Institute of Oceanology, Chinese Academy of Sciences
Article Information
- YANG Yue, XING Rong'e, LIU Song, QIN Yukun, LI Kecheng, YU Huahua, LI Pengcheng
- PI3K/Akt pathway is involved in the activation of RAW 264.7 cells induced by hydroxypropyltrimethyl ammonium chloride chitosan
- Journal of Oceanology and Limnology, 38(3): 834-840
- http://dx.doi.org/10.1007/s00343-019-9013-0
Article History
- Received Jan. 19, 2019
- accepted in principle Jun. 28, 2019
- accepted for publication Sep. 8, 2019
2 Laboratory for Marine Drugs and Bioproducts of Qingdao National Laboratory for Marine Science and Technology, Qingdao 266237, China;
3 University of Chinese Academy of Sciences, Beijing 100049, China
Immunity plays vital role in vertebrates. The immune system can broadly be divided into two branches: innate immunity and adaptive immunity (Fang and Zhang, 2016). Macrophages along with dendritic cells are two important innate immune system members. RAW 264.7 cells, a kind of macrophage derived from mouse ascites, are widely utilized as in vitro model to detect immunomodulatory effect of foreign substances. Upon activation, RAW 264.7 cells secrete cytokines through the activation of signaling pathways (Sun et al., 2015). The signaling pathways of RAW 264.7 cells have been elucidated by many researchers, among them, phosphoinositide 3-kinases (PI3K)/Akt, sarcoma (Src) family kinases, mitogen-activated protein kinase (MAPK) including p38, extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), Janus kinase/signal transducer and activator of transcription (JAK-STAT), and transcription factors such as activator protein (AP)-1 and nuclear factor (NF)-κB are vital pathways participating immunomodulatory process (Cantley, 2002; Johnson and Lapadat, 2002; Schindler et al., 2007; Youn et al., 2016). The PI3K family consists of four classes of enzymes: ⅠA, ⅠB, Ⅱ, Ⅲ (Wymann and Pirola, 1998; Katso et al., 2001). Akt is a direct downstream effector of PI3K (Cheever et al., 2001). Akt is a serine/threonine kinase which can be a transducer of signaling pathways initiated by growth factor receptor-activated PI3K (Kao et al., 2005). The role of PI3K/Akt signaling pathway in immunomodulatory effect has been implied (Hattori et al., 2003). Therefore, PI3K/Akt pathway is the focus of our study.
Chitin, which consists of β-(1-4)-poly-N-acetyl-Dglucosamine unit, is a kind of insoluble cationic amino polysaccharide. Chitin is plenteous in nature, it was isolated from mushroom for the first time in 1811 (Liaqat and Eltem, 2018). It also exists in bacterial, fungi, insect cuticles and exoskeleton crustacean shells and fungal cell walls (Pillai et al., 2009). Chitosan as the deacetylation production of chitin, are widely utilized in medical and food industry for its nonallergenic, biodegradable and low toxicity properties (Kurita, 2006; Li et al., 2016). The immunological properties of chitin and chitosan were studied in recent years (Lee et al., 2008). Chitin was found to be able to stimulate innate immunity of host to resist the invasion of viral and bacterial infections for the first time in 1980s (Nishimura et al., 1984). Some studies demonstrated chitin and chitosan activated macrophages and natural kill cells to secrete cytokines like interleukin-1β (IL-1β) and interferons (Chae et al., 2009). Researchers also found that chitin promoted innate and adaptive immune responses in a sizedependent manner (Lee et al., 2008). However, the solubility of chitosan and chitin in neutral environment was not satisfying for application. Therefore, the derivatives of chitin and chitosan were developed and studied by many researchers. Among them, the watersoluble hydroxypropyltrimethyl ammonium chloride chitosan (HACC) has attracted attention because of its high solubility and high charge density.
Hydroxypropyltrimethyl ammonium chloride chitosan as a derivative of chitosan has been demonstrated as a potential immunopotentiator in our previous study (Yang et al., 2019). We demonstrated that HACC promoted the secretion of nitric oxide (NO), interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α). We found the activation was partly through the Janus kinase/STAT and MAPK signaling pathway, while the Src tyrosine kinase was not affected by HACC. Therefore, in this study, we explored new signaling pathways to reveal the mechanisms of the immunomodulatory effects of HACC. We proposed that HACC could activate PI3K/Akt pathway and affect the translocation of activating protein 1 (AP-1) and nuclear factor kappa B (NF-κB) and conducted western blot assay and immunofluorescent staining to verify our hypothesis.
2 MATERIAL AND METHOD 2.1 Materialα-Chitosan with the molecular weight of 1856 kDa and the degree of deacetylation (DDA) of 86.0% was provided by Qingdao Yunzhou Biochemical Corp. (Qingdao, China). The penicillin-streptomycin was obtained from Gibco BRL (Life Technologies, Shanghai, China). Fetal bovine serum (FBS) and Roswell Park Memorial Institute (RPMI) medium 1640 were provided by HyClone (Thermo Fisher Scientific, Logan, Utah, USA). The nuclear protein isolation kits and BCA protein assay kits were from ComWin Biotech (Beijing, China). Primary antibodies to p85, Akt, phospho-specific p85, phospho-specific Akt, c-fos, c-Jun, p65, and β-actin were provided by the Cell Signaling Technology (Beverly, MA, USA). Horseradish peroxidase-labeled (HRP-labeled) antibodies were provided by Abcam (Cambridge, MA, USA). The donkey anti-rabbit IgG H&L (Alexa Fluor® 4647) secondary antibody were purchased from the Cell Signaling Technology (Beverly, MA, USA). Glycidyl trimethylammonium chloride was from Dongying Guofeng Fine Chemical Co. Ltd. (Shandong, China).
2.2 Preparation of 2-hydroxypropyltrimethyl ammonium chloride chitosanThe preparation of HACC was followed by the methods previous reported (Yang et al., 2019). Briefly, glycidyl trimethylammonium chloride and chitosan powder are added in a three-necked bottomed flask with distilled water at 80℃ for 24 h, and then the products are dialyzed, concentrated, and lyophilized to powder.
2.3 Cell cultureRAW 264.7 macrophages were provided by American Type Culture Collection (Manassas, VA, USA). After inactivated by heating, fetal bovine serum (FBS) was added to RPMI 1640 medium to the concentration of 10%. The antibiotics and glutamine were also added in the medium. Then the cells were cultured in the medium in an incubator with 5% CO2 at 37℃.
2.4 Western blottingTo determine the expression levels of proteins in signaling pathways, RAW 264.7 cells (5×106 cells/ well) were plated and incubated in 6-well plates. After 24 h, HACC (50 μg/mL) were added to each well except the blank control. After the treatment with HACC for indicated time points, the cells were respectively collected and lysed by the lysis buffer (ComWin Biotech, Beijing, China), and then the Roche complete protease inhibitor cocktail (Roche Diagnostics Ltd., Mannheim, Germany) were used to extract total proteins of cells. The nuclear protein isolation kits were used to extract nuclear proteins, and then BCA protein assay kits were utilized to determine the protein concentration. Equal amounts of supernatants were separated by SDS-PAGE and transferred onto polyvinylidene fluoride membranes, and then primary antibodies and HRP-labeled secondary antibodies were added, incubated and washed. The bands were finally visualized using ECL reagents. The densities of the bands were quantified by Quantity One software (Bio-Rad, Munich, Germany). Data were presented as mean±SD (n=3) from independent experiments.
2.5 Immunofluorescent stainingTo determine the effect of HACC on p65 nuclear translocation, RAW 264.7 cells (1×106 cells/mL) were seeded onto glass coverslips and incubated for 18 h in petri dishes. After pretreated with HACC (50 μg/mL), cells were washed by PBS and immobilized by paraformaldehyde. After washing three times with PBS, 0.5% Triton X-100 was added to permeabilize for 10 min, and then the slides were blocked with 3% BSA for 1 h. After washing with PBS, the monoclonal p65 antibody was introduced and incubated for 2 h. After another washing for three times, the slides were incubated with Donkey AntiRabbit IgG H&L secondary antibody and DAPI. The stained slides were observed using laser scanning confocal microscope (LSM 700, Zeiss, Jena, Germany).
2.6 Scanning electron microscopy (SEM)To observe the morphologic change of cells after treated with HACC, cells were seeded and cultured in petri dishes for 18 h. Then the cells were treated with HACC (50 μg/mL), fixed with glutaraldehyde, and washed three times with PBS. At last, cells were dewatered twice by 30% to 100% ethanol gradients and observed using SEM (FEI Quanta 450 FEG, Hillsboro, USA).
3 RESULT 3.1 HACC activated the PI3K/Akt pathwayBased on our previous study, we chose HACC with the molecular weight of 5003 Da to explore whether HACC activated PI3K/Akt pathway. The phosphorylation levels of p85, Akt and PDK1 were determined. Cells were pretreated with HACC (50 μg/ mL) for 0, 30, 60, 180, and 360 min. As shown in Fig. 1, the phosphorylation levels of PDK1 increased in a time-dependent manner. While the phosphorylation level of p85 increased within 60 min and remained for 360 min. The phosphorylated Akt levels were also determined by the western blotting. The results show that the expression of p-Akt peaked at 60 min and decreased gradually during 360 min.
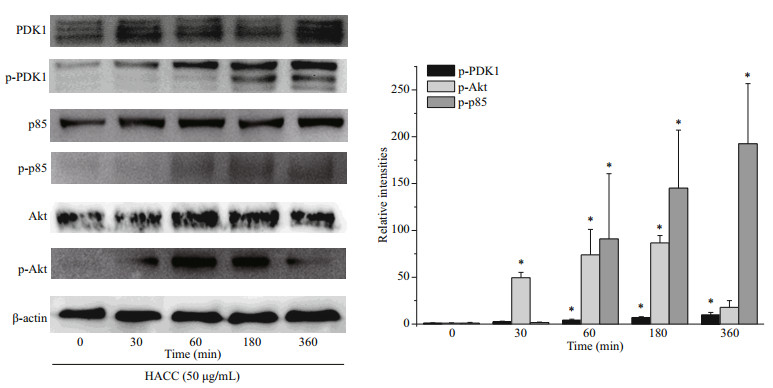
|
| Fig.1 Effect of hydroxypropyltrimethyl ammonium chloride chitosan (HACC) on PI3k/Akt signaling pathway Proteins were extracted from RAW 264.7 cells pretreated with 50 μg/mL of HACC for the indicated times (0-360 min). |
To reveal the molecular mechanism of HACC in RAW 264.7 cells, the levels of two vital transcription factors, nuclear factor (NF)-κB and AP-1 were investigated. Figure 2 shows that HACC promoted the translocation of p65 (NF-κB subnit). Furthermore, the translocation of c-Jun (AP-1 subnit) was also promoted by HACC after 60 min. However, the immunoblotting results indicate that the translocation c-Fos was not affected by HACC in 360 min. These results indicate that HACC induced the nuclear translocation of AP-1 and NF-κB.
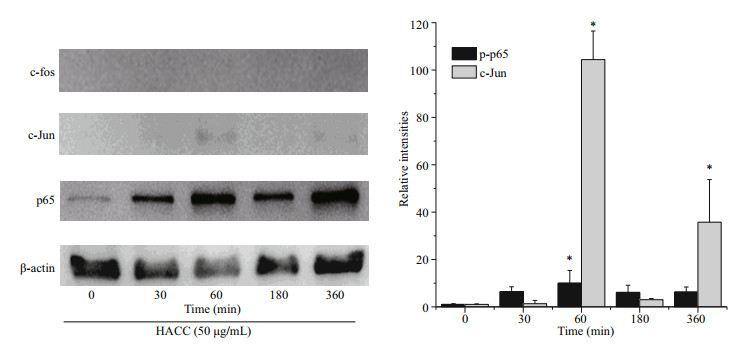
|
| Fig.2 Effects of the HACC (50 μg/mL) on the translocation of AP-1 and NF-κB Nucleus proteins were extracted from RAW 264.7 cells pretreated with 50 μg/mL of HACC for the indicated times (0-360 min). |
To visualize the translocation of NF-κB, immunofluorescence staining was conducted. The results (Fig. 3) show that p65 obviously translocated from cytoplasm to nucleus and accumulated after pretreatment with HACC (50 μg/mL).
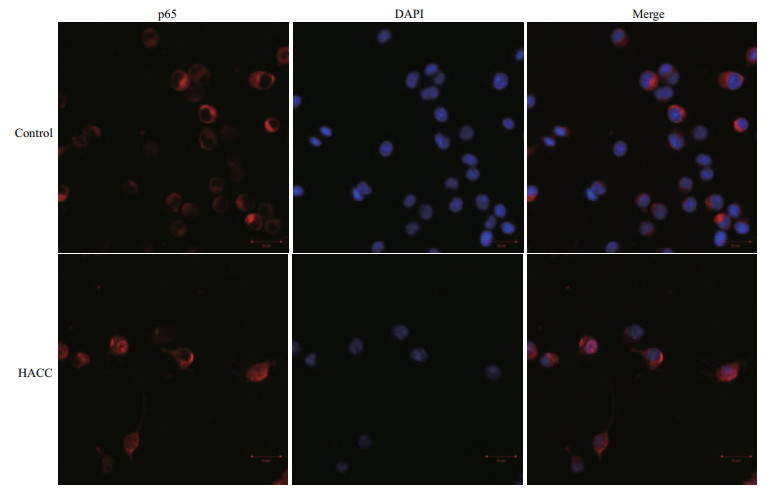
|
| Fig.3 Effects of HACC on p65 nuclear translocation in RAW 264.7 cells Cells were treated with control medium HACC (50 μg/mL) for 30 min. The localization of p65 was determined by fluorescence microscopy after staining with DAPI, anti-p65 and secondary antibody. |
To observe the morphology variation of RAW 264.7 cells after treated with HACC, SEM analysis was conducted. As shown in Fig. 4a, untreated cells are round in shape and have smooth surface, while the HACC-treated cells were activated and the morphology was obviously altered. As shown in Fig. 4b, cell differentiation is observed; the cells became polygon with projections, increased in size and are easier to detach from culture dishes.
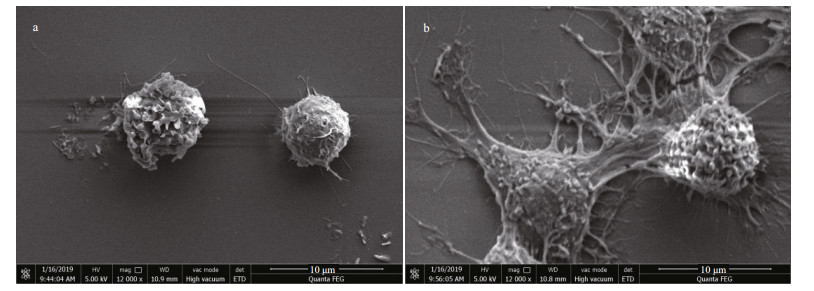
|
| Fig.4 Morphology of RAW 264.7 cell morphology visualized by SEM analysis a. control cells; b. cells treated with HACC (50 μg/mL). |
RAW 264.7 macrophages were derived from a tumor in BAB/14 mouse inoculated with Abelson murine leukemia virus (MuLV) about 30 years ago (Raschke et al., 1978). The cells have been commonly accepted as a model to study the immune regulation of candidates (Hartley et al., 2008; Ma et al., 2011; Yu et al., 2017). Therefore, we used RAW 264.7 cells as the in vitro model to further explore the mechanisms of HACC.
In our previous study (Yang et al., 2019), we demonstrated that HACC promoted the production of NO and proinflammatory cytokines by inducing the phosphorylation of ERK, JNK, p38, and STAT proteins in RAW 264.7 cells. However, the roles of other signaling pathways have not been explored. Therefore, we used HACC of 5003 Da to reveal the molecular mechanisms.
NF-κB is a eukaryotic transcription factor composed of RelA (p65), p50/p105 (NF-κB1), p52/p100 (NF- κB2), c-Rel, and RelB (Nyati et al., 2017; Wen et al., 2018; Zhang and Igwe, 2018). It has been reported that NF-κB is related to the secretion of various proinflammatory cytokines such as TNF-α, iNOS and IL-1β (Gukovsky et al., 1998; Chen et al., 2005; Zhang et al., 2017). Furthermore, it has been reported that NF-κB is activated by many cellular kinases including MAPK (Guha and Mackman, 2001). Our previous results showed that HACC activated the MAPK signaling pathway which resulted in the production of NO and TNF-α, therefore we explored the role of NF- κB in the HACC-induced activation of RAW 264.7 cells. P65 is a vital subunit of NF-κB complex, it is a vital signal for the initiation of the changes of NF-κB (Liang et al., 2018). The western blot analysis results showed that p65 participated the activation of cells which was consistent with the above reports. The confocal micrograph results (Fig. 3) exhibited the increase of p65 in nucleus, which is consistent with the western blotting results. Li et al. (2014) also performed immunofluorescence assay to determine the nuclear translocation of NF-κB (p65). Upon activation, NF- κB was translocated to cell nucleus and regulated the transcription of proinflammatory cytokines (Gugasyan et al., 2000; Li et al., 2018; Zhang and Igwe, 2018). Additional to NF-κB, AP-1 is another vital transcription factor regulating the immune responses (Poltorak et al., 1998). It is composed of c-Jun and c-Fos family (Karin et al., 1997; Shen et al., 2013). It has been demonstrated that AP-1 could be regulated by MAPK pathway (Musti et al., 1997). As our previous results (Yang et al., 2019) show, HACC activated MAPK pathway, and we determined the variation of AP-1 in the activation of RAW 264.7 cells. The results (Fig. 2) show that c-Jun was also promoted by HACC, but c-Fos was not affected by HACC, these results demonstrated that AP-1 was also affected by HACC.
PI3K has been reported to play vital functions because of the significance of cellular movement and membrane trafficking in the effector functions of immune cells (Koyasu, 2003). To determine the role of PI3K in the HACC-induced activation of RAW 264.7 cells, immunoblotting was conducted. The results (Fig. 1) indicate that PI3K activation is involved in the activation process of RAW 264.7 and the production of cytokines release. However, the different timedependent effects between Akt and p85 may be caused by two reasons. First, Akt could be activated through various signaling pathways in cells (Kao et al., 2005). Second, other subunits of PI3K such as p55, p50 also activated the downstream Akt. Our result is in accordance with the study in which PI3K was found involved in the signal transduction and resulted in the expression of iNOS and NO release induced by lipoteichoic acid (Kao et al., 2005). Tang et al. (2017) reported that the Akt phosphorylation was regulated by NF-κB; therefore, the phosphorylation level of Akt was also determined by the western blotting assay, and the results suggest that the phosphorylation of Akt was promoted by HACC. PDK1, the upstream protein of Akt, was also induced to phosphorylate by HACC (Fig. 1). Taken together, our results are consistent with the study reporting that PI3K activation resulted in the downstream activation of Akt, thus leading to the NO expression in macrophages (Hattori et al., 2003). The SEM results (Fig. 4) are in accordance with the results of the western blotting and further demonstrated that RAW 264.7 cells were activated by HACC.
5 CONCLUSIONIn this paper, we further studied the molecular mechanisms of HACC-induced activation of RAW 264.7 cells. The results show that HACC promoted the production of NO and proinflammatory cytokines by activating PI3K-Akt signaling pathway. Furthermore, the western blotting and immunofluorescence staining results demonstrated the activation was dependent on AP-1 and NF-κB activation. These results were helpful for providing the basis and illustrating the mechanisms for the immunostimulatory effect of HACC as an immunopotentiator.
6 DATA AVAILABILITY STATEMENTThe data that support the findings of this study are available from the corresponding author upon reasonable request.
7 ACKNOWLEDGMENTWe gratefully acknowledge Dr. Weicheng HU for proving cell culture room in Huaiyin Normal University (Jiangsu, China).
Cantley L C. 2002. The phosphoinositide 3-kinase pathway. Science, 296(5573): 1655-1657.
DOI:10.1126/science.296.5573.1655 |
Chae H S, Kang O H, Lee Y S, Choi J G, Oh Y C, Jang H J, Kim M S, Kim J H, Jeong S I, Kwon D Y. 2009. Inhibition of LPS-induced iNOS, COX-2 and inflammatory mediator expression by paeonol through the MAPKs inactivation in RAW 264.7 cells. Am. J. Chinese Med., 37(1): 181-194.
DOI:10.1142/S0192415X0900676X |
Cheever M L, Sato T K, de Beer T, Kutateladze T G, Emr S D, Overduin M. 2001. Phox domain interaction with PtdIns(3)P targets the Vam7 t-SNARE to vacuole membranes. Nature Cell Biology, 3(7): 613-618.
DOI:10.1038/35083000 |
Chen J J, Huang W C, Chen C C. 2005. Transcriptional regulation of cyclooxygenase-2 in response to proteasome inhibitors involves reactive oxygen species-mediated signaling pathway and recruitment of CCAAT/enhancerbinding protein δ and CREB-binding protein. Mol. Biol.Cell, 16(12): 5579-5591.
DOI:10.1091/mbc.e05-08-0778 |
Fang R H, Zhang L F. 2016. Nanoparticle-based modulation of the immune system. Annu. Rev. Chem. Biomol. Eng., 7(1): 305-326.
DOI:10.1146/annurev-chembioeng-080615-034446 |
Gugasyan R, Grumont R, Grossmann M, Nakamura Y, Pohl T, Nesic D, Gerondakis S. 2000. Rel/NF-κB transcription factors:key mediators of B-cell activation. Immunol.Rev., 176(1): 134-140.
|
Guha M, Mackman N. 2001. LPS induction of gene expression in human monocytes. Cellular Signalling, 13(2): 85-94.
|
Gukovsky I, Gukovskaya A S, Blinman T A, Zaninovic V, Pandol S J. 1998. Early NF-κB activation is associated with hormone-induced pancreatitis. Am. J. Physiol., 275(6): G1402-G1414.
|
Hartley J W, Evans L H, Green K Y, Naghashfar Z, Macias A R, Zerfas P M, Ward J M. 2008. Expression of infectious murine leukemia viruses by RAW264.7 cells, a potential complication for studies with a widely used mouse macrophage cell line. Retrovirology, 5: 1.
|
Hattori Y, Hattori S, Kasai K. 2003. Lipopolysaccharide activates Akt in vascular smooth muscle cells resulting in induction of inducible nitric oxide synthase through nuclear factor-kappa B activation. European Journal of Pharmacology, 481(2-3): 153-158.
DOI:10.1016/j.ejphar.2003.09.034 |
Johnson G L, Lapadat R. 2002. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science, 298(5600): 1911-1912.
DOI:10.1126/science.1072682 |
Kao S J, Lei H C, Kuo C T, Chang M S, Chen B C, Chang Y C, Chiu W T, Lin C H. 2005. Lipoteichoic acid induces nuclear factor-kappaB activation and nitric oxide synthase expression via phosphatidylinositol 3-kinase, Akt, and p38 MAPK in RAW 264.7 macrophages. Immunology, 115(3): 366-374.
|
Karin M, Liu Z G, Zandi E. 1997. AP-1 function and regulation. Curr. Opin. Cell Biol., 9(2): 240-246.
DOI:10.1016/S0955-0674(97)80068-3 |
Katso R, Okkenhaug K, Ahmadi K, White S, Timms J, Waterfield M D. 2001. Cellular function of phosphoinositide 3-kinases:implications for development, immunity, homeostasis, and cancer. Annual Review of Cell and Developmental Biology, 17(1): 615-675.
DOI:10.1146/annurev.cellbio.17.1.615 |
Koyasu S. 2003. The role of PI3K in immune cells. Nat.Immunol., 4(4): 313-319.
DOI:10.1038/ni0403-313 |
Kurita K. 2006. Chitin and chitosan:functional biopolymers from marine crustaceans. Mar. Biotechno., 8(3): 203-226.
DOI:10.1007/s10126-005-0097-5 |
Lee C G, Da Silva C A, Lee J Y, Hartl D, Elias J A. 2008. Chitin regulation of immune responses:an old molecule with new roles. Curr. Opin. Immunol., 20(6): 684-689.
DOI:10.1016/j.coi.2008.10.002 |
Li K K, Shen S S, Deng X Y, Shiu H T, Siu W S, Leung P C, Ko C H, Cheng B H. 2018. Dihydrofisetin exerts its antiinflammatory effects associated with suppressing ERK/p38 MAPK and Heme Oxygenase-1 activation in lipopolysaccharide-stimulated RAW 264.7 macrophages and carrageenan-induced mice paw edema. International Immunopharmacology, 54: 366-374.
|
Li L, Wang L Y, Wu Z Q, Yao L J, Wu Y H, Huang L, Liu K, Zhou X, Gou D M. 2014. Anthocyanin-rich fractions from red raspberries attenuate inflammation in both RAW264.7 macrophages and a mouse model of colitis. Sci. Rep., 4: 6234.
|
Li Y, Qin Y K, Liu S, Li P C, Xing R E. 2016. Preparation, characterization, and antifungal activity of hymexazollinked chitosan derivatives. Chinese Journal of Oceanology and Limnology, 35(5): 1079-1085.
|
Liang N, Sang Y X, Liu W H, Yu W L, Wang X H. 2018. AntiInflammatory effects of gingerol on lipopolysaccharidestimulated RAW 264.7 cells by inhibiting NF-κB signaling pathway. Inflammation, 41(3): 835-845.
|
Liaqat F, Eltem R. 2018. Chitooligosaccharides and their biological activities:a comprehensive review. Carbohydr.Polym., 184: 243-259.
DOI:10.1016/j.carbpol.2017.12.067 |
Ma P, Liu H T, Wei P, Xu Q S, Bai X F, Du Y G, Yu C. 2011. Chitosan oligosaccharides inhibit LPS-induced overexpression of IL-6 and TNF-α in RAW264.7 macrophage cells through blockade of mitogen-activated protein kinase (MAPK) and PI3K/Akt signaling pathways.Carbohydr. Polym., 84(4): 1391-1398.
|
Musti A M, Treier M, Bohmann D. 1997. Reduced ubiquitindependent degradation of c-Jun after phosphorylation by MAP Kinases. Science, 275(5298): 400-402.
DOI:10.1126/science.275.5298.400 |
Nishimura K, Nishimura S, Nishi N, Saiki I, Tokura S, Azuma I. 1984. Immunological activity of chitin and its derivatives. Vaccine, 2(1): 93-99.
DOI:10.1016/S0264-410X(98)90039-1 |
Nyati K K, Masuda K, Zaman M M U, Dubey P K, Millrine D, Chalise J P, Higa M, Li S L, Standley D M, Saito K, Hanieh H, Kishimoto T. 2017. TLR4-induced NF-κB and MAPK signaling regulate the IL-6 mRNA stabilizing protein Arid5a. Nucleic Acids Res., 45(5): 2687-2703.
DOI:10.1093/nar/gkx064 |
Pillai C K S, Paul W, Sharma C P. 2009. Chitin and chitosan polymers:chemistry, solubility and fiber formation. Progress in Polymer Science, 34(7): 641-678.
DOI:10.1016/j.progpolymsci.2009.04.001 |
Poltorak A, He X L, Smirnova I, Liu M Y, van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice:mutations in Tlr4 gene. Science, 282(5396): 2085-2088.
DOI:10.1126/science.282.5396.2085 |
Raschke W C, Baird S, Ralph P, Nakoinz I. 1978. Functional macrophage cell lines transformed by abelson leukemia virus. Cell, 15(1): 261-267.
DOI:10.1016/0092-8674(78)90101-0 |
Schindler C, Levy D E, Decker T. 2007. JAK-STAT signaling:from interferons to cytokines. J. Biol. Chem., 282(28): 20059-20063.
DOI:10.1074/jbc.R700016200 |
Shen T, Yang W S, Yi Y S, Sung G H, Rhee M H, Poo H, Kim M Y, Kim K W, Kim J H, Cho J Y. 2013. AP-1/IRF-3 targeted anti-Inflammatory activity of andrographolide isolated from Andrographis paniculata. Evid. Based Complement Alternat. Med., 2013(4): 210736.
|
Sun H X, Zhang J, Chen F Y, Chen X F, Zhou Z H, Wang H. 2015. Activation of RAW264.7 macrophages by the polysaccharide from the roots of Actinidia eriantha and its molecular mechanisms. Carbohydr.Polym., 121: 388-402.
|
Tang B, Li X, Ren Y, Wang J, Xu D, Hang Y, Zhou T, Li F, Wang L. 2017. MicroRNA-29a regulates lipopolysaccharide (LPS)-induced inflammatory responses in murine macrophages through the Akt1/NFkappaB pathway. Exp. Cell Res., 360(2): 74-80.
DOI:10.1016/j.yexcr.2017.08.013 |
Wen Q, Mei L Y, Ye S, Liu X, Xu Q, Miao J F, Du S H, Chen D F, Li C, Li H. 2018. Chrysophanol demonstrates antiinflammatory properties in LPS-primed RAW 264.7 macrophages through activating PPAR-γ. International Immunopharmacology, 56: 90-97.
|
Wymann M P, Pirola L. 1998. Structure and function of phosphoinositide 3-kinases. BBA-Mol. Cell. Biol. Lipids, 1436(1-2): 127-150.
DOI:10.1016/S0005-2760(98)00139-8 |
Yang Y, Xing R E, Liu S, Qin Y K, Li K C, Yu H H, Li P C. 2019. Hydroxypropyltrimethyl ammonium chloride chitosan activates RAW 264.7 macrophages through the MAPK and JAK-STAT signaling pathways. Carbohydr.Polym., 205: 401-409.
|
Youn G S, Lee K W, Choi S Y, Park J. 2016. Overexpression of HDAC6 induces pro-inflammatory responses by regulating ROS-MAPK-NF-κB/AP-1 signaling pathways in macrophages. Free Radical Biology and Medicine, 97: 14-23.
DOI:10.1016/j.freeradbiomed.2016.05.014 |
Yu Y, Shen M Y, Wang Z J, Wang Y X, Xie M Y, Xie J H. 2017. Sulfated polysaccharide from Cyclocarya paliurus enhances the immunomodulatory activity of macrophages. Carbohydr. Polym., 174: 669-676.
DOI:10.1016/j.carbpol.2017.07.009 |
Zhang Q, Wang L R, Chen B H, Zhuo Q, Bao C Y, Lin L. 2017. Propofol inhibits NF-κB activation to ameliorate airway inflammation in ovalbumin (OVA)-induced allergic asthma mice. International Immunopharmacology, 51: 158-164.
DOI:10.1016/j.intimp.2017.08.015 |
Zhang Y, Igwe O J. 2018. Exogenous oxidants activate nuclear factor kappa B through Toll-like receptor 4 stimulation to maintain inflammatory phenotype in macrophage. Biochem. Pharmacol., 147: 104-118.
DOI:10.1016/j.bcp.2017.11.012 |
 2020, Vol. 38
2020, Vol. 38


