., 2007b; Tang et al., 2010; Getchell, 2006). In addition, lower heterozygosity caused by inbreeding, toxic dinoflagellate, and shortage of food are also considered to be potential factors contributing to the severe mortality (Yan et al., 2001; Yang et al., 2001; Xiao et al., 2005). However, as a growing stressor worldwide and often a consequence of highdensity stocking and water quality deterioration, the effects of hypoxia has not been extensively studied.
Hypoxia is lethal to many marine organisms as mass mortalities often occur under severe hypoxia (Vaquer-Sunyer and Duarte, 2008). Hypoxic zones have significantly increased in the last few decades with the development of eutrophication and rising temperature (Diaz and Rosenberg, 2008; Howarth et al., 2011). In the culture area, the situation may become worse as the cultured organisms can produce large amounts of organic deposits, which may consume lots of oxygen in decomposition process. It is reported that in the Jiaozhou Bay, the culture of Zhikong scallops and bay scallops (Argopecten irradians) resulted in a decrease in dissolved oxygen (DO) levels to around 2.0 mg/L. In Sanggou Bay, another important culture area for Zhikong scallops, the risk of eutrophication and hypoxia have risen sharply due to aquaculture activities (Yang et al., 2018).
To survive hypoxia, metabolism is often depressed to save energy, as aerobic metabolism is limited when oxygen is insufficient. To save energy, the intensity of both physical and physiological activities, such as swimming, breathing, digestion, and heartbeat, are usually reduced to basal levels (Seibel, 2011; Breitburg et al., 2018). Furthermore, glucose, the main energy source for cells, can be broken down in multiple ways under anaerobic conditions: glucosesuccinate pathway, which features high efficiency but low rates; lactate and opine pathways, which have low efficiency but high rates. The former pathway is often considered the main strategy for hypoxiatolerant species due to the avoidance of acid metabolites, such as lactate, which may disrupt cell homeostasis (Larade et al., 2002). In addition, to control glycolytic flux under hypoxia, covalent modification of key glycolytic enzymes often takes place. For example, the downregulation of lactate dehydrogenase (LDH) and pyruvate kinase (PK) activities in Littorina littorea significantly reduced its metabolic rate under hypoxia (Smolinski, 2016).
Generally, scallops are less tolerant to hypoxia than other bivalves. For example, the bay scallop Argopecten irradians could only survive 8–24 h under the DO of 2–4 mg/L, although the DO is still suitable for most clams and oysters (Leverone, 1995). In addition to posing a great threat to survival, hypoxia is found to impart an inhibitory effect on the feeding, breathing, movement, and growth of bivalves. For example, the shell length and tissue dry weight of juvenile GreenLipped Mussel Perna viridis significantly decreased under hypoxic conditions, and the early developmental traits of the mussel Mytilus edulis were adversely affected by reduced oxygen (Wang et al., 2011; Kong et al., 2019). Besides, it is reported that hypoxia often lead to the increasing hemocyte mortality and reactive oxygen species production in bivalves, which may leave them vulnerable to diseases and other threats (Sui et al., 2016). For biochemical responses, the glucosesuccinate pathway that is popular in many bivalves, has been confirmed in the bay scallop, Argopecten irradians concentricus, based on the accumulation of succinate (Ivanina et al., 2016; Aguirre-Velarde et al., 2018).
For Zhikong scallop, mortality under moderate hypoxia has been reported, and several researchers have suggested that hypoxia may depress the immune responses of Zhikong scallop, increasing their sensitivity to pathogens (Chen et al., 2007a & c). However, none of these studies has investigated LC50 levels to assess their tolerance to hypoxia precisely, and the response of key enzyme activities involved in respiration metabolism remains unclear, whether for Zhikong scallops or most marine benthos. Besides, most studies on the behavioral responses of bivalves to hypoxia have focused on the slow-moving or infaunal species. For example, the Macoma balthica would reduce their burial depth to get more oxygen, and the anti-predatory responses of Perna viridis and Mytilus coruscus were often impaired by hypoxia (Long et al., 2008; Wang et al., 2013; Sui et al., 2016). In contrast, the behavioral responses of the free-living bivalves, including scallops, have not been studied extensively. Thus, to answer the questions that whether Zhikong scallop could survive a growing hypoxic threat and what kind of strategy it will adopt, a long-term experiment was conducted to determine the effects of hypoxia on the survival, behavior, physiological, and biochemical responses of Zhikong scallops. Determining the LC50 for DO may improve our understanding of the consequences of highdensity culture and water deterioration and provide scientific advice for the scallop farming industry.
2 MATERIAL AND METHOD 2.1 Animals and laboratorial hypoxiaZhikong scallop (mean shell height: 5.51±0.12 cm, mean weight: 23.86±2.79 g) were purchased from Mingsheng Farm, Qingdao, China on June 22, 2018. All of the scallops were transported to the laboratory within 2 h. During their transport, air was pumped continuously into the temporary containers filled with seawater to avoid any possible hypoxia-acclimation. In the laboratory, all of the scallops were brushed carefully to remove attached debris on shells and were placed in several 250-L-aquaria (80–90 scallops in one aquarium). In the holding period, Chlorella spp. (9.13±2.48 mg/L) were provided as food once a day. Water temperature was controlled at 18℃ (average temperature of the bottom water in most of their habitats during summer). DO levels were monitored in real time using an OX11875 meter manufactured by Loligo Systems (Viborg, Denmark). Air stones were placed at the bottom of the aquaria, and high-pressure bottle was opened to pump compressed air into the aquaria when DO dropped below 7.0 mg/L. Ammonia nitrogen, hydrogen sulfide, and pH were monitored daily using a multifunctional water quality analyzer HNT6S (Honart, Beijing, China), and one-third of the seawater was replaced every two days to ensure water quality. All of the aquaria were covered with a 2-mmthick black canvas to avoid light stimulation.
A circulating water system with DO- and temperature-controlled functions was designed for the experiments. Each DO concentration corresponded to a water supply tank and four replicate experimental tanks. The 800-L supply tank, which was equipped with a DO sensor, a temperature sensor, an ultraviolet lamp, a chiller, a heater, and air stones, served as the chamber to adjust DO and temperature of the sea water. The animals were housed in four 90-L experimental tanks, connected with the water supply tank. Between the water supply tank and the experimental tanks, a purifier was installed to filter the impurities and metabolic wastes. The water was continuously pumped from water supply tank to the experimental tanks, and then flowed back through the purifier. Solenoid valves, which were controlled by rely and a computer, automatically opened and closed according to the actual value and set value of the DO: if the actual value is higher than the setting value, then nitrogen will be pumped in; if the actual value is lower than the setting value, then compressed air will be pumped in. The time it took for the DO sensors to reach the exact value and for the oxygen to diffuse evenly in the tanks have been considered when designing the control program. The accuracy and stability of the DO in experimental tanks have been verified. The DO meter (OX1 1875) and sensors (OX11250) mentioned above were purchased from Loligo Systems (Viborg, Denmark). The purifiers were provided by Dalian Huixin Titanium Equipment Development Co., Ltd. (Dalian, China).
2.2 Survival and behavior observationIn the 20-day hypoxia tolerance experiment, DO concentrations were set as follows: 1.5, 2.0, 2.5, and 3.0 mg/L for the treatment groups, and 7.0 mg/L for the control group. In each group, four replicate experimental tanks were set. Among these, three tanks were used for survival, physiological, and biochemical studies, whereas the fourth tank was used for behavioral observation (Fig. 1). The temperature was set to 18℃; salinity was controlled at 30.0±1.0; the flow rate of each experimental tank was set as 200 L/h. All of the experimental tanks were covered with 2-mm-thick black canvas to avoid stimulus from the sun, except for the tanks used for behavior observation.
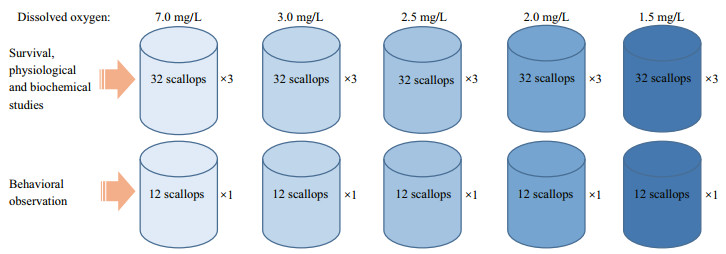
|
| Fig.1 Schematic diagram of the experimental design |
Before the scallops were transported from the holding aquaria to experimental tanks, they were carefully examined and only the ones with intact shells, tentacles, and outer mantle were selected for the experiments. The selected scallops were then assigned randomly to the experimental tanks with 32 or 12 (behavior observation) in each. In the first 72 h, the DO levels in all of the experimental tanks were controlled at 7.0 mg/L to allow the scallops to adapt to the new environment, and any scallops that died or abnormally behaved (i.e., shells were tightly closed or tentacles were not stretched) during this period were removed. The DO levels in all of the tanks were then slowly and evenly reduced to the target value within 48 h and maintained for 20 days (from July 8 to 27). Animals were fed once a day with Chlorella spp. (6.37±2.87 mg/L) during the entire experiment. To maintain the quality of the seawater, one-third was replaced every other day. The DO levels in all of the experimental tanks were monitored daily using the Winkler method, and ammonia nitrogen, hydrogen sulfide, and pH levels were analyzed every two days using the water quality analyzer earlier described.
In the pre-experiment, it is found that when a scallop is about to die under hypoxia, its shells will open and the outer mantle and tentacles will contract. Additionally, the gills often curl at some degree. Thus, during mortality check, the mantle and gill of the scallop that showed the symptoms described above would be touched using a glass rod for three times, and if the scallop did not respond, it would be considered dead. The dead scallops would be removed from the experimental tanks immediately to prevent it from destroying the water quality. Mortality was monitored every 12 h in all of the tanks during the acclimation period and the whole experiment.
For behavioral observations, five cameras were set to record the scallops in these specified tanks in real time. In addition, to better estimate the distance the scallops move, a vertical ruler was attached to the surface of the tank, and a cross-shaped ruler was installed at the bottom of the tank. Video camera master, a video recording software produced by ZG Technology Co., Ltd. (Tianjin, China) was adopted for video recording. The video files were then exported and analyzed to study the behavioral responses of the scallops. For each scallop, the horizontal and vertical moving distance were calculated, and the frequency of shell opening and closing was counted.
2.3 Physiological and biochemical measurementsTo measure the oxygen consumption rate (OCR) continuously, a 30-L tightly closed respiratory chamber was designed (Fig. 2). The water inlet was at the bottom of the cylindrical chamber, and the water outlet was at the top. A valve was connected to the water inlet to control the water flow. A DO sensor (OX11250) was placed in the middle of the chamber, and two stirrers were installed to ensure the oxygen to diffuse evenly. Before the scallops were deployed in the chamber, their volume was calculated using water displacement method. During measurements, the sea water from water supply tank was intermittently injected into the chamber, and the DO at the end of each injection and the beginning of next injection were recorded. In addition, a blank chamber carrying no scallop was set at the same time to measure the oxygen consumption by other microorganisms. The OCR was calculated as follows:
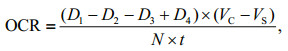
|
| Fig.2 Structure of the respiratory chamber The main structure includes the water inlet (middle of the bottom), the water outlet (upper side), the water valve (connected with the water inlet), the dissolved oxygen sensor (hung in the middle) and the two stirrers (hung on both sides of the sensor). |
where D1 is the DO of the treatment chamber at the end of one injection, and D2 is the DO of the treatment chamber at the beginning of next injection. D3 and D4 are the DO of the corresponding blank chamber at the same time when D1 and D2 were measured. VC and VS refer to the volume of the chamber and the scallops respectively. N is the number of scallops in the treatment chamber, and t is the time interval between the measurement of D1 (D3) and D2 (D4).
Ten respiratory chambers (diameter: 40 cm; height: 18 cm) were set with each DO concentration corresponding to a chamber carrying initially 28 scallops and a blank chamber. All of the scallops were checked, selected, and handled the same way as described above. Each measurement lasted for about 10 min. The time interval between two measurements was 2 h during the period of DO-decreasing process and the first day the 20-day tolerance experiment. In the remaining 19 days, it was extended to 12 h.
Heart rate (HR) and enzyme activities were analyzed at the end of the 20-day tolerance experiment. For HR measurement, eight scallops from each group were randomly designated. The sensors were attached to the shells with nail-free glue. Powerlab 8/35 produced by AD instrument (New South Wales, Australia) was used for the signal collection and analysis. The measurements started 2 h after the deployment of the sensors to avoid disturbance caused by the handling. The waveform files were then analyzed and only the segments with clear and stable waveform were adopted for HR counting. One heartbeat cycle was defined as the time from one wave crest to the next wave crest or from one trough to the next trough.
For enzyme activity measurements, the activities of phosphofructokinase (PFK), LDH, phosphoenolpyruvate carboxylase (PEPC), fumaric reductase (FR), and octopine dehydrogenase (ODH) were studied in the adductor muscle of the scallops. PFK is the most crucial rate-limiting enzyme in glycolysis. The activities of PEPC and FR usually reflect the pathway of phosphoenolpyruvate metabolism and the anaerobic metabolism. LDH and ODH are the common pyruvate reductase in bivalves. Eight random scallops were selected from each group, and the adductor muscle of each scallop was cut into several 3 mm×3 mm×3 mm sections. Each section was designated for the measurement of one enzyme and was temporarily stored in a centrifuge tube in the icebox. To avoid the potential bias caused by subjective consciousness, the experimenter responsible for the following analysis was blinded from the group information as the centrifuge tubes were re-numbered by another experimenter. For the measurements of PFK, LDH, and PEPC activities, commercial assay kits (A129, A020-2, and A130; Nanjing Jiancheng Bioengineering Institute, Nanjing, China) were used according to the manufacturer's instructions. For FR activity analysis, the method described in the Inhibition of Albendazole and Oxfendazole on the Activity of Fumaric Reductase in Cysticercus cellulosae (Gao et al., 2002) was adopted, whereas for ODH, the method in Further Studies on the Phylogenetic Distribution of Pyruvate Oxidoreductase Activities (Livingstone et al., 1990) was employed.
2.4 Post effect analysisSince hypoxic events may occur several times during the summer, frequent deoxygenation and oxygenation may bring extra oxidative damage to the scallops. To determine the survival rates of Zhikong scallops in a second hypoxic event, the DO of treatment tanks designated for survival observation were recovered to normoxic condition (7.0 mg/L) after physiological and biochemical sampling, and maintained for seven days. Then, the DO of all tanks, including the control ones in the previous tolerance experiment, were reduced to 1.5 mg/L evenly in 48 h and maintained for another 10 days. Other environmental settings and daily operations, including temperature, salinity, flow rates, sunshade canvas, and water changing frequency were the same as before. Mortality was monitored every 12 h.
2.5 Statistical analysesStatistical analyses were conducted in survival rates, moving distance, frequency of shell opening and closing, HR, and enzyme activities (Supplementary Tables S1–S2). The data were first tested for normality and homogeneity of variances. If the data successfully pass the two tests, then one-way ANOVA was adopted, and if the data failed to pass any of the above tests, then Kruskal-Wallis H-test would be adopted to ensure the reliability of the conclusion, though the sensitivity of Kruskal-Wallis H-test is lower than ANOVA. Survival rates were analyzed using the latter method, as the Kruskal-Wallis H-test is reliable when the number of replicates are not large enough. Tukey test was used for multiple comparisons corresponding to one-way ANOVA, whereas the Mann-Whitney U-test was used when Kruskal-Wallis H-test was adopted. Statistical significance of the results was determined when a P-value of < 0.05 was obtained. The 20-day LC50 was estimated using probit analysis (Finney, 2009). All of the above analyses were conducted using SPSS 16.0 (IBM, Armonk, USA).
3 RESULT 3.1 Survival and behavioral responseHypoxia posed a great threat to the survival of Zhikong scallops, as the survival rate was reduced with the decreasing DO (Fig. 3). The scallops exposed to 1.5 mg/L DO was apparently the most disrupted in this study, as the observed survival rate was < 50% and was significantly lower than the other treatment groups. The survival rates of the treatment groups exposed to 2.0, 2.5, and 3.0 mg/L DO are relatively close, ranging from 59% to 69%. Mortality occurred nearly at the beginning of the 20-day tolerance experiment. However, after about eight days, the survival rates of all of the groups came to a relatively stable stage, which lasted about four days. Then, the survival rates continued to drop until the end of the experiment. The scallops of the control group were all alive when the experiment was over, suggesting that there was no other environmental threat but hypoxia in this variable-controlled experiment. The LT50 at 1.5 mg/L DO was 432 h, and the 20-day LC50 for DO was calculated to be 1.8 mg/L using probit analysis.

|
| Fig.3 Changes in survival rates of Zhikong scallop in the 20-day hypoxia tolerance experiment The lines marked A, B, C, and D represent the treatment groups exposed to 1.5, 2.0, 2.5 and 3.0 mg/L DO. The line marked N represent the control group exposed to 7.0 mg/L DO. |
Figure 4 shows that hypoxia has a significant effect on the horizontal and vertical movements of the Zhikong scallops. Except for the first 96 h, the scallops exposed to 1.5, 2.0 and 2.5 mg/L DO were more active in terms of horizontal movements. The treatment group of 3.0 mg/L DO and the control group did not differ at most times. For vertical movements, the scallops of the treatment groups were quite active as the average moving distance often exceeded 160 cm per individual. The frequency of shell opening and closing did not differ much across different groups except that the control group showed a significant high value in the first 48 h.
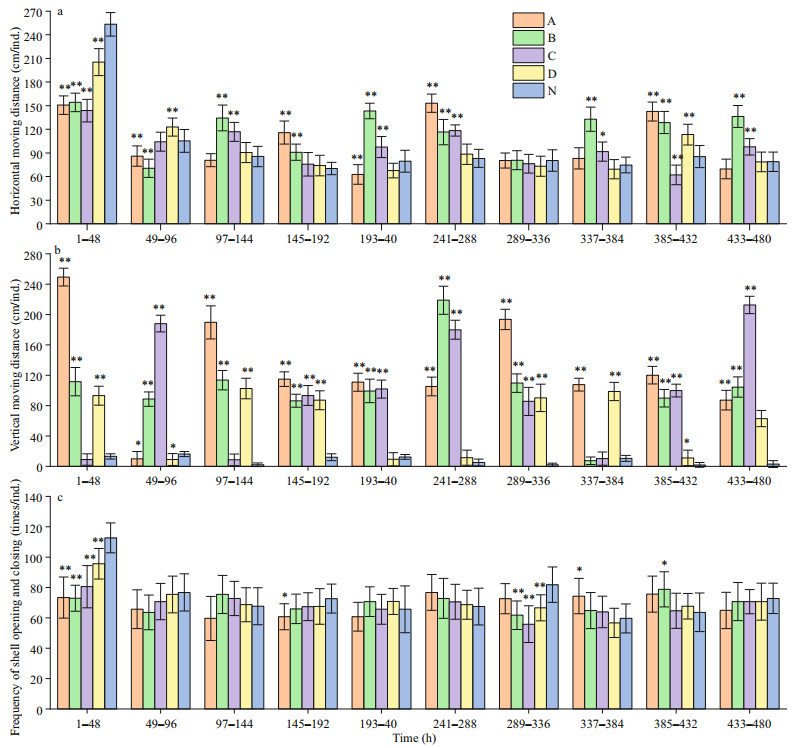
|
| Fig.4 Behavioral responses of Zhikong scallop to hypoxia a. average horizontal moving distance per scallop during the 20-day experiment; b. average vertical moving distance per scallop during the 20-day experiment; c. average frequency of shell opening and closing per scallop during the 20-day experiment. The columns marked A, B, C, and D represent the treatment groups exposed to 1.5, 2.0, 2.5 and 3.0 mg/L DO. The column marked N represent the control group exposed to 7.0 mg/L DO. Error bars indicate mean±SD (**indicates P < 0.01 and * indicates P < 0.05 compared with the control group). |
The OCR reduced immediately when the DO started to drop, and maintained relatively stable values after the target DO were reached. The lower the DO, the lower the OCR, though the differences between the treatment groups exposed to 2.0, 2.5, and 3.0 mg/L DO were small (Fig. 5). During the 48-h oxygen-decreasing period, the OCR did not decrease. A phenomenon that the OCR initially decreased, then rose and then decreased again was observed among all treatment groups. In the following period of the 20-day experiment, the OCR of all of the groups only slightly fluctuated.
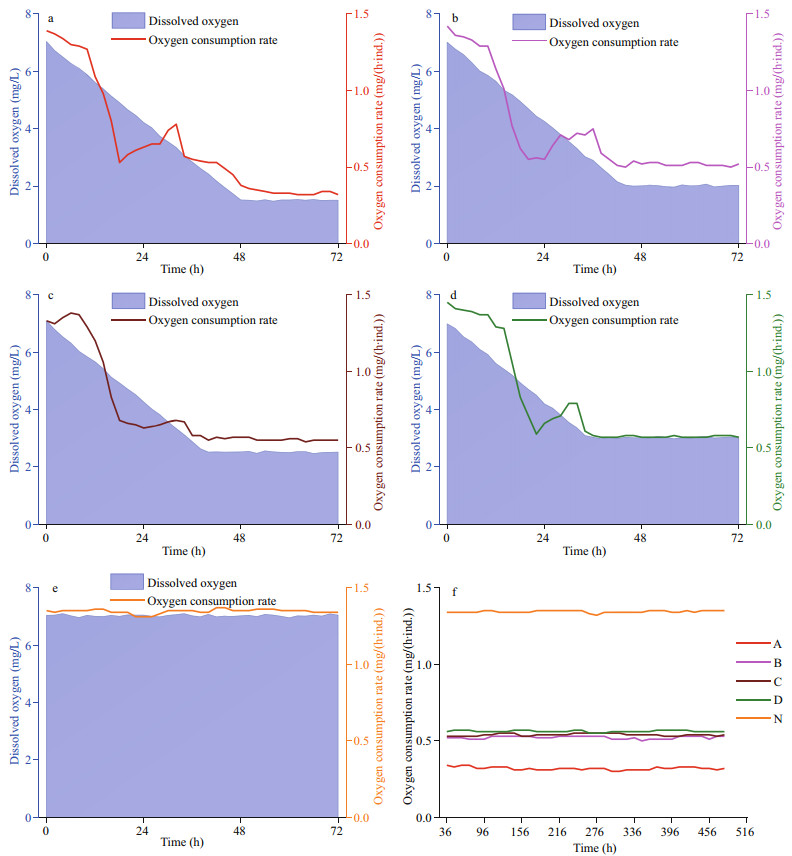
|
| Fig.5 Changes in oxygen consumption rate of Zhikong scallop a–e. oxygen consumption rates of the scallops exposed to 1.5, 2.0, 2.5, 3.0, and 7.0 mg/L DO in the 48-h oxygen-decreasing process and the first 24 h of the 20-day experiment; f. oxygen consumption rates of the scallops exposed to 1.5, 2.0, 2.5, 3.0, and 7.0 mg/L DO in the rest of the 20-day experiment. The lines marked A, B, C and D represent the scallops exposed to 1.5, 2.0, 2.5, and 3.0 mg/L DO. The line marked N represent the scallops exposed to 7.0 mg/L DO. |
The effect of hypoxia on heart rate was significant as the heart rate of the scallops exposed to 1.5 mg/L DO was depressed to around 20 beats per minute. However, there was no significant difference between the treatment groups exposed to 2.0, 2.5, and 3.0 mg/L DO and the control group (Fig. 6).
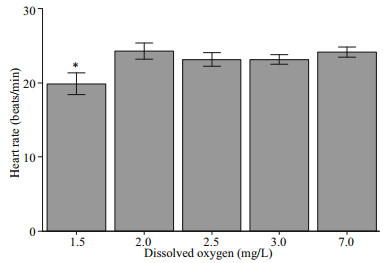
|
| Fig.6 Heart rates of Zhikong scallop at the end of the 20- day experiment, under different DO concentrations Error bars indicate mean±SE (* indicates P < 0.05). |
The effects of hypoxia on the activities of key enzymes involved in respiratory metabolism were significant (Fig. 7). FR activity in adductor muscle of Zhikong scallops responded drastically to decreasing oxygen levels as it peaked under the DO of 3.0 mg/L and gradually dropped with further decrease in DO. The FR activity under 1.5 mg/L DO was roughly equal to that under normoxic condition. The changes in LDH and PEPC activities showed a similar trend as the lower level of DO resulted in an increase in enzyme activities. Notably, the LDH activity of the control group showed almost no activity. The PFK activity decreased when DO dropped below 2.5 mg/L (P=0.033). ODH activity did not respond to hypoxia as no significant difference was detected between the treatment groups and the control group.
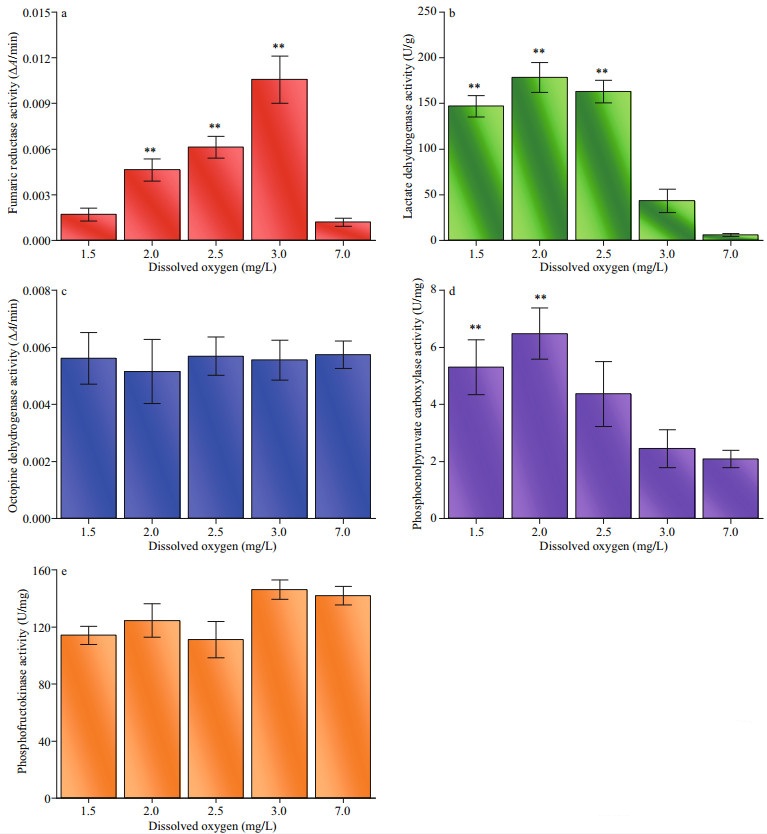
|
| Fig.7 Biochemical responses to hypoxia measured in adductor muscle of Zhikong scallop a–e: fumaric reductase, lactate dehydrogenase, octopine dehydrogenase, phosphoenolpyruvate carboxylase and phosphofructokinase activities at the end of the 20-day experiment. Error bars indicate mean±SE (** indicates P < 0.01). |
After the DO in all of the treatment groups were reset to normoxic level, mortality rates increases in the groups exposed to the reduced DO in the previous experiment (Fig. 8). At the end of the second hypoxia application, the survival rates of the groups once exposed to the reduced DO were 34%, 56%, 56%, and 55%, lower than that of the group once exposed to normoxia (71%). Besides, they were also lower than the survival rate on day 10 of the treatment group once exposed to 1.5 mg/L DO (67%). The treatment group once exposed to 1.5 mg/L DO had a minimum survival rate during the oxygen-decreasing period and the entire time of the second hypoxia.
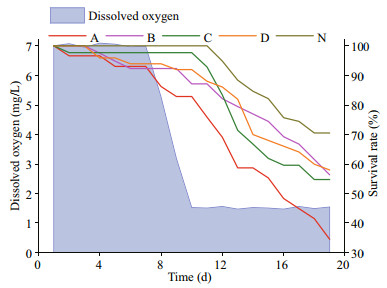
|
| Fig.8 Post effects of hypoxia The blue area represents the changes in dissolved oxygen, while the lines in different colors represent the survival rates of Zhikong scallop in different groups (A, B, C, D and N represent the scallops exposed to 1.5, 2.0, 2.5, 3.0 and 7.0 mg/L DO in the previous 20-day tolerance experiment). |
Hypoxia, a growing threat caused by eutrophication or high-density mariculture, significantly reduced the survival chance of Zhikong scallops, as shown in Fig. 3. The 20-day LC50 for DO was estimated to be 1.8 mg/L, indicating its tolerance to hypoxia was weaker than most other bivalves. For example, the Manila clam Ruditapes philippinarum is tolerant to 2 mg/L-hypoxia without mortality, whereas the Asian oyster Crassostrea ariakensis and the Eastern oyster Crassostrea virginica can survive more than 10 days under 0.5 mg/L DO (Lombardi et al., 2013; Zhang et al., 2017). Thus, to reduce economic losses, close DO monitoring was necessary and appropriate measures could be adopted to prevent the culture area from deadly hypoxia events.
An important phenomenon to notice is that the survival rate did not change linearly with DO. Figure 3 shows that the survival rate lines corresponding to the groups exposed to 2.0, 2.5, and 3.0 mg/L DO came pretty close, while the line corresponding to the group exposed to 1.5 mg/L DO was far apart. This may implicate there is an oxygen threshold between 1.5 and 2.0 mg/L for Zhikong scallop. It can be explained that the physical stress would break the limit of metabolic regulation when DO dropped below that threshold. In the study of another bivalve, Macoma balthica, the similar phenomenon was observed as mortality rates sharply increased when DO dropped below 2.5 mg/L (Long et al., 2008).
Another phenomenon worth paying attention to is that the survival rates did not always drop with time. This may indicate that after a certain time of hypoxia exposure, Zhikong scallops could adapt to the low DO concentrations to some degree. Furthermore, the time when mortality would come to a halt may represent the respond speed of adaptive mechanism. This phenomenon is quite meaningful, as it would protect Zhikong scallop population from complete destruction if DO concentration could rise after about 10 days. For another bivalve, Donax serra, the same trend was also observed (Laudien et al., 2002). However, since the temporary stop of mortality would come too late, it is meaningless for Donax serra population to recover.
In the summer, some coastal and estuarine hypoxia often occur more than once as the suitable hydrographical condition for water stratification may form and rupture several times within months. Thus, the survival of marine organisms may be challenged repeatedly. As an organism that is able to live in the intertidal zone fixing on rocks, Zhikong scallops are considered to have strong tolerance against the repeated deoxygenation and re-oxygenation processes like other intertidal benthos (Ivanina et al., 2016). However, in the present study, the scallops exposed to hypoxia for a second time have a survival rate 15% to 35% lower than those exposed to hypoxia for the first time. This result may again change people's understanding about the hypoxia tolerance of those intertidal organisms. The scallop culturists should also monitor water quality and take measures to prevent fluctuations in oxygen levels.
4.2 Strategy to survive hypoxiaTo increase survival chances under hypoxia, marine benthos would respond in terms of their behavior. Fish with strong swimming ability often initially try to escape hypoxic zones, and these attempts have been observed in some shrimps, such as the penaeid shrimp Metapenaeus ensis(Wu et al., 2002; Domenici et al., 2007). Scallops, with highly developed adductor muscle, are capable of swimming a short distance by rapidly opening and closing their shells.
For horizontal movements, the scallops were particularly active during the first 48 h, which may be attributed to adaptation efforts. In the following 18 days, the scallops exposed to hypoxia were generally more active than the scallops of the control group, which may be explained as the attempts to escape the challenging environments. However, although the differences were significant in statistics, the absolute values of the difference only ranged from about 40 to 50 cm, and it seemed useless for scallops to escape hypoxia in the wild. Here, we propose two assumptions: first, Zhikong scallop did attempt to escape hypoxia. However, the small experimental tanks limit their activity; another possible situation is that the increased horizontal movements were not due to the escape attempts, but due to the anxious response under hypoxia-stress. Thus, to further answer this question, it may be helpful to conduct further investigations in the wild or to conduct the experiments with huge tanks in the future.
Vertical movements in the control group were barely observed as most scallops were fixed to the bottom of the tanks and only make minor horizontal movements around the fixation points. The scallops exposed to hypoxia showed significantly high activity, with some individuals moving more than 240 cm. This fact seemed to support the first assumption described above, as the height of the experimental tank was longer than its diameter. From this perspective, Zhikong scallops may have a chance to avoid a hypoxic zone if the hypoxia is at its early stage or not spread to a large area. The frequency of shell opening and closing provides little implication.
However, if it is impossible to escape from hypoxic water, metabolic regulation is crucial. As the efficiency of anaerobic metabolism is much lower than that of aerobic metabolism, it is crucial to save energy when oxygen is insufficient. Oxygen consumption rate and heart rate, two important indicators of metabolic rate, are often depressed under hypoxia (Grieshaber et al., 1993; Gobler and Baumann, 2016). In 2014, Artigaud et al. (2014) studied the respiratory responses of the great scallop, Pecten maximus. Compared to the Zhikong scallop, the great scallops seemed to have stronger ability in respiratory regulation as the OCR barely changed when DO dropped from 100% to 40% saturation. As shown in Fig. 5, the OCR of Zhikong scallop decreased by more than a half when DO dropped from 7.0 to 3.0 mg/L. However, when DO further dropped from 3.0 to 2.0 mg/L, the OCR showed little response. This new strategy has rarely been reported. An advantage of this strategy could be that it is helpful for energy saving and storage, as feeding and digestion activities are often depressed under hypoxic conditions (Foss et al., 2002; Ripley and Foran, 2007). However, if an abnormal as well as depressed metabolism last for too long, then it may cause potential damage to the scallops.
In Fig. 5, it is easy to notice another interesting phenomenon that during the OCR-decreasing process, there was a little peak when DO dropped to about 3.0 mg/L. The phenomenon that OCR increases with decreasing DO was once reported in some fish. For example, the rainbow trout Salmo gairdneri was able to double its OCR under hypoxia. However, according to the conclusions, the utilization rate of oxygen reduced; the increased consumption of oxygen was not flowing into the metabolism of the body. Instead, it was the result of added efforts in breathing (Hughes and Sunders, 1970). For Zhikong scallops, the same thing could happen when they decide to regulate their respiration and prevent a further metabolic decline. To answer this question clearly, further investigations are warranted.
The cardiac response to hypoxia may differ across different species. In some shrimps, Nephrops norvegicus and Metapenaeus ensis for example, the HR were reduced with the decreasing DO (Hagerman and Uglow, 1985; Wu et al., 2002). However, in the scallop Pecten maximus, a gradually decreasing DO would result in a slight regulatory upswing of HR, and in the European sea bass, Dicentrarchus labrax, a strong hypoxia-tolerance was often correlated with higher rates of cardiac contraction and relaxation (Brand and Roberts, 1973; Joyce et al., 2016). Figure 6 shows that there was no significant decrease in HR when DO dropped to 2.0 mg/L. Even at the most severe hypoxic group (1.5 mg/L DO), HR was only slightly reduced, suggesting the lack of cardiac regulatory ability of Zhikong scallops. An increasing HR could pump more blood as well as more oxygen per unit time. However, the increase in heart activity itself will cost more energy, thus requiring more oxygen. Thus, the result we see here was part of a larger energy balance scheme.
As the major energy-generating reaction under hypoxia, glucose metabolism often has diversified pathways. At the beginning of hypoxia, glycolysis is usually coupled with the transamination of aspartate. During the process, pyruvate converts into succinate, leaving oxaloacetate, malate, and fumarate as intermediates, partially reversing the tricarboxylic acid cycle. With the depletion of aspartate, oxaloacetate will be produced directly from phosphoenolpyruvate instead of pyruvate. (Larade and Storey, 2002). Figure 7 shows an increase in FR and PEPC under hypoxia, which may prove the existence of the pathway described above. However, after the FR activity peaked under 3.0 mg/L DO, it began to decrease with DO, which may indicate that the glucose-succinate pathway was not selected as the main strategy when oxygen is seriously insufficient.
Additionally, it has been reported that pyruvate often reacts with arginine to form octopine, instead of lactate in invertebrates (Hardewing et al., 1993). This metabolic pathway is considered crucial as it can avoid the accumulation of acids and maintain cellular homeostasis. However, according to the present study, the ODH (the enzyme that catalyzed the production of octopine) activity did not change when DO dropped from 7.0 to 1.5 mg/L. Conversely, the LDH was greatly activated with decreasing DO, which may result in the excessive accumulation of lactate. Thus, the extremely high LDH activity may give us possible clues relating to the death of Zhikong scallops under hypoxia. Besides, the changes of PEPC activity was consistent with LDH activity, given that H+ is one of the activators of PEPC. Another important clue about the death of Zhikong scallop was that the PFK activity barely changed under hypoxia as it is often depressed to keep metabolism running at a low rate when oxygen is limited (Larade and Storey, 2002).
To better summarize the tolerance and response of Zhikong scallop under hypoxia, a summary figure (Fig. 9) is presented below.
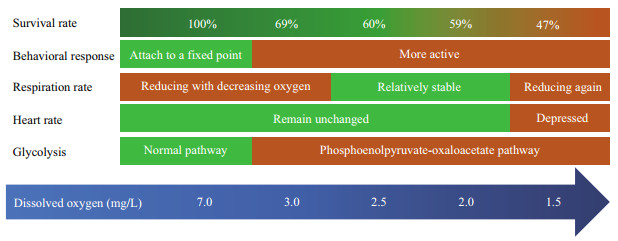
|
| Fig.9 Summary of survival and strategy under hypoxia for Zhikong scallop |
To investigate the effects of hypoxia on survival, behavior, physiological, and biochemical response of the commercially important Zhikong scallop, this 20- day experiment was conducted, and the implications of the results follow: (1) the survival of Zhikong scallop would be greatly threatened even in a moderate hypoxia, and a DO concentration lower than 2.0 mg/L could cause the population to collapse if the hypoxia lasts more than 10 days; (2) if the hypoxic event was within a limited water column, then the Zhikong scallops have a chance to escape by swimming; (3) the respiration of Zhikong scallops would be depressed to reduce metabolic rate when DO began to drop, but stay relatively stable when DO further drop from 3.0 to 2.0 mg/L; (4) the significant high LDH activity and unrepressed PFK activity may be responsible for the final death of Zhikong scallops; (5) Zhikong scallops may become less tolerant to a second hypoxia event.
6 DATA AVAILABILITY STATEMENTThe datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
7 ACKNOWLEDGMENTWe thank DAI Li for providing the unicellular algae, and ZHOU Li for providing the instruments for enzyme-activity analysis. We are grateful for the assistance of YU Nan who helped us greatly in materials preparation.
Aguirre-Velarde A, Jean F, Thouzeau G, Flye-Sainte-Marie J. 2018. Feeding behaviour and growth of the Peruvian scallop (Argopecten purpuratus) under daily cyclic hypoxia conditions. J. Sea Res., 131: 85-94.
DOI:10.1016/j.seares.2017.11.001 |
Artigaud S, Lacroix C, Pichereau V, Flye-Sainte-Marie J. 2014. Respiratory response to combined heat and hypoxia in the marine bivalves Pecten maximus and Mytilus spp. Comp. Biochem. Physiol. Part A:Mol. Integr. Physiol., 175: 135-140.
DOI:10.1016/j.cbpa.2014.06.005 |
Brand A R, Roberts D. 1973. The cardiac responses of the scallop Pecten maximus (L.) to respiratory stress. J. Exp.Mar. Biol. Ecol., 13(1): 29-43.
DOI:10.1016/0022-0981(73)90044-0 |
Breitburg D, Levin L A, Oschlies A, Grégoire M, Chavez F P, Conley D J, Garçon V, Gilbert D, Gutiérrez D, Isensee K, Jacinto G S, Limburg K E, Montes I, Naqvi S W A, Pitcher G C, Rabalais N N, Roman M R, Rose K. A, Seibel B A, Telszewski M, Yasuhara M, Zhang J. 2018. Declining oxygen in the global ocean and coastal waters. Science, 359(6371): eaam7240.
DOI:10.1126/science.aam7240 |
Chen J H, Mai K S, Ma H M, Wang X J, Deng D, Liu X W, Xu W, Liufu Z G, Zhang W B, Tan B P, Ai Q H. 2007a. Effects of dissolved oxygen on survival and immune responses of scallop (Chlamys farreri Jones et Preston). Fish Shellfish Immunol., 22(3): 272-281.
DOI:10.1016/j.fsi.2006.06.003 |
Chen M Y, Yang H S, Delaporte M, Zhao S J, Xing K. 2007c. Immune responses of the scallop Chlamys farreri after air exposure to different temperatures. J. Exp. Mar. Biol.Ecol., 345(1): 52-60.
DOI:10.1016/j.jembe.2007.01.007 |
Chen M Y, Yang H S, Delaporte M, Zhao S J. 2007b. Immune condition of Chlamys farreri in response to acute temperature challenge. Aquaculture, 271(1-4): 479-487.
DOI:10.1016/j.aquaculture.2007.04.051 |
Diaz R J, Rosenberg R. 2008. Spreading dead zones and consequences for marine ecosystems. Science, 321(5891): 926-929.
DOI:10.1126/science.1156401 |
Domenici P, Lefrançois C, Shingles A. 2007. Hypoxia and the antipredator behaviours of fishes. Philos. Trans. Roy. Soc.B:Biol. Sci., 362(1487): 2 105-2 121.
DOI:10.1098/rstb.2007.2103 |
Finney D. 2009. Probit Analysis. Cambridge University Press, Cambridge, UK. 272p.
|
Foss A, Evensen T H, Øiestad V. 2002. Effects of hypoxia and hyperoxia on growth and food conversion efficiency in the spotted wolffish Anarhichas minor (Olafsen). Aquacult. Res., 33(6): 437-444.
DOI:10.1046/j.1365-2109.2002.00693.x |
Gao J G, Gao X J, Hao Y H. 2002. Inhabition of albendazole and oxfendazole on the activity of fumaric reductase in Cysticercus cellulosae. Heilongjiang J. Anim. Sci. Vet.Med, (8): 8-9.
(in Chinese with English abstract) |
Gobler C J, Baumann H. 2016. Hypoxia and acidification in ocean ecosystems:coupled dynamics and effects on marine life. Biol. Lett., 12(5): 20150976.
DOI:10.1098/rsbl.2015.0976 |
Grieshaber M K, Hardewig I, Kreutzer U, Pörtner H O. 1993.Physiological and metabolic responses to hypoxia in invertebrates. In: Reviews of Physiology, Biochemistry and Pharmacology. Springer, Berlin, Heidelberg. p.43-147.
|
Guo X M, Luo Y S. 2006. Scallop culture in China. Dev.Aquacult. Fish. Sci., 35: 1 143-1 161.
|
Hagerman L, Uglow R F. 1985. Effects of hypoxia on the respiratory and circulatory regulation of Nephrops norvegicus. Mar. Biol, 87(3): 273-278.
DOI:10.1007/BF00397805 |
Howarth R, Chan F, Conley D J, Garnier J, Doney S C, Marino R, Billen G. 2011. Coupled biogeochemical cycles:eutrophication and hypoxia in temperate estuaries and coastal marine ecosystems. Front. Ecol. Environ., 9(1): 18-26.
DOI:10.1890/100008 |
Hughes G M, Sunders R L. 1970. Responses of the respiratory pumps to hypoxia in the rainbow trout (Salmo gairdneri). J. Exp. Biol., 53(3): 529-545.
|
Ivanina A V, Nesmelova I, Leamy L, Sokolov E P, Sokolova I M. 2016. Intermittent hypoxia leads to functional reorganization of mitochondria and affects cellular bioenergetics in marine molluscs. J. Exp. Biol., 219(11): 1 659-1 674.
DOI:10.1242/jeb.134700 |
Joyce W, Ozolina K, Mauduit F, Ollivier H, Claireaux G, Shiels H A. 2016. Individual variation in whole-animal hypoxia tolerance is associated with cardiac hypoxia tolerance in a marine teleost. Biol Lett., 12(1): 20150708.
DOI:10.1098/rsbl.2015.0708 |
Kong H, Jiang X Y, Clements J C, Wang T, Huang X Z, Shang Y Y, Chen J F, Hu M H, Wang Y J. 2019. Transgenerational effects of short-term exposure to acidification and hypoxia on early developmental traits of the mussel Mytilus edulis. Mar. Environ. Res., 145: 73-80.
DOI:10.1016/j.marenvres.2019.02.011 |
Larade K, Storey K B. 2002. A profile of the metabolic responses to anoxia in marine. Cell Mol. Response Stress, 3: 27-46.
DOI:10.1016/S1568-1254(02)80005-5 |
Laudien J, Schiedek D, Brey T, Pörtner H O, Arntz W E. 2002. Survivorship of juvenile surf clams Donax serra (Bivalvia, Donacidae) exposed to severe hypoxia and hydrogen sulphide. J. Exp. Mar. Biol. Ecol., 271(1): 9-23.
DOI:10.1016/S0022-0981(02)00030-8 |
Leverone J R. 1995. Diurnal dissolved oxygen in two Tampa Bay seagrass meadows:ramifications for the survival of adult bay scallops (Argopectin irradians concentricus). Fla. Sci., 58: 141-152.
DOI:10.2307/24320716 |
Livingstone D R, Stickle W B, Kapper M A, Wang S, Zurburg W. 1990. Further studies on the phylogenetic distribution of pyruvate oxidoreductase activities. Comp. Biochem.Physiol. Part B:Comp. Biochem., 97(4): 661-666.
DOI:10.1016/0305-0491(90)90104-2 |
Lombardi S A, Harlan N P, Paynter K T. 2013. Survival, acid-base balance, and gaping responses of the Asian oyster Crassostrea ariakensis and the eastern oyster Crassostrea virginica during clamped emersion and hypoxic immersion. J. Shellfish Res, 32(2): 409-416.
DOI:10.2983/035.032.0221 |
Long W C, Brylawski B J, Seitz R D. 2008. Behavioral effects of low dissolved oxygen on the bivalve Macoma balthica. J. Exp. Mar. Biol. Ecol., 359(1): 34-39.
DOI:10.1016/j.jembe.2008.02.013 |
McGladdery S E, Bower S M, Getchell R G. 2006. Diseases and parasites of scallops. Dev. Aquacult. Fish. Sci, 35: 595-650.
|
Ripley J L, Foran C M. 2007. Influence of estuarine hypoxia on feeding and sound production by two sympatric pipefish species (Syngnathidae). Mar. Environ. Res., 63(4): 350-367.
DOI:10.1016/j.marenvres.2006.10.003 |
Seibel B A. 2011. Critical oxygen levels and metabolic suppression in oceanic oxygen minimum zones. J. Exp.Biol., 214(2): 326-336.
DOI:10.1242/jeb.049171 |
Smolinski M B. 2016. Regulation of Pyruvate Kinase in Hypometabolic States. Carleton University, Ottawa, Ontario, Canada.
|
Sui Y M, Kong H, Shang Y Y, Huang X Z, Wu F L, Hu M H, Lin D H, Lu W Q, Wang Y J. 2016. Effects of short-term hypoxia and seawater acidification on hemocyte responses of the mussel Mytilus coruscus. Mar. Pollut. Bull., 108(1-2): 46-52.
DOI:10.1016/j.marpolbul.2016.05.001 |
Tang B J, Liu B Z, Wang X M, Yue X, Xiang J H. 2010. Physiological and immune responses of zhikong scallop Chlamys farreri to the acute viral necrobiotic virus infection. Fish Shellfish Immunol., 29(1): 42-48.
DOI:10.1016/j.fsi.2010.02.019 |
Vaquer-Sunyer R, Duarte C M. 2008. Thresholds of hypoxia for marine biodiversity. Proc. Natl. Acad. Sci. USA, 105(40): 15 452-15 457.
DOI:10.1073/pnas.0803833105 |
Wang M Q, Wang B J, Jiang K Y, Liu M, Shi X M, Wang L. 2018. A mitochondrial manganese superoxide dismutase involved in innate immunity is essential for the survival of Chlamys farreri. Fish Shellfish Immunol., 72: 282-290.
DOI:10.1016/j.fsi.2017.11.010 |
Wang Y J, Hu M H, Cheung S G, Shin P K S, Lu W Q, Li J L. 2013. Antipredatory responses of Perna viridis (Linnaeus, 1758) under acute hypoxia and low salinity. J. Molluscan Stud., 79(1): 42-50.
DOI:10.1093/mollus/eys035 |
Wang Y J, Hu M H, Wong W H, Cheung S G, Shin P K S. 2011. Combined effects of dissolved oxygen and salinity on growth and body composition of juvenile green-lipped mussel Perna viridis. J. Shellfish Res., 30(3): 851-857.
DOI:10.2983/035.030.0326 |
Wu R S S, Lam P K S, Wan K L. 2002. Tolerance to, and avoidance of, hypoxia by the penaeid shrimp (Metapenaeus ensis). Environ. Pollut., 118(3): 351-355.
DOI:10.1016/S0269-7491(01)00298-6 |
Xiao J, Ford S E, Yang H S, Zhang G F, Zhang F S, Guo X M. 2005. Studies on mass summer mortality of cultured zhikong scallops (Chlamys farreri Jones et Preston) in China. Aquaculture, 250(3-4): 602-615.
DOI:10.1016/j.aquaculture.2005.05.002 |
Yan T, Zhou M J, Fu M, Wang Y F, Yu R C, Li J. 2001. Inhibition of egg hatching success and larvae survival of the scallop, Chlamys farreri, associated with exposure to cells and cell fragments of the dinoflagellate Alexandrium tamarense. Toxicon, 39(8): 1 239-1 244.
DOI:10.1016/S0041-0101(01)00080-0 |
Yang H S, Wang J, Zhou Y, Wang P, He Y C, Zhang F S. 2001. Impact of starvation on survival, meat condition and metabolism of Chlamys farreri. Chin. J. Oceanol. Limnol., 19(1): 51-56.
DOI:10.1007/BF02842789 |
Yang S, Yang Q, Song X L, Liu S, Qu K M, Sun Y. 2018. A novel approach to evaluate potential risk of organic enrichment in marine aquaculture farms:a case study in Sanggou Bay. Environ. Sci. Pollut. Res., 25(17): 16 842-16 851.
DOI:10.1007/s11356-018-1828-2 |
Zhang F S, Yang H S. 1999. Analysis of the causes of mass mortality of farming Chlamys farreri in summer in coastal areas of Shandong, China. Mar. Sci, (1): 44-47.
(in Chinese with English abstract) |
Zhang Y, Wu H F, Wei L, Xie Z P, Guan B. 2017. Effects of hypoxia in the gills of the Manila clam Ruditapes philippinarum using NMR-based metabolomics. Mar.Pollut. Bull., 114: 84-89.
DOI:10.1016/j.marpolbul.2016.08.066 |
 2020, Vol. 38
2020, Vol. 38


