Institute of Oceanology, Chinese Academy of Sciences
Article Information
- TANG Changsheng, SUN Song, ZHANG Fang
- Intraguild predation by polyps of three scyphozoan jellyfish: Nemopilema nomurai, Aurelia coerulea, and Rhopilema esculentum
- Journal of Oceanology and Limnology, 38(6): 1755-1761
- http://dx.doi.org/10.1007/s00343-019-9079-8
Article History
- Received Mar. 22, 2019
- accepted in principle Jun. 30, 2019
- accepted for publication Oct. 25, 2020
2 Laboratory of Marine Ecology and Environmental Sciences, Qingdao National Laboratory for Marine Science and Technology, Qingdao 266071, China;
3 University of Chinese Academy of Sciences, Beijing 100049, China;
4 Center for Ocean Mega-Science, Chinese Academy of Sciences, Qingdao 266071, China;
5 Jiaozhou Bay Marine Ecosystem Research Station, Chinese Academy of Sciences, Qingdao 266071, China
In recent decades, jellyfish blooms are reported frequently from many coastal waters (Purcell et al., 2007; Pauly et al., 2009; Uye, 2014). Massive blooms of the giant jellyfish Nemopilema nomurai (Scyphozoa: Rhizostomea) have been observed in the Bohai Sea, Yellow Sea, and East China Sea every year since 2002, except for 2008, 2010, and 2011 (Dong et al., 2010; Kawahara et al., 2013; Sun et al., 2015b). The moon jellyfish Aurelia aurita sp. l. (Linnaeus) is a species of worldwide distribution in inner neritic waters between 70°N and 40°S (Lucas, 2001). The species found in China is A. coerulea (Feng et al., 2017), and it mainly occurs along the tourist coasts and coastal aquaculture regions (Dong et al., 2010). Blooms of these two species have had negative impacts on tourism, fisheries, coastal industries, and the marine ecosystem (Purcell et al., 2007; Uye, 2008). Blooming is thought to be related to climate change, overfishing, eutrophication, hypoxia, agriculture, and increasing amounts of anthropogenic hard substrate (Purcell et al., 2007; Purcell, 2012; Sun et al., 2015a). Another species common to China is the edible jellyfish, Rhopilema esculentum (Scyphozoa: Rhizostomea). It is one of the most important fishery species in China (Dong et al., 2014), and it inhabits a wide range in the Bohai Sea, Yellow Sea, East China Sea, and northern South China Sea (You et al., 2007).
The life cycle of the scyphozoan jellyfish consists of a pelagic sexual medusa stage and a benthic asexual polyp stage. Sexual fertilization occurs in the female or in the open seawater (Dong et al., 2008; Schiariti et al., 2012) and fertilized eggs develop into freeswimming planulae, which settle on hard substrate and metamorphose into sessile polyps (Van Walraven et al., 2016). The polyps are perennials (Lucas, 2001), and asexual strobilation of the sessile polyps directly determines the population dynamics of the medusa. Therefore, increasing attention is being paid to the important benthic polyp stage (Kawahara et al., 2006; Hoover and Purcell, 2009; Feng et al., 2015a; Lee et al., 2017). However, the polyps of many species of jellyfish have never been found in the wild (Ceh and Riascos, 2017; Feng et al., 2017), and they are known only from experiments using sexual and asexual propagation.
Considering the overlap of spatial distribution of the medusa stage of the three scyphozoan jellyfish species common to Chinese coastal seas, it is possible that the polyps of each species develop near each other. The polyps of A. aurita can reproduce by budding, fission, or podocysts (Thein et al., 2012), and the polyps of N. nomurai and R. esculentum reproduce mainly by podocysts (Dong et al., 2013; Feng et al., 2015b), which produce colonies of millions of individuals that extend the spatial distribution of the polyps. After settlement, the polyps are surprising mobile, using stolons to 'walk' around different surfaces or detaching and floating to new locations (Hoover and Purcell, 2009). As a result, it is likely that the polyps of different species encounter each other.
The polyps of Aurelia sp. l. have been found on natural substrates, such as bare rock, shells, amphipod and polychaete tubes, ascidians, and macroalgae and on artificial substrates, such as glass, ceramic, or plastic pieces in the sea (Lucas, 2001). The medusae of N. nomurai also breed and form polyps along coastal waters, but the polyps of N. nomurai have not yet been found in the field (Toyokawa et al., 2012). However, Feng et al. (2015a) suggested that the Changjiang River estuary and northern Liaodong Bay might be their nursery ground. Confirming the nursery ground of N. nomurai remains one of the most important topics of the Chinese National Basic Research Project (Sun et al., 2015a). The polyps of R. esculentum have not been found in the field, despite numerous field surveys along Chinese coastal waters (Dong et al., 2013). Why the habitat of polyps of these two species remains unknown is still a puzzle (Feng et al., 2017).
To better understand the dynamics of jellyfish blooms, it is essential to understand the intraguild predation relationship of polyps of the most common scyphozoan jellyfish species in the Bohai Sea, Yellow Sea, and East China Sea; determine their distribution patterns; and identify possible natural habitats that may host wild polyps of N. nomurai and R. esculentum. To our knowledge, no previous studies have focused on the intraguild predation relationship of the polyp stage of these jellyfish species at the individual level.
2 MATERIAL AND METHOD 2.1 MaterialMature medusae of N. nomurai (6 females, 4 males) were captured by hand nets in Jiaozhou Bay in September 2013 and then transferred to a 30-m3 pond for fertilization in the laboratory of the Institute of Oceanology, Chinese Academy of Sciences, Qingdao. The rearing temperature and salinity were maintained at 20±0.5℃ and 30±0.5, respectively. After planulae were detected, parent medusae were removed and polyethylene corrugated plates were placed in the pond for attachment. Polyps of N. nomurai (abbreviated to NP hereafter) with two to four tentacles formed on the plates within a week and developed fully to polyps with 16 tentacles one month later. Polyps of A. coerulea (abbreviated to AP hereafter) were also cultured at the Institute of Oceanology, Chinese Academy of Sciences, Qingdao. Polyethylene corrugated plates were used for attachment of planulae produced by mature medusae of A. coerulea collected from nearby Jiaozhou Bay in May 2014. Fully developed polyps of A. coerulea were formed after a month at 20±0.5℃ and 30±0.5 in sand-filtered seawater. Polyps of R. esculentum (abbreviated to RP hereafter) were bred at Yancheng Jinyang Aquatic Products Breeding Company in Jiangsu, China, in June 2014. Polyps of the three species were fed with newly hatched Artemia nauplii for 1–2 h every 3 d, and freshly sand-filtered seawater was used for replacement every 2 d before the experiment. The cultivation temperature and salinity were maintained at 18±0.5℃ and 30±0.5, respectively.
2.2 MethodIn the field, the medusae of the three species become mature at different periods of time (Wang, 2013; Dong et al., 2014; Sun et al., 2015b), indicating that the attachment sequence of the polyps would differ among different species. After formation, the body size of the polyps also differs among different species. To approximately simulate the encounter situation of the polyps of the three species in the field, polyps of each species were allowed to attach first, then they were fed polyps of another species. Additionally, the polyp size of the first species, relative to the second species, was experimentally manipulated.
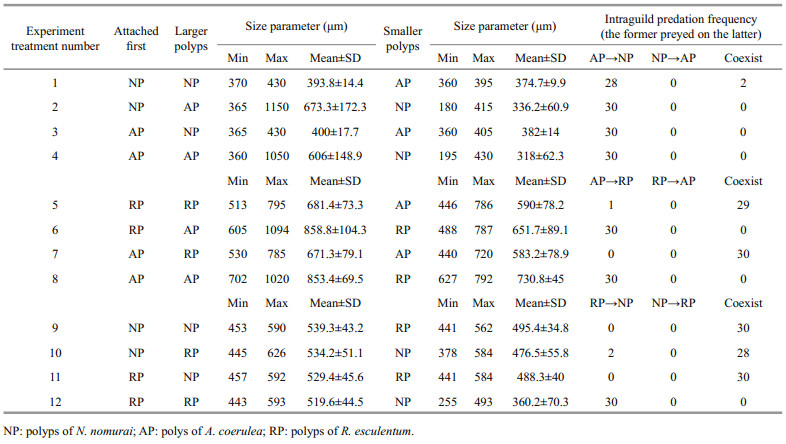
Fully developed polyps with 16 tentacles were used in the experiment. For each case, the polyps of one species were detached carefully from the root of the pedal disc with a dissecting needle under a dissecting microscope. Ten undamaged polyps were transferred to one well of a six-well cell culture plate containing 15-mL of freshly filtered seawater. After 2-d of acclimation without food in an incubator (18℃), all polyps were able to reattach. Ten polyps of another species, also detached carefully from the settle plates, then were collected into a capillary pipette and released within the tentacles of each polyp of the former species. The calyx diameter of all polyps was measured with the ocular micrometer, and the predation reactions of each polyp and its final state after 5 d were observed. Because the calyxes of the polyps were not uniformly circular, the approximate diameter was calculated by the average of maximum and minimum diameters. Twelve situations were tested, with three replicates containing 30 pairs of polyps in total per situation (Table 1). For every replicate, the predation percentage was recorded as follows:

The experiment was conducted in a temperature controlled room (18±0.5℃). The seawater in the culture plates was cautiously replaced with freshly filtered seawater of the same temperature and salinity daily. The experiment began on September 23, 2014 and lasted for 50 d.
2.3 Overview of the sea areaThe sea surface temperature and average salinity of the Bohai Sea, Yellow Sea, and East China Sea ranges from -1.5 to 29.7℃ and 30 to 34, respectively. The major ocean currents include the Kuroshio, Yellow Sea Warm Current, Yellow Sea Cold Water Mass, Bohai Sea circulation, and longshore current. The area is characterized by a monsoon climate, which mainly includes a warm temperate monsoon and a subtropical monsoon climate. The most remarkable feature of the topography is the wide stretch of continental shelf, which tilts from northwest to southeast (Su, 2005). The seafloor mainly consist of argillaceous sediment in the nearshore area and arenaceous sediment in the central and offshore areas (Liu, 1992).
2.4 Statistical analysisBinary logistic regression using SPSS 23.0 was used to test the following three null hypotheses, and GraphPad Prism 8 was used to plot the results:
H01:whether or not AP preyed on NP was not related to attachment sequence and size relationship;
H02: whether or not AP preyed on RP was not related to attachment sequence and size relationship;
H03: whether or not RP preyed on NP was not related to attachment sequence and size relationship.
3 RESULTNeither asexual reproduction nor cyst formation was observed in any treatment during 5 d under the given experimental condition. Polyps of A. coerulea preyed on polyps of N. nomurai in all treatments containing this pair (Fig. 1a) and digested them into white flocculent residues, some of which were released from the A. coerulea mouth, within 24 h. The calyx diameter of polyps of N. nomurai and A. coerulea were 180–430 μm and 360–1 150 μm, respectively. AP predation on NP did not differ significantly with attachment sequence or size relationship (Table 2); thus, H01 was not refused.
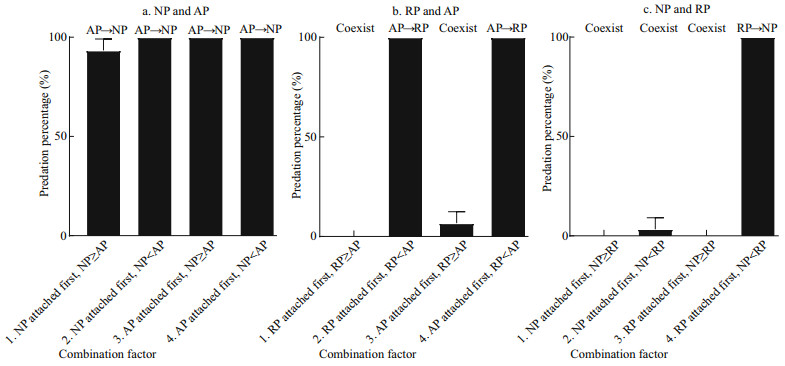
|
| Fig.1 Mean predation percentage of polyps (mean±SD) for two attachment sequences and two size relationships of calyx diameter a. polyps of N. nomurai and A. coerulea; b. polyps of R. esculentum and A. coerulea; c. polyps of N. nomurai and R. esculentum. The former preyed on the latter. |

|
Polyps of A. coerulea preyed on polyps of R. esculentum when polyps of A. coerulea were larger than polyps of R. esculentum and digested them into white flocculent residues within 24 h, regardless of which polyp species attached first. However, the two species could coexist when the polyps of A. coerulea were smaller than the polyps of R. esculentum for more than 5 d (Fig. 1b). The calyx diameter of polyps of R. esculentum and A. coerulea were 488–795 μm and 440–1 094 μm, respectively. AP predation on RP differed depending on calyx size (Table 2), and therefore, H02 was rejected. Further analysis of the variables revealed that the predation reaction difference was caused by the calyx size relationship of RP and AP (Table 2).
The polyps of R. esculentum preyed on polyps of N. nomurai only when RP attached first and the calyx diameter of RP was larger than that of NP. Under these conditions, RP digested NP into white flocculent residues within 24 h. When these condition were not present, polyps of the two species could coexist for more than 5 d (Fig. 1c). The calyx diameter of polyps of N. nomurai and R. esculentum were 255–592 μm and 441–626 μm, respectively. RP predation on NP differed depending on conditions (Table 2), thus H03 was rejected. Further analysis of the variables revealed that the predation reaction difference was caused by both the attachment sequence and the calyx size relationship of NP and AP (Table 2).
4 DISCUSSIONIn this study, the polyps of A. coerulea preyed on the polyps of N. nomurai. When AP attached first, NP were captured by the tentacles of AP and placed in the mouth of the polyps within a few hours. When NP attached first, polyps of both species exhibited the capture reaction of the tentacles, but ultimately AP were able to turn upside down, swallow NP, and digest them into white flocculent residues within 24 h. The calyx diameter size relationship of AP and NP did not significantly affect the predation reaction. This finding is not unique among cnidarians, as Kaliszewicz (2013) concluded that larger size did not guarantee competitive superiority among hydras, which are also sessile sit-and-wait predators. Our data showed that the newly formed polyps of N. nomurai were preyed upon by the polyps of A. coerulea, even when the A. coerulea polyps were present for only a short time. Even when the colony of polyps of N. nomurai formed first, the newly formed A. coerulea polyps preyed on them and took over the living space. These results indicate that colonies of N. nomurai polyps could not survive in locations inhabited by A. coerulea.
The polyps of A. coerulea are distributed widely in coastal waters (Malej et al., 2012). Our scuba diving group found A. coerulea polyps along the coast of Shandong Province (Qingdao), Liaoning Province (Dalian), and Hebei Province (Qinghuangdao), and this distribution might explain why it is so difficult to find N. nomurai polyps in the field. Russell (1970) reported that A. aurita polyps were not found deeper than 20 m, and a variety of in situ experiments carried out at depths between 0.3 m and 25 m reported the same finding (Brewer, 1978; Hernroth and Gröndahl, 1985; Keen, 1987; Feng et al., 2017). Relative to the medusae of A. aurita, which occur mainly in nearshore waters (Dong et al., 2012; Wan and Zhang, 2012) and are rare in deep waters (Zhang et al., 2012), N. nomurai is a deep sea species that shows diurnal vertical movement and migration between depths of over 100 m and the surface (Honda et al., 2009). Consequently, we speculate that the polyps of N. nomurai might be located in their natural habitat at a depth >20 m in Chinese coastal waters or in locations where polyps of A. coerulea are not present. Similarly, Ceh and Riascos (2017) proposed that the polyps of the scyphozoan jellyfish Chrysaora plocamiai, whose natural habitat remains unknown, might be located up to 20-m deep based on the results of planulae settlement preference for substrate color.
The results of our experiment showed that polyps of A. coerulea preyed on polyps of R. esculentum when the calyx diameter size of the former was larger than that of the latter. However, AP could not prey on RP when the calyx diameter of AP was smaller than that of RP; slight damage to the tentacles of RP was detected, but both polyp types survived and coexisted for more than 5 d. Similarly, Li et al. (2012) and Chi et al. (2013) reported that AP and RP could coexist for 24 h. Our results suggest that the ability of AP to prey on RP is determined by the calyx size relationship between the two polyp types, but overall AP is competitively superior to RP, even though colonies of the two species can coexist to some extent.
Polyps of R. esculentum and N. nomurai coexisted for more than 5 d in most cases, except when R. esculentum polyps attached first and when the calyx diameter was larger than that of N. nomurai. This result indicates that the polyps of R. esculentum are slightly more competitive than those of N. nomurai. It might be attributed to the same class and order (Scyphozoan: Rhizostomea) they shared and similar morphological characteristics (Liu et al., 2009).
The polyps of A. coerulea aggressively outcompeted polyps of other species, and this pattern might be true in other coastal areas of the world, especially in areas where blooms of different species of jellyfish co-occur. Intraguild predation by the polyps might be useful for the study of searching for polyps of other species in other areas as well.
5 CONCLUSIONIn this study, A. coerulea and R. esculentum polyps were able to coexist to some extents, and the same was true for R. esculentum and N. nomurai polyps. However, it was almost impossible for N. nomurai polyps to settle and form colonies in locations inhabited by A. coerulea. Considering the wide distribution of polyps of A. coerulea along Chinese coastal seas at a depth of less than 20 m, we propose that N. nomurai polyps might be located in the natural habitat at a depth of more than 20 m. Future studies should search for the polyps of N. nomurai at greater depths and confirm the location of nursery grounds. The results of this study offer valuable insights into competition between polyps of different co-occurring species of jellyfish that likely will be applicable to other locales.
6 DATA AVAILABILITY STATEMENTAll data generated and/or analyzed during this study are available upon request by contacting with the corresponding author.
Brewer R H. 1978. Larval settlement behavior in the jellyfish Aurelia aurita (Linnaeus) (Scyphozoa: Semaeostomeae). Estuaries, 1(2): 120.
DOI:10.2307/1351601 |
Ceh J, Riascos J M. 2017. Cryptic life stages in scyphozoan jellyfish: larval settlement preferences of the South American sea nettle Chrysaora plocamia. Journal of Experimental Marine Biology and Ecology, 490: 52-55.
DOI:10.1016/j.jembe.2017.02.007 |
Chi X P, You K, Ma C H, Yuan Y H, Chen S Q, Yang Y F, Liu X T. 2013. Preliminary study on the competition in the larvae stage between Aurelia aurita and Rhopilema esculentum during a short term. Acta Oceanologica Sinica, 35(6): 140-146.
(in Chinese with English abstract) |
Dong J, Jiang L X, Sun M, Wang B, Li Y L, Tan K F, Chai Y, Sun S. 2013. Research on the Biological of Large Jellyfish in the Bohai Sea and Northern Yellow Sea. Ocean Press, Beijing.
(in Chinese)
|
Dong J, Jiang L X, Tan K F, Liu H Y, Purcell J E, Li P J, Ye C C. 2008. Stock enhancement of the edible jellyfish (Rhopilema esculentum Kishinouye) in Liaodong Bay, China: a review. Hydrobiologia, 616(1): 113-118.
|
Dong Z J, Liu D Y, Keesing J K. 2010. Jellyfish blooms in China:dominant species, causes and consequences. Marine Pollution Bulletin, 60(7): 954-963.
DOI:10.1016/j.marpolbul.2010.04.022 |
Dong Z J, Liu D Y, Keesing J K. 2014. Contrasting trends in populations of Rhopilema esculentum and Aurelia aurita in Chinese waters. In. Pitt P A, Lucas C H eds. Jellyfish Blooms. Springer, Dordrecht. p.207–218.
|
Dong Z J, Liu D Y, Wang Y J, Di B P, Song X K, Shi Y J. 2012. A report on a moon jellyfish Aurelia aurita bloom in Sishili Bay, northern Yellow Sea of China in 2009. Aquatic Ecosystem Health & Management, 15(2): 161-167.
|
Feng S, Wang S W, Sun S, Zhang F, Uye Si. 2017. Strobilation of three scyphozoans (Aurelia coelurea, Nemopilema nomurai and Rhopilema esculentum) in the field at Jiaozhou Bay, China. Marine Ecology Progress Series, 591: 141-153.
|
Feng S, Wang S W, Zhang G T, Sun S, Zhang F. 2017. Selective suppression of in situ proliferation of scyphozoan polyps by biofouling. Marine Pollution Bulletin, 114(2): 1 046-1 056.
DOI:10.1016/j.marpolbul.2016.10.062 |
Feng S, Zhang F, Sun S, Wang S W, Li C L. 2015a. Effects of duration at low temperature on asexual reproduction in polyps of the scyphozoan Nemopilema nomurai (Scyphozoa: Rhizostomeae). Hydrobiologia, 754(1): 97-111.
DOI:10.1007/s10750-015-2173-9 |
Feng S, Zhang G T, Sun S, Zhang F, Wang S W, Liu M T. 2015b. Effects of temperature regime and food supply on asexual reproduction in Cyanea nozakii and Nemopilema nomurai. Hydrobiologia, 754(1): 201-214.
DOI:10.1007/s10750-015-2279-0 |
Hernroth L, Gröndahl F. 1985. On the biology of Aurelia aurita (L.) 3. Predation by Coryphella verrucosa (gastropoda, opisthobranchia), a major factor regulating the development of Aurelia populations in the Gullmar Fjord, western Sweden. Ophelia, 24(1): 37-45.
DOI:10.1080/00785236.1985.10426617 |
Honda N, Watanabe T, Matsushita Y. 2009. Swimming depths of the giant jellyfish Nemopilema nomurai investigated using pop-up archival transmitting tags and ultrasonic pingers. Fisheries Science, 75(4): 947-956.
DOI:10.1007/s12562-009-0114-0 |
Hoover R A, Purcell J E. 2009. Substrate preferences of scyphozoan Aurelia labiata polyps among common dockbuilding materials. Hydrobiologia, 616(1): 259-267.
DOI:10.1007/s10750-008-9595-6 |
Kaliszewicz A. 2013. Is larger better in sit-and-wait predators? Competitive superiority in Hydra. Hydrobiologia, 714(1): 105-114.
DOI:10.1007/s10750-013-1527-4 |
Kawahara M, Ohtsu K, Uye S I. 2013. Bloom or non-bloom in the giant jellyfish Nemopilema nomurai (Scyphozoa: Rhizostomeae): roles of dormant podocysts. Journal of Plankton Research, 35(1): 213-217.
DOI:10.1093/plankt/fbs074 |
Kawahara M, Uye S I, Ohtsu K, Iizumi H. 2006. Unusual population explosion of the giant jellyfish Nemopilema nomurai (Scyphozoa: Rhizostomeae) in East Asian waters. Marine Ecology Progress Series, 307: 161-173.
DOI:10.3354/meps307161 |
Keen S L. 1987. Recruitment of Aurelia aurita (Cnidaria: Scyphozoa) larvae is position-dependent, and independent of conspecific density, within a settling surface. Marine Ecology Progress Series, 38: 151-160.
DOI:10.3354/meps038151 |
Lee H E, Han C H, Kim B, Yoon W D. 2017. Effects of temperature and salinity on the asexual reproduction of Nemopilema nomurai (Scyphozoa: Rhizostomeae). Ocean Science Journal, 52(4): 573-579.
DOI:10.1007/s12601-017-0040-5 |
Li Z F, Liu C S, Zhuang Z M, Zou A G, Chen S Q, Yan J P, Liu C L. 2012. The mutual predatory relationship of jellyfishes in different life stages between Aurelia sp.1 and Rhopilema esculenta. Oceanologia et Limnologia Sinica, 43(3): 539-544.
|
Liu C Y, Wang W B, Dong J, Wang Y Q, Zhou H, Sun M, Lu Z C, Yu X G. 2009. The life history of Rhopilema hispidum and comparison of morphological characteristics among some scyphistomae. Progress in Fishery Sciences, 30(4): 102-107.
(in Chinese with English abstract) |
Liu X Q. 1992. Map of the newest bottom-material types of china offshore continental shelf. Marine Geology & Quaternary Geology, 12(4): 11-20.
(in Chinese with English abstract) |
Lucas C H. 2001. Reproduction and life history strategies of the common jellyfish, Aurelia aurita, in relation to its ambient environment. Hydrobiologia, 451(1-3): 229-246.
|
Malej A, Kogovšek T, Ramšak A, Catenacci L. 2012. Blooms and population dynamics of moon jellyfish in the northern Adriatic. Cahiers de Biologie Marine, 53(3): 337-342.
|
Pauly D, Graham W, Libralato S, Morissette L, Palomares M L D. 2009. Jellyfish in ecosystems, online databases, and ecosystem models. Hydrobiologia, 616(1): 67-85.
DOI:10.1007/s10750-008-9583-x |
Purcell J E, Uye S I, Lo W T. 2007. Anthropogenic causes of jellyfish blooms and their direct consequences for humans:a review. Marine Ecology Progress Series, 350: 153-174.
DOI:10.3354/meps07093 |
Purcell J E. 2012. Jellyfish and ctenophore blooms coincide with human proliferations and environmental perturbations. Annual Review of Marine Science, 4: 209-235.
DOI:10.1146/annurev-marine-120709-142751 |
Russell F S. 1970. The Medusae of the British Isles II. Pelagic Scyphozoa, with a Supplement to the First Volume of Hydromedusae. Cambridge University Press, New York. 284p.
|
Schiariti A, Christiansen E, Morandini A C, Da Silveira F L, Giberto D A, Mianzan H W. 2012. Reproductive biology of Lychnorhiza lucerna (Cnidaria: Scyphozoa: Rhizostomeae): Individual traits related to sexual reproduction. Marine Biology Research, 8(3): 255-264.
DOI:10.1080/17451000.2011.616897 |
Su J L. 2005. Offshore Hydrology of China. Ocean Press, Beijing.
(in Chinese)
|
Sun S, Sun X X, Jenkinson I R. 2015a. Preface:giant jellyfish blooms in Chinese waters. Hydrobiologia, 754(1): 1-11.
DOI:10.1007/s10750-015-2320-3 |
Sun S, Zhang F, Li C L, Wang S W, Wang M X, Tao Z C, Wang Y T, Zhang G T. 2015b. Breeding places, population dynamics, and distribution of the giant jellyfish Nemopilema nomurai (Scyphozoa: Rhizostomeae) in the Yellow Sea and the East China Sea. Hydrobiologia, 754(1): 59-74.
DOI:10.1007/s10750-015-2266-5 |
Thein H, Ikeda H, Uye S I. 2012. The potential role of podocysts in perpetuation of the common jellyfish Aurelia aurita s.l. (Cnidaria: Scyphozoa) in anthropogenically perturbed coastal waters. Hydrobiologia, 690(1): 157-167.
DOI:10.1007/s10750-012-1045-9 |
Toyokawa M, Shibata M, Cheng J H, Li H Y, Ling J Z, Lin N, Liu Z L, Zhang Y. 2012. First record of wild ephyrae of the giant jellyfish Nemopilema nomurai. Fisheries Science, 78(6): 1 213-1 218.
DOI:10.1007/s12562-012-0550-0 |
Uye S I. 2008. Blooms of the giant jellyfish Nemopilema nomurai: a threat to the fisheries sustainability of the East Asian Marginal Seas. Plankton and Benthos Research, 3(S1): 125-131.
|
Uye S I. 2014. The giant jellyfish Nemopilema nomurai in East Asian Marginal Seas. In. Pitt P A, Lucas C H eds. Jellyfish Blooms. Springer, Dordrecht. p.185–205.
|
Van Walraven L, Driessen F, Van Bleijswijk J, Bol A, Luttikhuizen P C, Coolen J W P, Bos O G, Gittenberger A, Schrieken N, Langenberg V T, Van Der Veer H W. 2016. Where are the polyps? Molecular identification, distribution and population differentiation of Aurelia aurita jellyfish polyps in the southern North Sea area. Marine Biology, 163(8): 172.
DOI:10.1007/s00227-016-2945-4 |
Wan A Y, Zhang G T. 2012. Annual occurrence of moon jellyfish Aurelia sp.1 in the Jiaozhou Bay and its impacts on zooplankton community. Oceanologia et Limnologia Sinica, 43(3): 494-501.
|
Wang Y T, Zheng S, Sun S, Zhang F. 2015. Effect of temperature and food type on asexual reproduction in Aurelia sp.1 polyps. Hydrobiologia, 754(1): 169-178.
DOI:10.1007/s10750-014-2020-4 |
Wang Y T. 2013. Study on the Key Process of Life Cycle of Aurelia coerulea, Institute of Oceanology. Chinese Academy of Sciences: 9-10.
(in Chinese) |
You K, Ma C H, Gao H W, Li F Q, Zhang M Z, Qiu Y T, Wang B. 2007. Research on the jellyfish (Rhopilema esculentum Kishinouye) and associated aquaculture techniques in China: current status. Aquaculture International, 15(6): 479-488.
DOI:10.1007/s10499-007-9114-1 |
Zhang F, Sun S, Jin X S, Li C L. 2012. Associations of large jellyfish distributions with temperature and salinity in the Yellow Sea and East China Sea. Hydrobiologia, 690(1): 81-96.
DOI:10.1007/s10750-012-1057-5 |
 2020, Vol. 38
2020, Vol. 38