Institute of Oceanology, Chinese Academy of Sciences
Article Information
- LI Hao, MENG Fanping, WANG Yuejie, LIN Yufei
- Removal of phenol by Isochrysis galbana in seawater under varying temperature and light intensity
- Journal of Oceanology and Limnology, 38(3): 773-782
- http://dx.doi.org/10.1007/s00343-019-9125-6
Article History
- Received May. 6, 2019
- accepted in principle Jul. 22, 2019
- accepted for publication Sep. 5, 2019
2 College of Environmental Science and Engineering, Ocean University of China, Qingdao 266100, China;
3 National Marine Hazard Mitigation Service, Ministry of Natural Resources of the People's Republic of China, Beijing 100194, China
Phenol is an important chemical raw material, and it is widely used in chemical, pharmaceutical, petroleum, leather, and other industries. It has an annual output of approximately 7 million tons (Senthilvelan et al., 2014). Phenol is one of the most common hazardous noxious substances (HNS) involved in accidental marine spills due to its high market demand and transportation volume (Cunha et al., 2015; Duan et al., 2017). For example, a total of 400 tonnes of phenol leaked into the seawater in the Port of Gothenburg, Sweden in 1973, because a cistern into which the phenol was loaded was suddenly ruptured (HELCOM, 2002). Phenol is soluble in water (Massalha et al., 2010), and it is highly toxic to aquatic organisms (Calabrese and Kenyon, 1991). Phenol is currently listed as a priority pollutant by the US Environmental Protection Agency (USEPA) (Du et al., 2009). French McCay et al. (2006) used prediction models to show that phenol is one of the most dangerous chemicals for aquatic organisms used in bulk transport.
Conventional physical and chemical environmental restoration methods are not effective after the leakage of dissolved HNS in large-area waters, such as an ocean. The only thing that can be done for an HNS leakage is to take measures to speed up the natural dilution process. Microalgae are a primary producer in marine ecological environments, and they are an important part of the marine ecosystem. Microalgae can not only maintain autotrophic growth using photosynthesis but also utilize organic matter from the environment as its carbon source for heterotrophic growth (Perez-Garcia et al., 2011). Many marine microalgae can biodegrade specific pollutants, especially for phenols (Yang et al., 2002; Lima et al., 2004; Gao et al., 2011; Gao and Chi, 2015; Wang et al., 2019). Thus, microalgae can be used to remove organic contaminants from seawater due to its significant advantages, including being driven by solar energy, environmental friendliness, its role in the fixation and turnover of carbon, cheap food for animals (Wijffels et al., 2013; Matamoros et al., 2015; Xiong et al., 2017). However, the environmental conditions of oceans are complicated and diverse, and changing marine environmental conditions have a great impact on the removal of organic pollutants by microorganisms (Caracciolo et al., 2015). Temperature and light intensity are two basic factors that will affect biodegradation by microorganisms to a large extent (Bradley and Writer, 2014; Zhou et al., 2015; Surkatti and El-Naas, 2018).
Isochrysis galbana (a common feed microalga used widely in aquaculture) has been screened by Wang et al. (2019) as an excellent phenol-degrader. In this study, the removal of phenol by I. galbana is studied under different temperatures and light intensities in seawater. The kinetics is also studied to discuss their influence on the removal of phenol by temperature and light.
2 MATERIAL AND METHOD 2.1 Chemicals, microalga source, and culture mediumPhenol was obtained from the SinoPharm Chemical Reagent Co., Ltd. (Shanghai, China). All of the other solvents and reagents were analytical grade. Natural seawater was used in this study, which was taken from the coastal waters of Qingdao, China. Seawater was filtered using a 0.45-μm microporous membrane, and then sterilized under 120℃ for 20 min.
Golden alga (Isochrysis galbana MACC/H59) was obtained from the algal culture collection at the Ocean University of China (OUC). The pure-cultured microalga was cultivated in a sterilized F/2 medium (Guillard, 1975) with the following composition (/L): NaNO3, 75 mg; Na2HPO4·H2O, 5 mg; EDTA·Na2, 4.36 mg; a solution of trace elements (ZnSO4·4H2O, 0.023 g/L; CoCl2·6H2O, 0.012 g/L; FeCl3·6H2O, 3.2 g/L; MnCl2·4H2O, 0.178 g/L; CuSO4·5H2O, 0.010 g/L; Na2MoO4·2H2O, 0.006 g/L), 1 mL; and a solution of vitamins (Vitamin B12, 0.000 5 g/L; Vitamin B1, 0.100 g/L; biotin, 0.000 5 g/L) 1 mL. The experiments and sampling processes were carried out in a superclean bench, and the flasks were sealed with ventilated membranes (pore-size is 0.45 μm) to ensure air entry and axenic environments. The microalga was cultivated to the exponential phase for next-step inoculation operation.
2.2 The effect of temperature on the growth of microalga and the biodegradation of phenolThe experiment was conducted in light incubators. The initial inoculation density of each microalga cell was 5×105 cells/mL. Each 100-mL culture was cultivated in a 250-mL flask. There were five temperature groups, and each group contained 0 (control), 50, and 100 mg/L of phenol (50 mg/L of phenol is higher than LC50 values of most marine fishes and crustaceans (Duan et al., 2018), so phenol above 50 mg/L can be considered as a serious hazard to the marine environment). There are three repetitions in each temperature. First, all of the groups were preincubated in an incubator for 24 h at a temperature of 20℃, 60 μmol/(m2·s) of light intensity, and a 14-h light/10-h dark cycle. Then the five groups were incubated at temperatures of 10℃, 15℃, 20℃, 25℃, and 30℃. The other factors at this stage were the same as during pre-incubation. The flasks were shaken with a speed of 150 r/min. The density of the microalga and concentration of phenol were measured every 2 h.
2.3 The effect of light intensity on the growth of microalga and biodegradation of phenolThere were five groups established to investigate the effects of light intensity. Each group contained 0 (control), 50, and 100 mg/L of phenol. The inoculum and pre-incubation was the same as before. Afterward, the five groups were incubated in 0 (dark), 60, 120, 180, and 240 μmol/(m2·s) of light intensity, and there are three repetitions in each illumination. In addition, one group without inoculum (containing 50 and 100 mg/L of phenol) was set as a control of photolysis in each illumination. Other factors were the same as during pre-incubation. The density of the microalga and the concentration of phenol were measured every 2 h.
2.4 The effect of kinetics on the biodegradation of phenol by I. galbanaThe exponential rate model is widely applied to the removal process of organics as a result of its good applicability and fitting effect (Liu et al., 2014). There are zero-order model, first-order model and pseudo first-order model widely used for modeling the degradation of organic contaminants (Saravanan et al., 2009; Wan et al., 2010; Silambarasan and Abraham, 2013). The zero-order model and pseudo first-order model were used to describe the degradation kinetics of phenol removal in this study (Saravanan et al., 2009; Wang et al., 2015), as the following Eqs.1 & 2:
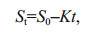 (1)
(1)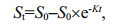 (2)
(2)where S0 is the initial concentration of phenol (mg/L); and St is the concentration of phenol (mg/L) at time t. In order to estimate kinetic parameters for phenol removal, the Eqs.1 & 2 were solved using the linear and nonlinear regression analysis, respectively. The attenuation concentration of phenol was determined by Eq.3:
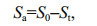 (3)
(3)where Sa is the attenuated concentration of phenol (mg/L). In zero-order kinetics, t is the half-life when St/S0=1/2. In first-order kinetics, the half-life (t1/2) of phenol removal can be measured using Eq.4 (Sinkkonen and Paasivirta, 2000):
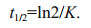 (4)
(4)Cell densities of microalga were measured using a haemocytometer.
The concentrations of phenol were analyzed using the 4-aminoantipyrine spectrophotometric method at 510 nm (Emerson, 1943). Before measuring the phenol concentrations, a 2-mL sample was withdrawn from each flask and centrifuged at 4 400×g for 5 min and then filtered through a 0.45-μm filter. The supernatants were used to monitor the residual phenol concentrations.
2.6 Statistical analysisThe means and standard deviations of all the results were obtained from three independent replicates. Statistical analysis was performed using a one-way analysis of variance (ANOVA), and a probability P < /italic> < 0.05 was considered as statistically significant.
3 RESULT 3.1 Removal of phenol by I. galbana under different temperatures 3.1.1 The effect of temperature on I. galbana growth and the removal of phenolThe attenuation curve of phenol by I. galbana is shown in Fig. 1, and the corresponding curve of cell growth is shown in Fig. 2. The most suitable temperature for the growth of I. galbana among the five temperatures was 25℃, and the removal efficiency of phenol was the highest at the same temperature. The growth of the microalga was inhibited with a decrease in temperature, and the removal efficiency of the substrate decreased accordingly. The high temperature (30℃) also slowed down the growth. The microalga nearly completely removed 100 mg/L of phenol in 24 h at 25℃ and 20℃. The removal of phenol by I. galbana was inhibited at lower temperatures, such as 10℃.
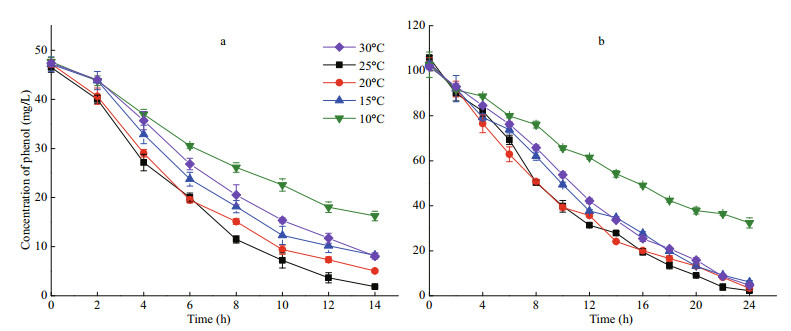
|
| Fig.1 Removal of 50 mg/L (a) and 100 mg/L (b) of phenol using I. galbana under different temperatures |
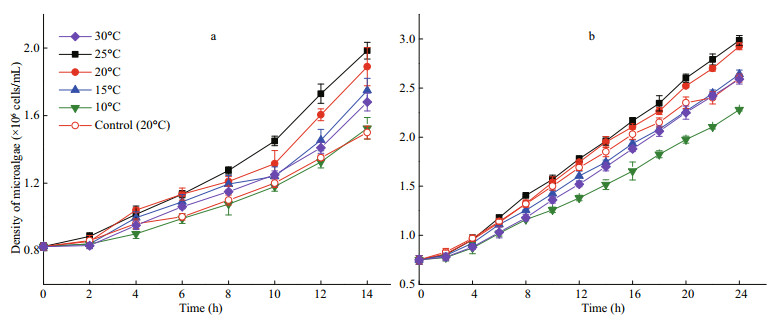
|
| Fig.2 Growth curves of I. galbana exposed to 50 mg/L (a) and 100 mg/L (b) of phenol under different temperatures |
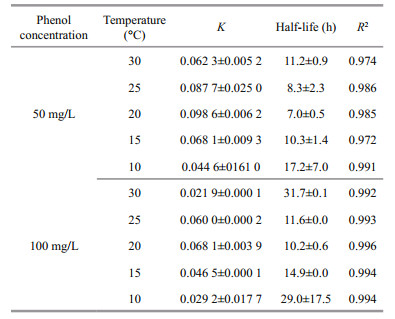
The exponential rate model was used to characterize the attenuation process of phenol by I. galbana in this study. The aim was to compare the degradation effect under diverse temperatures using a constant degradation rate. Hence, zero-order and first-order kinetic models were applied to fit the degradation process of microalga at different temperatures, and the best-fit exponential rate model was chosen to describe the removal process of phenol. The fitting curves of the kinetics are shown in Fig. 3, and the corresponding parameters are detailed in Table 1. The degradation kinetics of I. galbana in two concentrations of phenol both fit to the first-order model, and all of the R2 values were higher than 0.972.
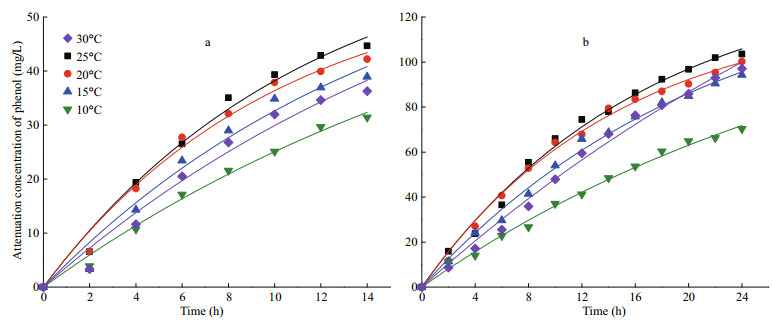
|
| Fig.3 Pseudo first-order profiles for the biodegradation of phenol with 50 mg/L (a) and 100 mg/L (b) using I. galbana under different temperatures |
|
A comparison with the K values under different temperatures (Table 1) showed that the optimal temperatures of phenol removal for 50 and 100 mg/L of phenol were both 20℃, and the values of K were 0.098 6 and 0.068 1/h, respectively. In addition, the half-lives of phenol were 7.0 and 10.2 h. Although the results were consistent with those shown in Fig. 1, the removal efficiency of phenol at 20℃ was close to 25℃ (Fig. 1).
3.2 Removal of phenol by I. galbana under different light intensities 3.2.1 The effect of light intensity on I. galbana growth and the degradation of phenolThe attenuation curves of phenol and growth curves of microalga under different light intensities are shown in Fig. 4. The curves of microalga in complete darkness are shown in Fig. 5. According to the growth curves, 180 μmol/(m2·s) was most suitable for the growth of I. galbana among the four light intensities. The growth of microalga was inhibited at a low illumination condition. However, the removal trend of phenol by microalga was different from the growth change at the various illuminations. The degradation of phenol under 180 μmol/(m2·s) was similar to the result of 120 and 240 μmol/(m2·s), and their reduction curves were almost coincident. The 50 and 100 mg/L of phenol were nearly completely removed by I. galbana in 14 and 24 h, respectively, under the three higher light intensities. However, the degradation effect of phenol and the growth of microalga at 60 μmol/(m2·s) were less satisfactory in comparison to the other light intensities.
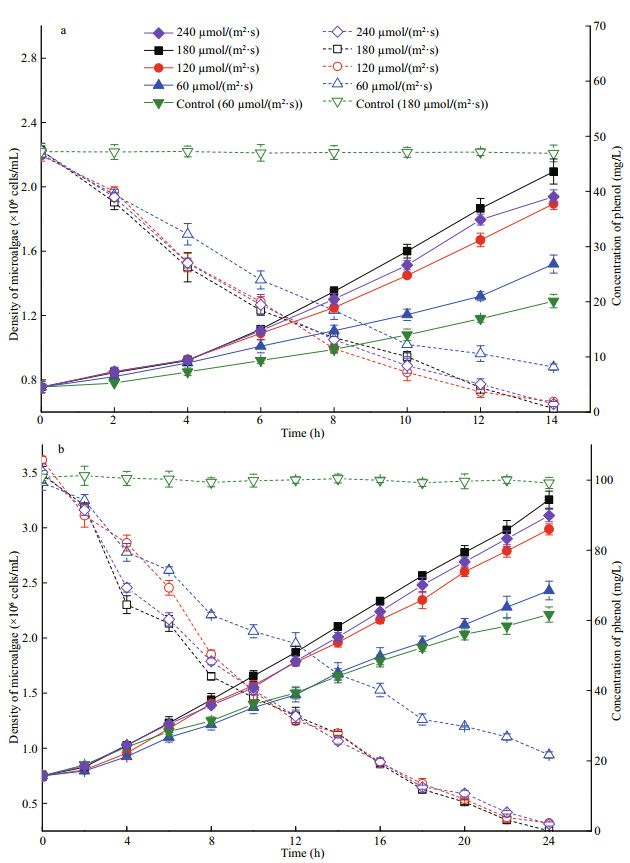
|
| Fig.4 Phenol removal and cell growth of I. galbana in 50 mg/L (a) and 100 mg/L (b) of phenol under different light intensities Solid points represent the cell densities, and empty points represent the phenol concentrations. Control (60 μmol/(m2·s)) represents the control without phenol, and control (180 μmol/(m2·s)) represents the control without microorganism. |
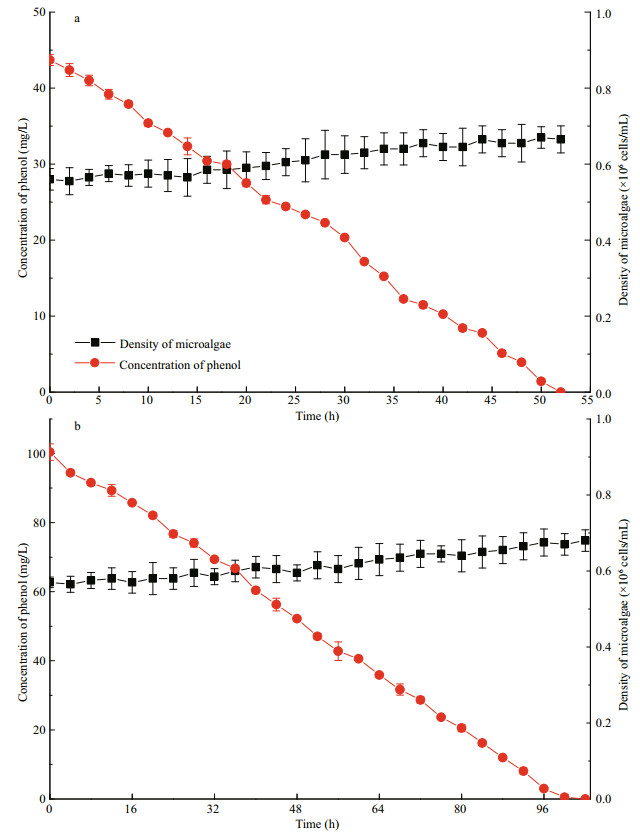
|
| Fig.5 Removal in 50 mg/L (a) and 100 mg/L (b) of phenol with I. galbana, and cell growth under dark conditions |
Phenol could be removed by microalga under dark conditions (Fig. 5), and light may be not an essential factor for the removal of phenol. However, 50 and 100 mg/L of phenol were completely removed in 52 and 102 h, respectively. Hence, the degradation time was much longer than the time under the light condition (14 and 24 h at 180 μmol/(m2·s)).
3.2.2 Kinetics of phenol removal by I. galbana under different light intensitiesThe kinetic fitting curves are shown in Fig. 6, and the corresponding parameters are detailed in Table 2. The kinetics in two concentrations of phenol both fit to the first-order model under different light intensities, and all of the coefficients of determination (R2) were higher than 0.986. The kinetics of phenol removal by I. galbana under dark conditions fit to the zero-order model.
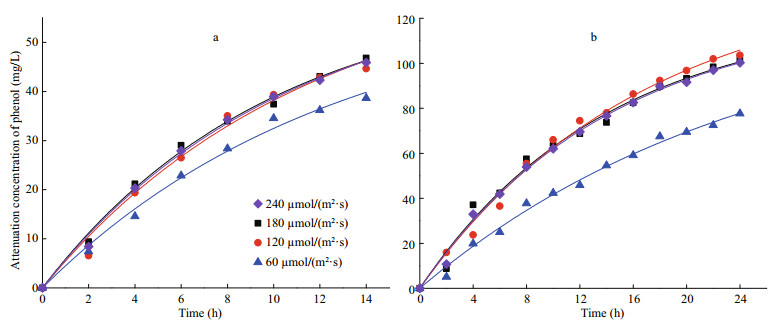
|
| Fig.6 Pseudo first-order profiles for the removal of phenol with 50 mg/L (a) and 100 mg/L (b) using I. galbana under different light intensities |

|
As shown in Table 2, the most suitable light intensity for the removal by I. galbana was 180 μmol/ (m2·s), the constants of the degradation rate (K) were 0.100 9 and 0.072 0/h at 50 and 100 mg/L of phenol, respectively, and half-lives were 6.9 and 9.6 h, respectively. The optimal light intensities for the microalga growth and removal of phenol were the same.
4 DISCUSSIONLow concentrations of organic pollutants may promote the growth of microorganisms. According to Figs. 2 & 4, 50 mg/L and 100 mg/L of phenol can promote the growth of I. galbana compare with control experiments. However, Wang et al. (2019) indicated a phenol concentration ≥125 mg/L inhibits the reproduction of I. galbana. Di Caprio et al. (2018) drew a similar result that phenols concentration higher than 100 mg/L have an inhibition effect on Scenedesmus sp.
The optimal temperatures for removal of phenol and growth of microalga are 25℃ and 20℃ respectively through experiments and kinetic model. Considering the removal effects of phenol at 25℃ and 20℃ were close (Fig. 1 and Table 1), the temperature range between 20℃ to 25℃ can be seen as appropriate temperatures for the removal of phenol by I. galbana. But the removal of phenol is inhibited by low temperatures, the reason may be that lower temperatures inhibited the activities of enzymes in the microbes so that their growth and metabolism of microorganisms were influenced (Siddiqui, 2015). Higher temperatures improve the activities of the enzymes related to photosynthesis, and relevant processes, such as the diffusion of CO2, were also boosted (Raven and Geider, 1988). In addition, higher temperatures may speed up the process of cellular metabolism to promote the growth of microalga. When the temperature continues to increase and exceed the optimum temperature, irreversible physiological reactions may happen in cells, such as a decrease in the photosynthetic rate and growth rates of microorganisms. Also, the alga may be susceptible to other environmental factors, such as irradiance and salinity (Wernberg et al., 2010; Andersen et al., 2013). So the growth of I. galbana and phenol removal became to be inhibited at 30℃. Thus, it can be seen that the removal process of phenol by I. galbana is affected by temperature of the marine environment.
The effect of temperature on the removal of phenol with microorganisms has been studied in many reports. Among them, studies of bacteria have been the most prevalent. Onysko et al. (2000) found that the optimum temperature for biodegradation of phenol by Pseudomonas putida was 25℃, and the kinetic parameters of degradation showed an increasing trend with an increase in temperature. Polymenakou and Stephanou (2005) studied the removal effect of phenol by a native Pseudomonas sp. between 10℃ and 40℃ and confirmed 30℃ was best for the degradation of phenol for Pseudomonas sp. Lu et al. (2009) studied the degradation of phenol by Phanerochaete chrysosporium at different temperatures, and the results showed that first-order kinetics were suitable for the biodegradation process, and the optimal temperature was 37℃ for the removal of phenol. In comparison with these temperatures, the most suitable temperature of I. galbana (20-25℃) is relatively low, and this temperature can be more easily achieved in actual marine environments.
Wang et al. (2017a) studied the natural attenuation of phenol under simulated marine conditions, the results indicated that photolysis was not the main pathway of phenol removal. And according to Fig. 4, the phenol concentration of control groups (containing 50 mg/L and 100 mg/L of phenol without microalga) did not change in the whole process. Therefore, the effect of photolysis is neglectable in this study. As shown in Fig. 4, the best effect of growth of I. galbana and removal of phenol is obtained at 180 μmol/(m2·s) (Fig. 4), and the kinetic model agree with the results (Table 2). However, in a marine environment, the intensity of light decreases with an increase in the water depth, and the irradiance in deeper waters is generally very weak (Wang et al., 2017b). By inference, the degradation efficiency of phenol by I.galbana will decrease as water depth increases. This phenomenon indicates that the removal process progressed slowly instead of terminating in a dark environment. At the scene of an actual marine accident, the dark or weak light conditions will slow down the process of phenol removal by microalga, instead of completely inhibiting it. Moreover, the growth of the microalga under dark conditions was extremely inhibited, and there was very little growth in the cell densities of the microalga at 96 h (Fig. 5) because the growth of I. galbana was inhibited in heterotrophy (Alkhamis and Qin, 2013). It is speculated that I. galbana could remove phenol under dark conditions, but phenol would not be utilized as the carbon source for the growth of this microbe. Nazos et al. (2017) studied the biodegradation of phenol by Chlamydomonas reinhardtii under different light intensities. Their results showed that degradation was a process of energy consumption. The metabolism of phenol by C. reinhardtii only occurred under aerobic and light conditions. Hence, the phenol was not degraded during anaerobic or dark conditions. This conclusion is different from the results of this study, and this may be due to the different microalgae species.
The removal mechanism of organic pollutants by microalgae mainly includes biosorption, biodegradation and bioaccumulation. According to Song et al. (2019) and Xiong et al. (2017), biodegradation is the main pathway on the removal of organic contaminants while biosorption and bioaccumulation have little contribution to the removal process. However, the mechanism of phenol removal by I. galbana will be studied in the future. Furthermore, most microalgae inhabit the surface water due to their phototaxis. Therefore, in situ bioremediation via microalgae is suitable for the removal of phenol pollution in shallow water and coastal seawater. New biotechnologies, such as immobilization, are in urgent need for treating phenol contaminations in deeper seawater. In addition, microalgae is expected to be used in wastewater treatment, e.g. a Leptolyngbya sp. strain was applied to efficiently remove 100 mg/L of phenol from cokeoven wastewater (Thakurta et al., 2018). Hence I.galbana can also be used to the ex situ treatment of saline phenol-containing wastewater.
5 CONCLUSIONThe effects of temperature and light intensity on the removal of phenol by I. galbana in seawater were experimentally studied in this research. The results showed that the kinetics under different temperatures and light intensities agreed with the first-order model. Temperature and illumination have the ability to significantly influence the bioremediation effect. The most suitable temperature and light intensity were 20℃ and 180 μmol/(m2·s), respectively. Phenol was slowly removed by I. galbana in dark conditions, and light was not essential for the removal of I. galbana, but the microalga did not grow under dark conditions. Although the inhibition could occur, I. galbana was shown in this study to remove high concentrations of phenol at low-temperatures or under low-light (or dark) conditions. Hence, I. galbana has a promising potential for applications in the bioremediation of phenol pollution in actual marine environments.
6 DADA AVAILABILITY STATEMENTThe data generated or analyzed during the current study are available from the corresponding author on reasonable request.
Alkhamis Y, Qin J G. 2013. Cultivation of Isochrysis galbana in phototrophic, heterotrophic, and mixotrophic conditions. BioMed. Res. Int., 2013: 983.
|
Andersen G S, Pedersen M F, Nielsen S L. 2013. Temperature acclimation and heat tolerance of photosynthesis in Norwegian Saccharina latissima (Laminariales, Phaeophyceae). J. Phycol., 49(4): 689-700.
DOI:10.1111/jpy.12077 |
Bradley P M, Writer J H. 2014. Effect of light on biodegradation of estrone, 17β-estradiol, and 17α-ethinylestradiol in stream sediment. J. Am. Water Resour. Assoc., 50(2): 334-342.
DOI:10.1111/jawr.12157 |
Calabrese E J, Kenyon E M. 1991. Air Toxics and Risk Assessment. Lewis Publishers, Chelsea.Caracciolo A B, Topp E, Grenni P. 2015. Pharmaceuticals in the environment:biodegradation and effects on natural microbial communities. A review. J. Pharm. Biomed.Anal., 106: 25-36.
|
Cunha I, Moreira S, Santos M M. 2015. Review on hazardous and noxious substances (HNS) involved in marine spill incidents-An online database. J. Hazard. Mater., 285: 509-516.
DOI:10.1016/j.jhazmat.2014.11.005 |
Di Caprio F, Scarponi P, Altimari P, Iaquaniello G, Pagnanelli F. 2018. The influence of phenols extracted from olive mill wastewater on the heterotrophic and mixotrophic growth of Scenedesmus sp. J. Chem. Technol. Biotechnol., 93(12): 3619-3626.
DOI:10.1002/jctb.5743 |
Du W, Zhao F Q, Zeng B Z. 2009. Novel multiwalled carbon nanotubes-polyaniline composite film coated platinum wire for headspace solid-phase microextraction and gas chromatographic determination of phenolic compounds. J. Chromatogr. A., 1216(18): 3751-3757.
DOI:10.1016/j.chroma.2009.03.013 |
Duan W Y, Meng F P, Cui H W, Lin Y F, Wang G S, Wu J Y. 2018. Ecotoxicity of phenol and cresols to aquatic organisms:a review. Ecotoxicol. Environ. Saf., 157: 441-456.
DOI:10.1016/j.ecoenv.2018.03.089 |
Duan W Y, Meng F P, Lin Y F, Wang G S. 2017. Toxicological effects of phenol on four marine microalgae. Environ.Toxicol. Pharmacol., 52: 170-176.
DOI:10.1016/j.etap.2017.04.006 |
Emerson E. 1943. The condensation of aminoantipyrine. II. A new color test for phenolic compounds. J. Org. Chem., 8(5): 417-428.
|
French McCay D P, Whittier N, Ward M, Santos C. 2006. Spill hazard evaluation for chemicals shipped in bulk using modeling. Environ. Modell. Softw., 21(2): 156-169.
DOI:10.1016/j.envsoft.2004.04.021 |
Gao J, Chi J. 2015. Biodegradation of phthalate acid esters by different marine microalgal species. Mar. Pollut. Bull., 99(1-2): 70-75.
DOI:10.1016/j.marpolbul.2015.07.061 |
Gao Q T, Wong Y S, Tam N F Y. 2011. Removal and biodegradation of nonylphenol by different Chlorella species. Mar. Pollut. Bull., 63(5-12): 445-451.
DOI:10.1016/j.marpolbul.2011.03.030 |
Guillard R R L. 1975. Culture of phytoplankton for feeding marine invertebrates. In: Smith W L, Chanley M H eds.Culture of Marine Invertebrate Animals. Springer, Boston.p.29-60.
|
HELCOM. 2002. Response to Accidents at Sea Involving Spills of Hazardous Substances and Loss of Packaged Dangerous Goods. Baltic Marine Environment Protection Commission, Helsinki, Finland.
|
Lima S A C, Raposo M F J, Castro P M L, Morais R M. 2004. Biodegradation of p-chlorophenol by a microalgae consortium. Water Res., 38(1): 97-102.
DOI:10.1016/j.watres.2003.09.005 |
Liu M J, Fu Q, Fu H L, Shu G, Zhang W, Deng F Y, Hu J. 2014. A new kinetic approach.Stability of cefquinome sulfate under variable pH and temperature. Indian J. Pharm.Educ. Res., 48(S1): 27-33.
|
Lu Y, Yan L H, Wang Y, Zhou S F, Fu J J, Zhang J F. 2009. Biodegradation of phenolic compounds from coking wastewater by immobilized white rot fungus Phanerochaete chrysosporium. J. Hazard. Mater., 165(1-3): 1091-1097.
DOI:10.1016/j.jhazmat.2008.10.091 |
Matamoros V, Gutiérrez R, Ferrer I, García J, Bayona J M. 2015. Capability of microalgae-based wastewater treatment systems to remove emerging organic contaminants:a pilot-scale study. J. Hazard. Mater., 288: 34-42.
DOI:10.1016/j.jhazmat.2015.02.002 |
Massalha N, Shaviv A, Sabbah I. 2010. Modeling the effect of immobilization of microorganisms on the rate of biodegradation of phenol under inhibitory conditions. Water Res., 44(18): 5252-5259.
DOI:10.1016/j.watres.2010.06.042 |
Nazos T T, Kokarakis E J, Ghanotakis D F. 2017. Metabolism of xenobiotics by Chlamydomonas reinhardtii:phenol degradation under conditions affecting photosynthesis. Photosynth. Res., 131(1): 31-40.
DOI:10.1007/s11120-016-0294-2 |
Onysko K A, Budman H M, Robinson C W. 2000. Effect of temperature on the inhibition kinetics of phenol biodegradation by Pseudomonas putida Q5. Biotechnol.Bioeng., 70(3): 291-299.
DOI:10.1002/1097-0290(20001105)70:3<291::AID-BIT6>3.0.CO;2-Y |
Perez-Garcia O, Escalante F M E, De-Bashan L E, Bashan Y. 2011. Heterotrophic cultures of microalgae:metabolism and potential products. Water Res., 45(1): 11-36.
DOI:10.1016/j.watres.2010.08.037 |
Polymenakou P N, Stephanou E G. 2005. Effect of temperature and additional carbon sources on phenol degradation by an indigenous soil Pseudomonad. Biodegradation, 16(5): 403-413.
DOI:10.1007/s10532-004-3333-1 |
Raven J A, Geider R J. 1988. Temperature and algal growth. New Phytol., 110(4): 441-461.
DOI:10.1111/j.1469-8137.1988.tb00282.x |
Saravanan P, Pakshirajan K, Saha P. 2009. Batch growth kinetics of an indigenous mixed microbial culture utilizing m-cresol as the sole carbon source. J. Hazard. Mater., 162(1): 476-481.
DOI:10.1016/j.jhazmat.2008.05.069 |
Senthilvelan T, Kanagaraj J, Panda R C, Mandal A B. 2014. Biodegradation of phenol by mixed microbial culture:an eco-friendly approach for the pollution reduction. Clean.Technol. Environ. Policy, 16(1): 113-126.
|
Siddiqui K S. 2015. Some like it hot, some like it cold:temperature dependent biotechnological applications and improvements in extremophilic enzymes. Biotechnol.Adv., 33(8): 1912-1922.
DOI:10.1016/j.biotechadv.2015.11.001 |
Silambarasan S, Abraham J. 2013. Kinetic studies on enhancement of degradation of chlorpyrifos and its hydrolyzing metabolite TCP by a newly isolated Alcaligenes sp. JAS1. J. Taiwan Inst. Chem. Eng., 44(3): 438-445.
DOI:10.1016/j.jtice.2012.12.002 |
Sinkkonen S, Paasivirta J. 2000. Degradation half-life times of PCDDs, PCDFs and PCBs for environmental fate modeling. Chemosphere, 40(9-11): 943-949.
DOI:10.1016/S0045-6535(99)00337-9 |
Song C F, Wei Y L, Qiu Y T, Qi Y, Li Y, Kitamura Y. 2019. Biodegradability and mechanism of florfenicol via Chlorella sp.UTEX1602 and L38:experimental study. Bioresour. Technol., 272: 529-534.
DOI:10.1016/j.biortech.2018.10.080 |
Surkatti R, El-Naas M H. 2018. Competitive interference during the biodegradation of cresols. Int. J. Environ. Sci.Technol., 15(2): 301-308.
DOI:10.1007/s13762-017-1383-2 |
Thakurta S G, Aakula M, Chakrabarty J, Dutta S. 2018. Bioremediation of phenol from synthetic and real wastewater using Leptolyngbya sp.:a comparison and assessment of lipid production. 3 Biotech, 8(4): 206.
|
Wan S G, Li G Y, An T C, Guo B, Sun L, Zu L, Ren A L. 2010. Biodegradation of ethanethiol in aqueous medium by a new Lysinibacillus sphaericus strain RG-1 isolated from activated sludge. Biodegradation, 21(6): 1057-1066.
DOI:10.1007/s10532-010-9366-8 |
Wang T, Xu Z Y, Wu L G, Li B R, Chen M X, Xue S Y, Zhu Y C, Cai J. 2017b. Enhanced photocatalytic activity for degrading phenol in seawater by TiO2-based catalysts under weak light irradiation. RSC Adv., 7(51): 31921-31929.
DOI:10.1039/C7RA04732K |
Wang X Q, Wang Q L, Li S J, Li W. 2015. Degradation pathway and kinetic analysis for p-xylene removal by a novel Pandoraea sp.strain WL1 and its application in a biotrickling filter. J. Hazard. Mater., 288: 17-24.
|
Wang Y J, Meng F P, Li H, Zhao S L, Liu Q Q, Lin Y F, Wang G S, Wu J Y. 2019. Biodegradation of phenol by Isochrysis galbana screened from eight species of marine microalgae:growth kinetic models, enzyme analysis and biodegradation pathway. J. Appl. Phycol., 31(1): 445-455.
DOI:10.1007/s10811-018-1517-z |
Wang Y J, Meng F P, Lin Y F, Duan W Y, Liu Q Q. 2017a. Four types of attenuation of phenol and cresols in microcosms under simulated marine conditions:a kinetic study. Chemosphere, 185: 595-601.
DOI:10.1016/j.chemosphere.2017.07.045 |
Wernberg T, Thomsen M S, Tuya F, Kendrick G A, Staehr P A, Toohey B D. 2010. Decreasing resilience of kelp beds along a latitudinal temperature gradient:potential implications for a warmer future. Ecol. Lett., 13(6): 685-694.
DOI:10.1111/j.1461-0248.2010.01466.x |
Wijffels R H, Kruse O, Hellingwerf K J. 2013. Potential of industrial biotechnology with cyanobacteria and eukaryotic microalgae. Curr. Opin. Biotech., 24(3): 405-413.
DOI:10.1016/j.copbio.2013.04.004 |
Xiong J Q, Kurade M B, Jeon B H. 2017. Biodegradation of levofloxacin by an acclimated freshwater microalga, Chlorella vulgaris. Chem. Eng. J., 313: 1251-1257.
DOI:10.1016/j.cej.2016.11.017 |
Yang S, Wu R S S, Kong R Y C. 2002. Biodegradation and enzymatic responses in the marine diatom Skeletonema costatum upon exposure to 2, 4-dichlorophenol. Aquat.Toxicol., 59(3-4): 191-200.
DOI:10.1016/S0166-445X(01)00252-1 |
Zhou D D, Xu Z X, Dong S S, Huo M X, Dong S S, Tian X D, Cui B, Xiong H F, Li T T, Ma D M. 2015. Intimate coupling of photocatalysis and biodegradation for degrading phenol using different light types:visible light vs UV light. Environ. Sci. Technol., 49(13): 7776-7783.
DOI:10.1021/acs.est.5b00989 |
 2020, Vol. 38
2020, Vol. 38


