Institute of Oceanology, Chinese Academy of Sciences
Article Information
- MA Chengbo, LI Wenjun, GE Baosheng, LIN Jian, QIN Song
- Biosynthesis of phycocyanobilin in recombinant Escherichia coli
- Journal of Oceanology and Limnology, 38(2): 529-538
- http://dx.doi.org/10.1007/s00343-019-9060-6
Article History
- Received Mar. 12, 2019
- accepted in principle May. 8, 2019
- accepted for publication Aug. 14, 2019
2 Yantai Institute of Coastal Zone Research, Chinese Academy of Sciences, Yantai 264003, China;
3 Center for Bioengineering and Biotechnology, China University of Petroleum (East China), Qingdao 266580, China;
4 Center for Ocean Mega-Science, Chinese Academy of Sciences, Qingdao 266071, China
To capture effciently light energy, photosynthetic organisms have developed light-harvesting antenna systems with unique structures and functions, such as the light-harvesting antenna systems in chlorophyll proteins of higher plants and that in phycobiliproteins of cyanobacteria and red algae (phycobilisomes) (MacColl, 1998; Adir, 2005). The light-harvesting function of phycobiliproteins is mainly due to the presence of phycobilins. As a type of phycobilin, PCB is a linear tetrapyrrole derived from heme as a light-harvesting pigment in cryptophycean algae and cyanobacteria (Alvey et al., 2011). It has been found that PCB in Spirulina could inhibit strongly the activity of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (Sotiroudis and Sotiroudis, 2013). Thus, supplementation of PCB may help prevention and therapy against various diseases with NADPH oxidase in affected tissues. In addition, studies have also shown that PCB can effectively inhibit the proliferation of inflammatory cells and the production of inflammatory factors (Basdeo et al., 2016).
The PCB chromophore can be cleaved from phycocyanin apoprotein by hot methanol for a long time (Carra and Heocha, 1966; Beuhler et al., 1976), which is the key technology for extracting PCB from cyanobacteria. Because Spirulina is rich in phycocyanin, it can be used as a good raw material for extracting PCB (Marín-Prida et al., 2013). However, this long-term pyrolysis of phycocyanin consumes much methanol and energy. Therefore, a good genetic engineering technology has been demanded, in which single and pure product of PCB can be extracted via fermentation of recombinate E. coli, thus avoiding complicated process of extracting phycocyanin from Spirulina, saving the cost in the cleavage of phycocyanin.
In cyanobacteria, ferredoxin-dependent heme oxygenase (Ho1) and phycocyanobilin: ferredoxin oxidoreductase (PcyA) are two key enzymes used to generate PCB in two-step enzymatic reaction (Willows et al., 2000; Frankenberg and Lagarias, 2003). First, Ho1 catalyzes the ring-opening reaction of cyclic tetrapyrrole heme at the α-mesocarbon bridge, yielding open-chain tetrapyrrole biliverdin IX α (BV) (De Montellano, 2000). Second, BV is further reduced to PCB by PcyA. Gambetta and Lagarias. (2001) had successfully co-expressed ho1 and pcyA two genes from the bilin biosynthetic pathway of Synechocystis together with PCB from the same organism to produce holophytochome in E. coli. Recently, Ge et al. (2013) produced recombinant PCB via shake-flask culture by fermentation. Under the optimized conditions of culture medium, induction time, concentration of isopropyl β-D-thiogalactoside (IPTG), and the addition of 5-aminolevulinic acid (ALA), the rate of PCB production could reach ~3 mg/L.
IPTG is a very effective inducer of lactose operon. However, due to the potential toxicity of IPTG to humans, if IPTG is used to induce the expression of recombinant drugs, the final product may harm the human body. In addition, IPTG is expensive, so is the fermentation. On the contrast, lactose is a disaccharide, non-toxic and inexpensive; it is likely to be used as the inducer to substitute IPTG and as a carbon source for metabolism as well. After enters the cells, lactose is converted to allolactose via catalyzing β-galactosidase after which the Lac promoter is activated (Jobe and Bourgeois, 1972; Müller-Hill et al., 1964). The non-toxic and low-cost nature makes lactose as a potential material for industrial production of recombinant genetic engineering drugs.
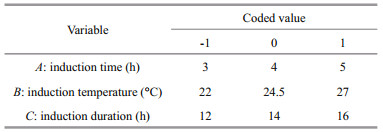
In addition to the inducer, many other factors may affect the expression effciency of recombinant E. coli, such as the time, duration, and temperature of the induction. To determine the optimum fermentation conditions for PCB production and possible combined effects of other variables, the response surface methodology (RSM) proposed by Lee and Gilmore (2006) was introduced for the biotechnology production. Learnt from RSM design, three interactive parameters (time, duration, and temperature) are chosen for the combined impact on the absorbance of PCB at 680 nm to optimize the condition of PCB production.
The aim of this study is to determine the optimum conditions in the course of induction by the inducer lactose; the time, induction duration, and temperature of the induction are determined for achieving a higher production of recombinant PCB. First, we introduced the philosophy of RSM for getting a specific protocol of recombinant PCB, and then the purification of recombinant PCB is extracted with chloroform effciently. Finally, the recombinant PCB with high anti-oxidative activity is established by DPPH anti-oxidant experiment.
2 MATERIAL AND METHOD 2.1 Recombinant E. coli constructionGe et al.(2013, 2018) described the plasmid pET-28a-ho1-pcyA. According to their studies, genes ho1 and pcyA that cloned in the pET28a expression system are both controlled by a T7 promoter. In addition, the plasmid is transformed into E. coli BL21 (DE3) to cause overexpress. The bioengineer E. coli is cultured at 37℃ in the LB medium with 50 mg/L kanamycin until OD600 nm=0.8–1.0. IPTG is added into fermentation liquid until the concentration with 0.1 mmol/L and the expression is induced in 20 h at 27℃. Cells are obtained by centrifugation of 13 000× g for 10 min at 4℃.
2.2 Quantitative analysis of PCB productionCells are collected in a 100-mL fermentation broth via above-mentioned centrifugation. The cells are then heated in 5-mL methanol at 50℃ for 1 to 2 h in a water bath until the cell pellets turn white, centrifuged again at 13 000×g for 10 min, and the supernatant is taken. The supernatant is added with appropriate amount of concentrated hydrochloric acid (VCH3OH:VHCl=95:5) and the absorption spectrum is measured with UV-Vis spectrophotometer at 0.5 wavelength intervals (Bennett and Bogorad, 1973; Ge et al., 2018). The molecular of PCB diacids is weighted as 586 and 588 Da (Beuhler et al., 1976). The production of PCB is determined from the A680 value with the following equation (Glazer and Fang, 1973):
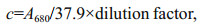 (1)
(1)in which c is the molarity of PCB solution in unit of mmol/L; A680 is the absorbance of PCB solution at 680 nm; and the dilution factor can be determined by the dilution ratio of the original PCB solution.
2.3 Single-factor optimization of expression conditionAn amount of 1-mL strain stored in -80℃ refrigerator is taken for culture in 100-mL LB medium at 37℃ until OD600 nm=0.8–1.0 and shaken, which is then used as the seed liquid and inoculated in 100-mL TB medium at 1% inoculum. Every 100-mL culture medium contains 100-μL kanamycin.
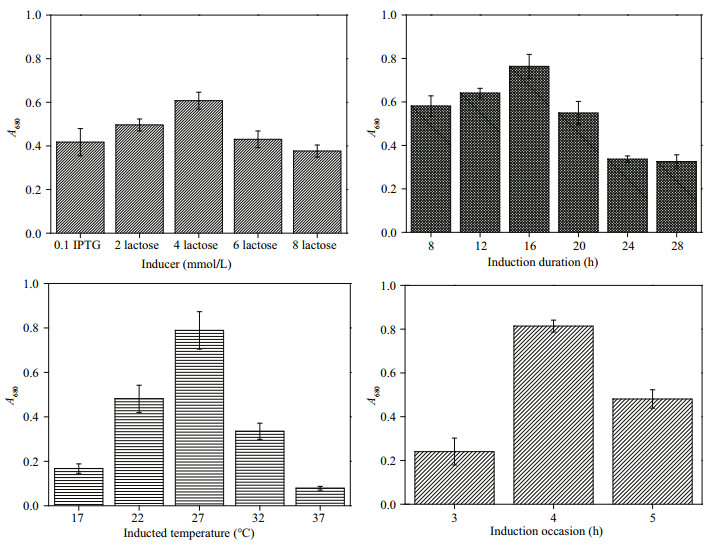
To optimize the culture conditions for PCB production with recombinant E. coli, different inducer concentration, induction duration, induction temperature, and induction time are tested. The cultures of TB medium are shaken at 170 r/min and 37℃ until OD600 nm reaches 0.6–0.8, cool down to 25℃, and be induced at 0.1 mmol/L IPTG and 2, 4, 6, or 8 mmol/L lactose for 20 h, individually. The corresponding inducer is added into the culture when OD600 nm of the fermentation broth reaches 1.0–1.2, and be induced at 27℃ for 8, 12, 16, 20, 24, and 28 h. And the optimal induction temperature and induction time are determined in a procedure similar to that of induction duration at the points of 17, 22, 27, 32, and 37℃, and 3 h (OD600=0.6–0.8), 4 h (OD600=1.0–1.2), and 5 h (OD600=more than 1.5) after inoculation.
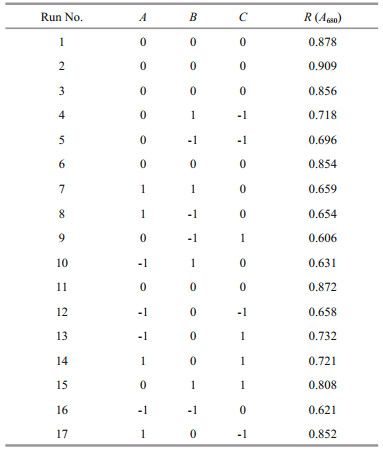
2.4 Response surface optimizationA model of 33 RSM is designed to optimize the A680 of PCB in the experimental range. The induction time (A), temperature (B), and duration (C) are chosen as three independent input variables, and the A680 of PCB (R) is the output variable (Tables 1 and 2). Seventeen experiments were conducted with 5 points at the center and the impact of random errors on the experimental results is accessed.
The expression of the strain is induced under the optimized conditions, i.e., induction time 4.60 h, 24.69℃, and in 13.57 h. After 1-L fermentation broth is centrifuged at 13 000×g for 10 min, bacteria are suspended in 100-mL methanol to form a blue methanol solution. The supernatant is concentrated to 10-mL using a rotary evaporator and filtered through 0.22-μm membrane. Multiple extractions are carried out in 10-mL chloroform and 20-mL water until the color of chloroform layer turned into any colors but blue. The chloroform layer is washed several times in 20-mL water until the color of water layer stays and then dried in N2. In all programs, PCB is covered with aluminum foil to protect it from light.
2.6 Absorbance and fluorescence spectrometryAbsorbance spectra are measured using a computer-controlled spectrophotometer (Agilent Cary 60 UV-Vis, Agilent, USA). The fluorescence emission spectra are measured by Cary Eclipse Fluorescence Spectrophotometer (excitation wavelength: 370 nm; emission wavelength range: 380–700 nm; emission slit width: 10 nm; and excitation wavelength: 10 nm).
2.7 High pressure liquid chromatography (HPLC) analysisHPLC analysis is performed using an UltiMate 3000 (Thermo Scientific, USA). The reversed phase C18 Eclipse (5 μm, 250 mm×4.6 mm) column was used. HPLC chromatogram is recorded at 380 nm. The mobile phase consisted of buffer A (deionized H2O containing 0.1% formic acid) and buffer B (acetonitrile containing 0.1% formic acid). The following gradient is used: 40% B at 0 min and 55% B at 30 min. The column temperature is 25℃ and the flowrate is 0.8 mL/min.
2.8 Liquid chromatography - mass spectrometry (LC-MS) analysisFurther analysis of the results of the liquid phase is performed via LC-MS. The same column and solvent gradient as in HPLC analysis (see Section 2.7) were used. LC-MS analysis was performed on a LCQ FLEET (Thermo Scientific, USA) device.
2.9 Ability of DPPH (2, 2-diphenyl-1-picrylhydrazyl) free-radical scavengingThe method of scavenging free radicals with DPPH was proposed by Blois (1958) and since then it has been widely used to determine the antioxidant ability of biological samples and foods. The method takes advantage that DPPH free radical has a single electron and a strong absorption at 517 nm; and its alcohol solution is purple. When a free radical scavenger is present, its absorption gradually disappears due to its single electron pairing, and its degree of fading is related quantitatively to the number of electrons it receives. Therefore, a rapid quantitative analysis can be performed with a microplate reader. The radical scavenging activity is determined using DPPH method, i.e., 100-μL test sample is mixed with DPPH at a different density. After being vigorously mixed and incubated for 30 min, A517 is measured using microplate reader, and the scavenging activity is calculated using Eq.1 above-mentioned.
To calculate DPPH scavenging activity, 0.1-mmol/L DPPH solution in absolute ethanol is prepared and preserved in the dark, and Eq.2 is used:
 (2)
(2)in which A is the absorbance of 100-μL DPPH plus 100-μL sample at 517 nm; A0 is the absorbance of 100-μL DPPH plus 100-μL alcohol and B is the absorbance of 100-μL sample plus 100-μL alcohol.
3 RESULT 3.1 Characterization analysis of recombinant PCBThe fermentation broth was centrifuged after induction of expression, from which bacterial sludge was obtained (Fig. 1a). The blue slime was treated with acidic methanol, from which a blue methanol solution formed and its absorption spectrum is shown in Fig. 1b. The absorption spectrum shows two absorption peaks at about 680 nm and 380 nm. Comparing previous studies in the color and spectrum of the solution (O'Carra and Heocha, 1980; Ge et al., 2013), we could confirm that the recombinant strain was successfully constructed and the expression product was PCB. The expression level of PCB can be characterized by measuring the A680.

|
| Fig.1 The fermentation liquid (a) and the absorption spectrum (b) in test tubes The green one is centrifuged cells with the inducer added, while the brown one without. |
To improve the production of PCB, the culture conditions are optimized. Due to the high background absorbance of cell lysate at 680 nm, the expression conditions are scrutinized using absorbance at 680 nm. As shown in Fig. 2, A680 reached a maximum when induced by 4-mmol/L lactose after 4 h of inoculation at 27℃ for 16 h.
|
| Fig.2 Optimization of culture conditions for high-level expression of recombinant PCB in E. coli |
For a single-factor optimization involving inducer, induction duration, induction temperature, and induction time, we conducted 17 experiments in different variable combinations according to the central composite design. Equation 3 is the result of regression that fits the empirical evidence of A680 (R):
 (3)
(3)in which R is A680; A is the induction time (h); B is the temperature (℃); and C is the duration (h).
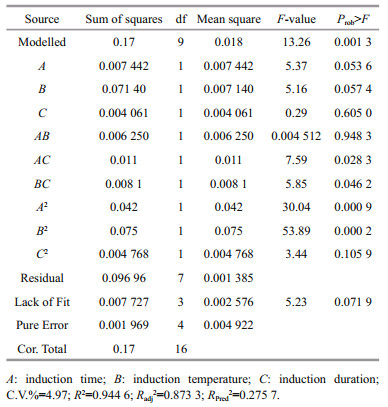
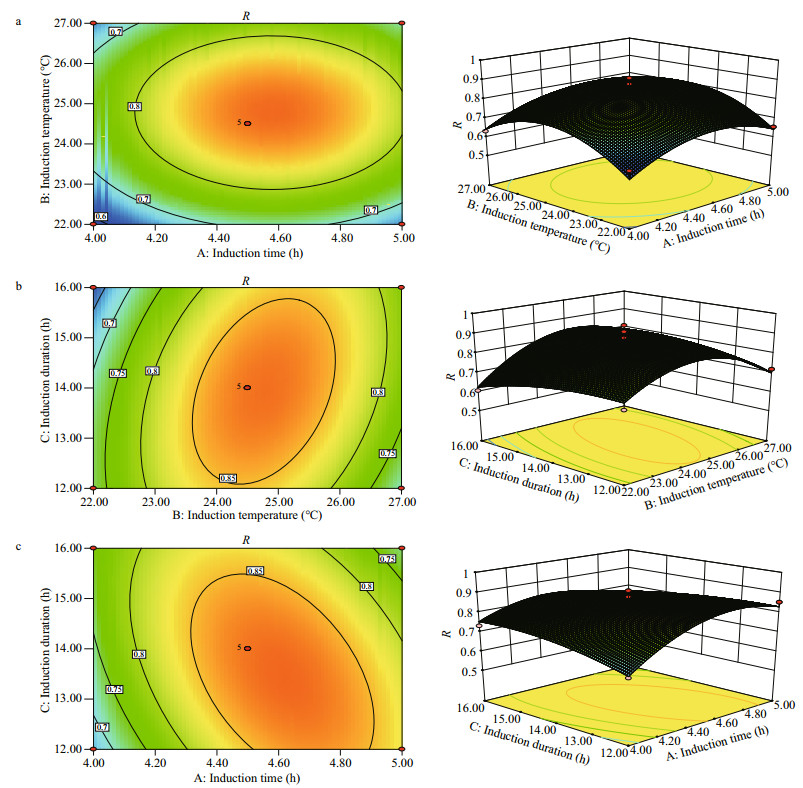
The predicted results and the regression model in ANOVA by Eq.3 are shown in Table 3. The value of ''Prob > F" below 0.01 suggests that the model term is extremely significant, and above 0.05 indicates that the regression equation has a good fit. CV%=4.97 indicates that the operation can be trusted. The corrected Radj2 of the model is 0.873 3, which explains 87.33% of the response variability. Figure 3 is the three-dimensional contour plots for the optimization of formulation. As shown in Fig. 3a, R increases first and then decreases as the value of A or B increases. However, the curves of A and R are similar to the steepness of the curves of B to R. This indicates that the interaction between A and B has a little effect on R. Figure 3b shows that the interaction between B and C is greater, and the effect of B on R is greater than the effect of C on R. Figure 3c shows that the effect of C on R is less than A. As shown in Table 3, the order of influence of each factor on the response is A > B > C. Moreover, there is interaction among the factors. P values of the interaction terms AC and BC are below 0.05, and the impact is significant. In addition, P values of the secondary terms A2 and B2 are less than 0.01. The effect is extremely significant, while the other items are not significant. The significance of the interaction term and the quadratic term further indicates that the influence of each influence factor on the response value is not in a simple linear relationship and cannot be optimized by single-factor experiment alone.
|
| Fig.3 The three-dimensional contour plots of RSM for the maximum R The plots are generated using the data shown in Table 3. The inputs are the 17 experimental runs carried out under the conditions established in the RSM design. a. A vs. B on absorbance of 680 nm; b. B vs. C on absorbance of 680 nm; c. A vs. C on absorbance of 680 nm. |
Therefore, we applied the RSM method to reduce the workload and determine the optimal conditions of multiple factors, i.e., the induction time, induction temperature, and induction duration in our case. The predicted optimum conditions are: induction time at 4.60 h, the temperature at 24.69℃, and the duration of 13.57 h, under which the predicted maximum A680 is 0.879. The predicted optimum conditions have been verified in a trial run, reaching excellent agreement with the predicted value of the A680 for 0.862 with a relative error of 2%.
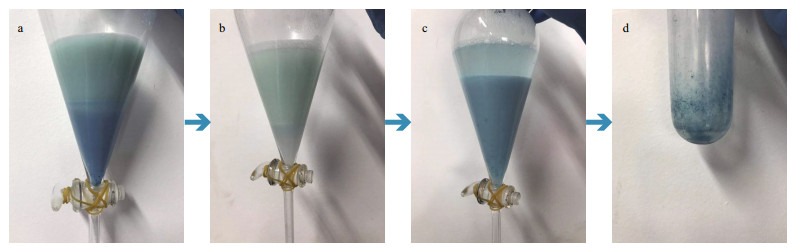
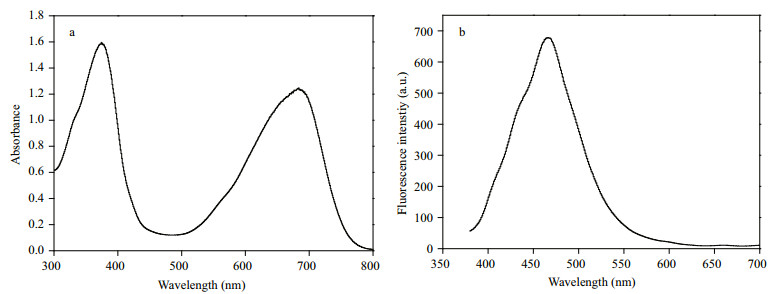
3.3 PCB extraction and spectral analysisThe PCB extracted in hot methanol contains many impurities. To purify the PCB, we use chloroform to extract the PCB and remove its water-soluble impurities and other substances. On the other hand, as chloroform is highly volatile, a blue PCB powder can be obtained by nitrogen-blowing. The procedure of the PCB powder extraction is shown in Fig. 4. An amount of 1-L fermentation broth can produce about 10.6 mg of blue PCB powder. The blue powder was dissolved in acidic methanol, and its absorption spectrum and fluorescence emission spectrum were analyzed (Fig. 5). The absorption spectrum of PCB has two absorption peaks at 380 and 680 nm (Fig. 5a), which is consistent with the report by O'Carra et al. (1980) and Ge et al. (2013). When excited at 370 nm, the fluorescence emission spectrum shows a strong emission at about 470 nm (Fig. 5b).
|
| Fig.4 The procedure of the PCB powder extraction a. extract PCB from the upper methanol layer to the lower chloroform layer; b. keep doing the extraction until the color of the lower chloroform layer turns no longer blue; c. keep washing the chloroform layer until the color of the upper water layer stays; d. nitrogen-blow the chloroform layer to obtain solid-state PCB. |
|
| Fig.5 The absorption spectrum (a) and fluorescence emission spectrum (b) of the PCB powder The UV-Vis spectra are recorded using standard equipment. Fluorescence emission spectra are acquired from 380 to 700 nm with excitation at 370 nm. |
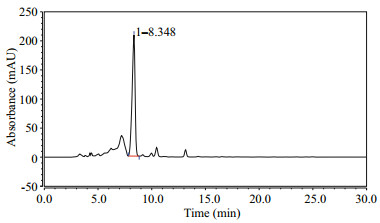
The chromatogram of the extracted PCB is shown in Fig. 6. The main peak found in all cases has retention time of ~8.348 min. Upon LC-MS analyses, this respective peak gives a signal at m/z=587 (positive ionization). The molecular weight of the PCB is 586– 588 Da, thus the product we obtained was PCB.
|
| Fig.6 Analytical HPLC chromatograms of the purified recombinant PCB |
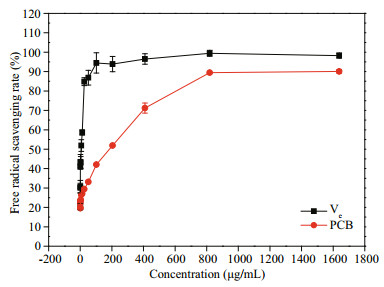
Vitamin E (Ve) is a fat-soluble vitamin with a strong antioxidant effect. As shown in Fig. 7, the recombinant PCB has a good scavenging effect on DPPH free radicals. As the concentration increases, the ability of the recombinant PCB to scavenge DPPH free radicals gradually increases. When the concentration of recombinant PCB was 0.1–819.2 μg/mL, the free radical scavenging rate was 19.8%–89.5%. However, when the concentration of the recombinant PCB is greater than 819.2 μg/mL, the DPPH free radical scavenging rate will remain at 90%.
|
| Fig.7 Comparison in anti-oxidant activity between Ve and recombinant PCB As the concentration increases, the ability of the recombinant PCB to scavenge DPPH free radicals gradually increases. When the concentration of the recombinant PCB is greater than 819.2 μg/mL, the DPPH free radical scavenging rate will remain at 90%. |
It is known that C-phycocyanin has antioxidant and anti-inflammatory properties (Romay et al., 1998, 2003). As the only chromophore of C-PC, PCB plays an important role in antioxidant activities (Benedetti et al., 2010). Orally administered PCB may have considerable potential for preventing or slowing down the progression of a range of neurodegenerative disorders which may be related to neuronal NADPH oxidase (McCarty et al., 2010). In addition, as the demand for natural foods is continuously growing, food and beverage industries turned to use natural pigments instead of synthetic colorants. This trend has been increasing after the publication of a study linking synthetic pigments to the changes in childhood behavior (McCann et al., 2007). As a natural pigment with antioxidant activity, the addition of PCB to food and cosmetics may be of concern.
The PCB chromophore can be cleaved from the phycocyanin apoprotein by hot methanol for a long time (Carra and Heocha, 1966; Beuhler et al., 1976). This is the key technology for extracting PCB from cyanobacteria. Because Spirulina is rich in phycocyanin, it can be used as a good raw material for extracting PCB (Marín-Prida et al., 2013). However, this long-term pyrolysis of phycocyanin consumes a great amount of methanol and energy. Therefore, a good genetic bio-engineering technology should be developed, with which single and pure product of PCB can be extracted via fermentation of recombinate E. coli, unlike complicated procures of extracting phycocyanin from Spirulina, thus saving cost in the cleavage of phycocyanin. In addition, there are some other advantages to use recombinant PCB with E. coli. Since PCBs have been proved to have antioxidant effects reported in our research and other studies (Benedetti et al., 2010; Sotiroudis and Sotiroudis, 2013). And PCB can also effectively inhibit the proliferation of inflammatory cells and the production of inflammatory factors (Basdeo et al., 2016). Therefore, supplementation of PCB is important for human health. E. coli is a popular bacterium in humans and animals, and generally has no pathogenicity to the human body (Qaiser et al., 2018). Importantly, recombinant E. coli cells expressing recombinant PCB may be propagated in the intestine, and the expressed PCB may improve the intestinal environment.
In order to obtain recombinant PCB, the method of combinant fermentation needs to be established. In a previous trial by Ge et al. (2013), the recombinant PCB's production condition was optimized in a single-factor scheme, while the interaction among factors was ignored, so the yield of restructured PCB was low. In fact, there are many factors affecting the production of PCB via recombinant E. coli fermentation, and the interaction among multiple factors can affect the PCB production.
Using RSM optimization method, we combine several influencing factors and improved the yield of recombinant PCB. Our results indicate that the interaction among several influential factors on the yield of recombinant PCB is significant. Ge et al. (2018) explored the biosynthetic pathway of PCB and found a feedback inhibition process that leads to low expression of recombinant PCB in E. coli. However, if the interaction of external factors affecting the production of recombinant PCB fermentation is fully considered, even if there is feedback inhibition, the production of recombinant PCB may be increased greatly to a relatively high level. Of course, solving the feedback suppression effect on the PCB synthesis process is a critical issue for realizing large-scale production of reorganized PCB, and may become a hot spot for PCB research.
5 CONCLUSIONIn order to improve the recombinant PCB production, fermentation optimization method is established and implemented. The optimum culture conditions determined in the response surface methodology are: induction time 4.60 h, temperature 24.69℃, and induction duration 13.57 h, and the predicted maximum A680 at 0.879. Under the conditions, the total PCB production reached about 13 mg/L. Additionally, recombinant PCB could be purified using methanol reflux followed by chloroform extraction. Finally, the purified recombinant PCB exhibited a good antioxidative activity and a promising potential for industrial application.
6 DATA AVAILABILITY STATEMENTThe data supporting the findings of this study are shown in this article. Otherwise, please contact the author for more information.
Adir N. 2005. Elucidation of the molecular structures of components of the phycobilisome: reconstructing a giant. Photosynthesis Research, 85(1): 15-32.
|
Alvey R M, Biswas A, Schluchter W M, Bryant D A. 2011. Attachment of noncognate chromophores to CpcA of Synechocystis sp. PCC 6803 and Synechococcus sp. PCC 7002 by heterologous expression in Escherichia coli. Biochemistry, 50(22): 4890-4902.
|
Basdeo S A, Campbell N K, Sullivan L M, Flood B, Creagh E M, Mantle T J, Fletcher J M, Dunne A. 2016. Suppression of human alloreactive T cells by linear tetrapyrroles; relevance for transplantation. Translational Research, 178: 81-94.
DOI:10.1016/j.trsl.2016.07.011 |
Benedetti S, Benvenuti F, Scoglio S, Canestrari F. 2010. Oxygen radical absorbance capacity of phycocyanin and phycocyanobilin from the food supplement Aphanizomenon flos-aquae. Journal of Medicinal Food, 13(1): 223-227.
DOI:10.1089/jmf.2008.0257 |
Bennett A, Bogorad L. 1973. Complementary chromatic adaptation in a filamentous blue-green alga. Journal of Cell Biology, 58(2): 419-435.
DOI:10.1083/jcb.58.2.419 |
Beuhler R J, Pierce R C, Friedman L, Siegelman H W. 1976. Cleavage of phycocyanobilin from C-phycocyanin. Separation and mass spectral identification of the products. Journal of Biological Chemistry, 251(8): 2405-2411.
|
Blois M S. 1958. Antioxidant determinations by the use of a stable free radical. Nature, 181(4617): 1199-1200.
DOI:10.1038/1811199a0 |
Carra P ó, Heocha C ó. 1966. Bilins released from algae and biliproteins by methanolic extraction. Phytochemistry, 5(5): 993-997.
DOI:10.1016/S0031-9422(00)82796-X |
De Montellano P R O. 2000. The mechanism of heme oxygenase. Current Opinion in Chemical Biology, 4(2): 221-227.
DOI:10.1016/S1367-5931(99)00079-4 |
Frankenberg N, Lagarias J C. 2003. Phycocyanobilin: ferredoxin oxidoreductase of Anabaena sp. PCC 7120 biochemical and spectroscopic characterization. Journal of Biological Chemistry, 278(11): 9219-9226.
|
Gambetta G A, Lagarias J C. 2001. Genetic engineering of phytochrome biosynthesis in bacteria. Proceedings of the National Academy of Sciences of the United States of America, 98(19): 10566-10571.
DOI:10.1073/pnas.191375198 |
Ge B S, Chen Y, Yu Q, Lin X J, Li J Q, Qin S. 2018. Regulation of the heme biosynthetic pathway for combinational biosynthesis of phycocyanobilin in Escherichia coli. Process Biochemistry, 71: 23-30.
DOI:10.1016/j.procbio.2018.05.011 |
Ge B S, Li Y, Sun H X, Zhang S, Hu P J, Qin S, Huang F. 2013. Combinational biosynthesis of phycocyanobilin using genetically-engineered Escherichia coli. Biotechnology Letters, 35(5): 689-693.
DOI:10.1007/s10529-012-1132-z |
Glazer A N, Fang S. 1973. Chromophore content of blue-green algal phycobiliproteins. Journal of Biological Chemistry, 248(2): 659-662.
|
Jobe A, Bourgeois S. 1972. Lac repressor-operator interaction: VI. The natural inducer of the lac operon. Journal of Molecular Biology, 69(3): 397-408.
|
Lee K M, Gilmore D F. 2006. Statistical experimental design for bioprocess modeling and optimization analysis. Applied Biochemistry and Biotechnology, 135(2): 101-115.
|
Müller-Hill B, Rickenberg H V, Wallenfels K. 1964. Specificity of the induction of the enzymes of the lac operon in Escherichia coli. Journal of Molecular Biology, 10(2): 303-318.
DOI:10.1016/S0022-2836(64)80049-8 |
MacColl R. 1998. Cyanobacterial phycobilisomes. Journal of Structural Biology, 124(2-3): 311-334.
DOI:10.1006/jsbi.1998.4062 |
Marín-Prida J, Pavón-Fuentes N, Llópiz-Arzuaga A, Fernández-Massó J R, Delgado-Roche L, Mendoza-Marí Y, Santana S P, Cruz-Ramírez A, Valenzuela-Silva C, Nazábal-Gálvez M, Cintado-Benítez A, Pardo-Andreu G L, Polentarutti N, Riva F, Pentón-Arias E, Pentón-Rol G. 2013. Phycocyanobilin promotes PC12 cell survival and modulates immune and inflammatory genes and oxidative stress markers in acute cerebral hypoperfusion in rats. Toxicology and Applied Pharmacology, 272(1): 49-60.
DOI:10.1016/j.taap.2013.05.021 |
McCann D, Barrett A, Cooper A, Crumpler D, Dalen L, Grimshaw K, Kitchin E, Lok K, Porteous L, Prince E, Sonuga-Barke E, Warner J O, Stevenson J. 2007. Food additives and hyperactive behaviour in 3-year-old and 8/9-year-old children in the community: a randomised, double-blinded, placebo-controlled trial. The Lancet, 370(9598): 1560-1567.
DOI:10.1016/S0140-6736(07)61306-3 |
Mccarty M F, Barroso-Aranda J, Contreras F. 2010a. NAPDH oxidase mediates glucolipotoxicity-induced beta cell dysfunction-clinical implications. Medical Hypotheses, 74(3): 596-600.
DOI:10.1016/j.mehy.2008.09.062 |
McCarty M F, Barroso-Aranda J, Contreras F. 2010b. Oral phycocyanobilin may diminish the pathogenicity of activated brain microglia in neurodegenerative disorders. Medical Hypotheses, 74(3): 601-605.
DOI:10.1016/j.mehy.2008.09.061 |
O'Carra P, Murphy R F, Killilea S D. 1980. The native forms of the phycobilin chromophores of algal biliproteins. A clarification. Biochemical Journal, 187(2): 303-309.
|
Qaiser H, Aslam F, Iftikhar S, Farooq A. 2018. Construction and recombinant expression of Pseudomonas aeruginosa truncated exotoxin A in Escherichia coli. Cellular and Molecular Biology, 64(1): 64-69.
DOI:10.14715/cmb/2018.64.1.12 |
Romay C, Armesto J, Remirez D, González R, Ledon N, García N L. 1998. Antioxidant and anti-inflammatory properties of C-phycocyanin from blue-green algae. Inflammation Research, 47(1): 36-41.
DOI:10.1007/s000110050256 |
Romay C, González R, Ledón N, Remirez D, Rimbau V. 2003. C-phycocyanin: a biliprotein with antioxidant, anti-inflammatory and neuroprotective effects. Current Protein & Peptide Science, 4(3): 207-216.
|
Sotiroudis T G, Sotiroudis G T. 2013. Health aspects of Spirulina (Arthrospira) microalga food supplement. Journal of the Serbian Chemical Society, 78(3): 395-405.
DOI:10.2298/JSC121020152S |
Willows R D, Mayer S M, Foulk M S, DeLong A, Hanson K, Chory J, Beale S I. 2000. Phytobilin biosynthesis: the Synechocystis sp. PCC 6803 heme oxygenase-encoding ho1 gene complements a phytochrome-deficient Arabidopsis thaliana hy1 mutant. Plant Molecular Biology, 43(1): 113-120.
|
 2020, Vol. 38
2020, Vol. 38