Shanghai University
Article Information
- Fatima REHMAN, Muhammad FAISAL
- Toxic hexavalent chromium reduction by Bacillus pumilis, Cellulosimicrobium cellulans and Exiguobacterium
- Chinese Journal of Oceanology and Limnology, 2015, 33(3): 585-589
- http://dx.doi.org/10.1007/s00343-015-4155-1
Article History
- Received May 28, 2014
- accepted in principle Sep. 19, 2014;
- accepted for publication Oct. 10, 2014
Anthropogenic activities lead to the introduction ofheavy metal pollutants into the environment(Das and Mishra, 2010). Signifi cant environmental harm, ofglobal concern, is caused by non-degradable, toxicheavy metals introduced by direct or secondarydisposal, particularly when at high concentration(Agrafioti et al., 2014). Chromium(Cr)is widely usedin textile dyeing, wood treatment, electroplating, corrosion inhibition and tanning, and results in twosignifi cant oxidation states hexavalent and trivalentchromium in industrial discharges(Tahri Joutey et al., 2014). The removal of high concentrations of heavymetal pollutants from liquid wastes by ion-exchange, chemical precipitation, membrane processes, electroplating and evaporation are expensive and oflimited effi cacy(Loukidou et al., 2004; Preetha and Viruthagiri, 2005). Reference levels of 0.5 mg/L Cr(WHO)cannot be reduced by the above methods(Onyancha et al., 2008).
Biosorption is more cost-effective, practicable and environmentally friendly, and has gained interest forthe removal of toxic metals from dilute aqueoussolution(Romera et al., 2007). One importantpotential for remediation of environmental metalpollution uses alternatives to existing methods, including the use of several microorganisms asbiosorbents for metal binding by living as well asdead algae(Horsfall et al., 2004; Han et al., 2007).Bacteria, algae, yeast and fungi have been investigatedfor heavy metals removal(Naja et al., 2005). Severalbacterial strains have the ability to convertcarcinogenic hexavalent chromium into less toxic and immobile trivalent chromium which can be recovered and reused. Keeping this in view, we will use thechromium-resistant bacterial strains Bacillus pumilus -CrK08, Cellulosimicrobium cellulans -CrK16 and Exiguobacterium -CrK19 for the reduction of CR(VI)to CR(III)under various environmental conditionsi.e., pH, incubation time, temperature, other metallicions and media. 2 MATERIAL AND METHOD 2.1 Bacterial strains and culture conditions
Three chromium-resistant bacterial strains Bacilluspumilus -CrK08, Cellulosimicrobium cellulans -CrK16 and Exiguobacterium -CrK19 were isolatedfrom chromium-contaminated tannery effluent.Various physico-chemical properties of soil sampleswere also analyzed. Initially, strains were streaked onLuria Bertani agar medium(1% NaCl, 1% tryptone, 0.5% yeast extract and 1.5% agar) and their minimuminhibitory concentration(MIC)was checked atvarious concentrations of K2CrO4. Strains wereroutinely cultured and maintained on Luria Bertaniagar plates(pH 7)at 37 °C amended with 500 μg/mLof K2CrO4 and stored at 4°C. 2.2 Cr(VI)reduction experiments
For the estimation of Cr(VI)reduction ability ofthese bacterial strains, DeLeo and Ehrlich medium(1994)(g/L: tryptone, 10.0; yeast extract, 5.0; NaCl, 5.0; citric acid, 1.0; Na2HPO4, 6.9)was used. Themedium was supplemented with two initial K2CrO4 concentrations, 200 and 400 μg/mL, and wereinoculated and incubated overnight at 37°C withshaking at 150 r/min. For analysis of Cr(VI)reduction, samples were taken aseptically after 24 h of incubation and centrifuged(12 000 r/min for 10 min). Supernatantwas taken and then processed for the analysis ofhexavalent chromate reduced usingDiphenylcarbazide. Diphenylcarbazide reacted withhexavalent chromium present in the supernatant ofthe cultures in an acidic solution of phosphoric acid.Absorption was measured at 540 nm using a st and ardspectrophotometric method(Clesceri et al., 1998).
Reduction of toxic hexavalent chromium was alsomonitored at 200 and 400 μg/mL by optimizing atvarious temperature, pH and time duration and inpresence of other metallic ions. Chromate reductionagainst other metallic ions was assessed by using lowconcentrations 50 μg/mL each of arsenate, cadmium and cobalt. The effect of Lauria Bertani medium onCr(VI)reduction was checked by using Luria Bertanimedium at two initial K2CrO4 concentrations, 200 and 400 μg/mL. All these experiments were run in triplicate. 3 RESULT
Three bacterial strains(Bacillus pumilus-CrK08, Cellulosimicrobium cellulans -CrK16, Exiguobacterium -CrK19)already isolated, characterized and identifi ed, were used in the present study to reduce the toxichexavalent chromium to the less toxic trivalentchromium. The MIC for strains Cellulosimicrobiumcellulans -CrK16 and Exiguobacterium -CrK19 was8 mg/mL while it was 5 mg/mL for Bacillus pumilus -CrK08. The strains were isolated from chromiumcontaminatedtannery waste at Kasur, Pakistanlatitude(N)3 106′18.53′′ and longitude(E)7 427′3417′′. The pH of a sample of this material was6.4 and the organic matter content was 7.9±0.023%.The Cr(VI)content was 4.21±0.043 μg/g. 3.1 Cr(VI)reduction
Initially the chromate reduction by three bacterialstrains i.e. Bacillus pumilus -CrK08, Cellulosimicrobium cellulans -CrK16 and Exiguobacterium -CrK19 at two different initialK2CrO4 concentrations, 200 and 400 μg/mL, wasmeasured(Fig. 1). Maximum reduction was shownby Bacillus pumilus -CrK08 i.e., 51% at 200 μg/mL and 24% at 400 μg/mL after 24 h of incubation at37°C(Fig. 1). The strains Cellulosimicrobiumcellulans -CrK16 and Exiguobacterium -CrK19reduced 49 and 39% of chromate after 24 h wheninitially supplied with 200 μg/mL, respectively(Fig. 1).
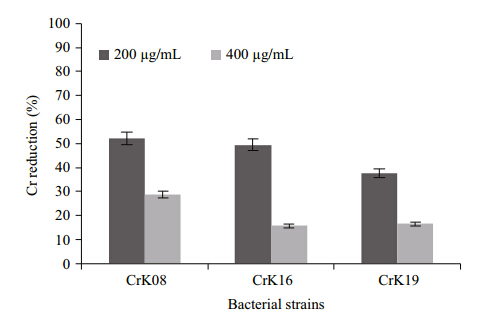 |
| Fig. 1 Chromium reduction by three bacterial strains, CrK08, CrK16 and CrK19, at an initial chromateconcentration of 200 and 400 μg/mL after 24 h ofincubation at 37°C |
Figure 2 shows Cr(VI)reduction by these strainsat various pH(3, 5, 7 and 9)when initiallysupplemented with 200 and 400 μg/mL at 37°C. Theoptimum pH was found to be 7 where strainCellulosimicrobium cellulans -CrK16 reduced Cr(VI)91% at 200 μg/mL and 54.9% at 400 μg/mL after 24 h of incubation. Strains Bacillus pumilus -CrK08reduced by 84% and 55% at initial chromateconcentrations of 200 and 400 μg/mL, respectively, while the reduction percentages were 77% and 46%for Exiguobacterium -CrK19.
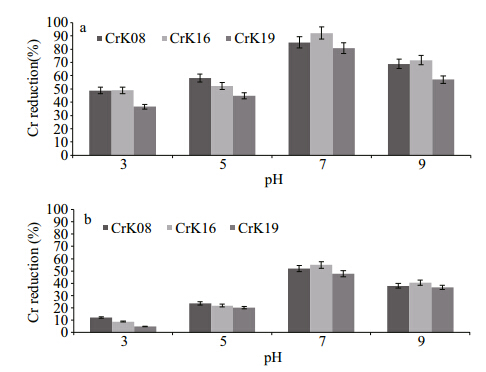 |
| Fig. 2 Hexavalent chromium reduction by three bacterialstrains at pH 3, 5, 7 and 9 when initially supplied with(a)200 and (b)400 μg/mL of potassium chromateafter 24 h of incubation at 37°C |
Inoculated reduction medium supplemented withtwo initial hexavalent chromium concentrations of200 and 400 μg/mL was incubated at 37°C at threetime intervals, 24, 48 and 72 h. The strain Bacilluspumilus -CrK08 showed 64% and 43% Cr(VI)reduction after 72 h at initial Cr(VI)concentrations of200 and 400 μg/mL, respectively(Fig. 3a, b). Almostthe same trends were observed in other two strains, Cellulosimicrobium cellulans -CrK16 and Exiguobacterium -CrK19, after 72 h of incubation.
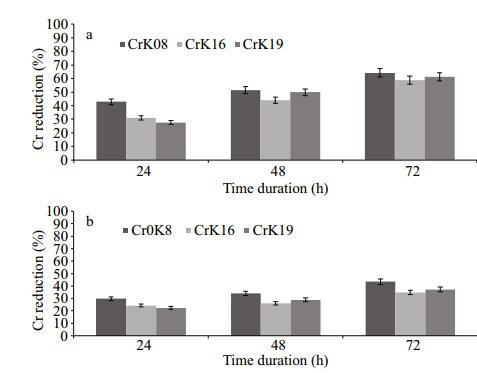 |
| Fig. 3 Hexavalent chromium reduction by three bacterialstrains at various time duration(24, 48 and 72 hrs)when initially supplied with(a)200 and (b)400 μg/mL of potassium chromate at 37°C |
Three varying temperatures, 25, 37, and 45°C, were chosen at two different chromium concentrations, 200 and 400 μg/mL. Figure 4 shows that maximumCr(VI)reduction of 92%, 78%, and 72% was observedat 37°C by strains Bacillus pumilus -CrK08, Cellulosimicrobium cellulans -CrK16 and Exiguobacterium -CrK19, respectively after 24 hincubation period at an initial chromate concentrationof 200 μg/mL(Fig. 4a). The reduction by these strainswas decreased at both 25°C and 45°C compared tothat at 37°C(Fig. 4a, b).
 |
| Fig. 4 Hexavalent chromium reduction by three bacterialstrains at temperatures 25, 37 and 45°C, wheninitially supplied with(a)200 and (b)400 μg/mL ofpotassium chromate after 24 h of incubation |
As the industrial effluent contains many othermetallic salts along with the target metal(hexavalentchromium), the strains should resist these othermetallic salts as well as any antibiotics when used foreffluent treatment. Figure 5 shows that the presenceof the metallic ions Co2+, Ni2+, and As3-(50 μg/mLeach)have no major impact on Cr(VI)reduction bythese strains when given 200 and 400 μg/mL ofK2CrO4 . The strain Cellulosimicrobium cellulans -CrK16 showed maximum reduction of 34% at 200 μg/mL but only 27% at 400 μg/mL after 24 h incubationat 37°C. In the same time period the strainExiguobacterium -CrK19 was able to convert 34% and 18% of chromate supplied i.e., 200 and 400 μg/mL, respectively(Fig. 5).
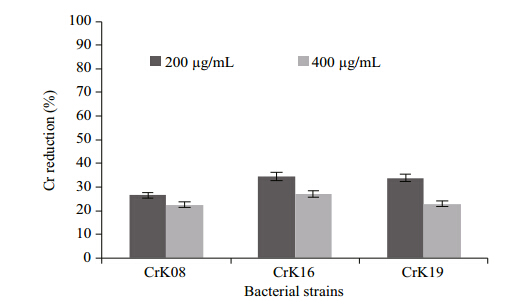 |
| Fig. 5 Chromate reduction by three strains in the presence ofthree metallic ions Ar, Co, and Cd at a concentrationof 50 μg each after 24 h of incubation at 37°C wheninitially supplied with 200 and 400 μg/mL |
Bacterial strains grown in enriched medium i.e.L-broth supplemented with 200 and 400 μg/mL ofchromate in contrast to the DeLeo and Ehrlichmedium, did not show a marked impact on Cr(VI)reduction. The strain Bacillus pumilus -CrK08 showedmaximum a reduction of 54% at 200 μg/mL and 19%at 400 μg/mL after 24 h at 37°C(Fig. 6). StrainsCellulosimicrobium cellulans -CrK16 and Exiguobacterium -CrK19 reduced 21% and 32% ofchromate when supplemented with 200 and 400 μg/mL, respectively.
 |
| Fig. 6 Hexavalent chromium reduction by three bacterialstrains in L. broth after 24 h of incubation at 37°Cwhen initially supplied with 200 and 400 μg/mL |
A serious environmental problem is the presence oftoxic and carcinogenic hexavalent chromium and itsseepage and leaching from the soil into the groundwater at industrial sites(Zayed and Terry, 2003). Apotential substitute to the conventional chemicalmethods of bioremediation is conversion of toxic Cr(VI)to less toxic trivalent chromium(Mukherjee et al., 2014).
The present study deals with chromate reductionby three chromium resistant bacterial strains, Bacilluspumilus -CrK08, Cellulosimicrobium cellulans -CrK16 and Exiguobacterium -CrK19. These strainshave the highest MIC values as compared to otherstrains reported in the literature. Some of the isolatestolerated Cr(VI)with MIC values >600 μg/mLwhereas the majority showed an MIC value of<600 μg/mL Cr(VI)(Pal and Paul, 2004). Theresistance level against chromate was 500 μg/mL inthe case of strain Cr-10(Zhang and Li, 2011).Synechocystis sp. MK(S), a cyanobacterium, toleratedmore chromate, up to 250 μg/mL(Hameed and Hasnain, 2012).
Higher chromate reduction was observed at lowerinitial chromate concentration, 200 μg/mL. Similarresults were obtained in another study where due tohigher cell density chromium removal was maximumat a low concentration of Cr(VI), 25 μg/mL than athigher concentrations of hexavalent chromium(Essahale et al., 2012). As chromate concentrationincreases, chromium reduction by bacterial strainsdecreases, and vice versa. At higher initial chromatelevel the reduction was carried by enzymatic reactionin various bacterial strains(Thacker et al., 2006; Lee et al., 2008).
Maximum chromium reduction was observed at37°C in all the three strains at both initial chromateconcentrations of 200 and 400 μg/mL after 24 h ofincubation, but the reduction potential decreases atother temperatures, 25°C and 45°C. The same trendwas observed in the case of pH, where pH7 was theoptimum, and where maximum reduction wasobserved at both the initial chromate concentrationused. With increasing incubation time there, therewas a gradual increase in cell mass which leads toincreased reduction compared to at low cell mass atearly incubation time period. At an initial hexavalentchromium concentration of 100 μg/mL, after 144 hthe chromate reduction in Bacillus sp. was more than90%(Dhal et al., 2010).
The addition of other metallic ions had no majorimpact on the chromate reduction by these threestrains while Cellulosimicrobium cellulans -CrK16showed more reduction in the presence of thesemetallic ions. These metallic ions at low concentration help strain CrK16 to reduce more chromate in thesame time period at 200 and 400 μg/mL of chromate.In another study Faisal and Hasnain(2004)observedthat the addition of different metallic ions, Ni, Mn, Zn, Cu and Co, did not show any impact on thereduction by O. intermedium CrT-1. Bacterial strainsisolated from contaminated sites mainly showedmultiple metal and antibiotic resistances(Faisal and Hasnain, 2006). Metal resistance for Bacillus sp.JDM-2-1 and S . capitis was also observed at 4 000 μg/mL of Ni(II)(Zahoor and Rehman, 2009). Theoptimum pH for chromate reduction was found to be7. For Pseudomonas fluorescens and Bacillus sp. themaximal hexavalent chromium reduction wasobserved at a pH range of 7 to 8(Parameswari et al., 2009). Less chromate was reduced in L-broth mediumas compared to other reduction medium i.e., DeLeo and Ehrlich medium at pH7 and temperature 37°Cafter 24 h incubation period. 5 CONCLUSION
From the above results it can be concluded thatthese three strains have high chromate reductionpotential under the various environmental parametersinvestigated. Hence these strains are good c and idatesfor the removal of toxic chromate from industrialeffluent. This is a promising technology that can beapplied at industrial scale as it is inexpensive, effective, and harmless.
| Agrafioti E, Kalderis D, Diamadopoulos E. 2014. Arsenic and chromium removal from water using biochars derived from rice husk, organic solid wastes and sewage sludge. J.Environ. Manage., 133 : 309-314. |
| Das A P, Mishra S. 2010. Biodegradation of the metallic carcinogen hexavalent chromium Cr (VI) by an indigenously isolated bacterial strain. J. Carcinog., 9 (1): 6, http://dx.doi.org/10.4103/1477-3163.63584. |
| DeLeo P C, Ehrlich H L. 1994. Reduction of hexavalent chromium by Pseudomonas fluorescens LB 300 in batch and continuous cultures. Appl. Microbiol. Biotechnol., 40 : 756-759. |
| Dhal B, Thatoi H, Das N, Pandey B D. 2010. Reduction of hexavalent chromium by Bacillus sp. isolated from chromite mine soils and characterization of reduced product. J. Chem. Technol. Biotechnol., 85 (11): 1471-1479. |
| Essahale A, Malki M, Marín I, Moumni M. 2012. Hexavalent chromium reduction and accumulation by AcinetobacterAB1 isolated from Fez Tanneries in Morocco. Indian J.Microbiol., 52 (1): 48-53. |
| Faisal M, Hasnain S. 2004. Comparative study of Cr (VI) uptake and reduction in industrial effluent by Ochrobactrum intermedium and Brevibacterium sp.Biotechnol. Lett., 26 (21): 1 623-1 628. |
| Faisal M, Hasnain S. 2006. Growth stimulatory effect of Ochrobactrum intermedium and Bacillus cereus on Vigna radiata plants. Lett. Appl. Microbiol., 43 (4): 461-466. |
| Hameed A, Hasnain S. 2012. Isolation and molecular identification of metal resistant Synechocystis from polluted areas. Afr. J. Microbiol. Res., 6 (3): 648-652. |
| Han X, Wong Y S, Wong M H, Tam N F Y. 2007. Biosorption and bioreduction of Cr(VI) by a microalgal isolate,Chlorella miniata. J. Hazard. Mater., 146 (1): 65-72. |
| Horsfall M J, Arbia A, Spiff A. 2004. Removal of Cu (II) and Zn (II) ions from wastewater by cassava (Manihot esculenta Cranz) waste biomass. Afr. J. Biotechnol., 2 (10): 360-364. |
| Lee S, Lee J-U, Chon H, Lee J. 2008. Reduction of Cr (VI) by indigenous bacteria in Cr-contaminated sediment under aerobic condition. J. Geochem. Explor., 96 (2): 144-147. |
| Loukidou M X, Zouboulis A I, Karapantsios T D, Matis K A. 2004. Equilibrium and kinetic modeling of chromium (VI) biosorption by Aeromonas caviae. Colloid Surfac A, 242 (1): 93-104. |
| Mukherjee K, Nandi R, Saha D, Saha B. 2014. Surfactantassisted enhancement of bioremediation rate for hexavalent chromium by water extract of Sajina (Moringa oleifera) flower. Desalin. Water Treat, http://dx.doi.org/ 10.1080/19443994.2014.884477. |
| Naja G, Mustin C, Volesky B, Berthelin J. 2005. A highresolution titrator: a new approach to studying binding sites of microbial biosorbents. Water Res., 39 (4): 579-588. |
| Onyancha D, Mavura W, Ngila J C, Ongoma P, Chacha J. 2008. Studies of chromium removal from tannery wastewaters by algae biosorbents, Spirogyra condensata and Rhizoclonium hieroglyphicum. J. Hazard. Mater., 158 (2): 605-614. |
| Pal A, Paul A. 2004. Aerobic chromate reduction by chromiumresistant bacteria isolated from serpentine soil. Microbiol.Res., 159 (4): 347-354. |
| Parameswari E, Lakshmanan A, Thilagavathi T. 2009.Chromate resistance and reduction by bacterial isolates.Aus. J. Basic Appl. Sci., 3 (2): 1 363-1 368. |
| Preetha B, Viruthagiri T. 2005. Biosorption of zinc (II) by Rhizopus arrhizus : equilibrium and kinetic modelling.Afr. J. Biotechnol., 4 (6): 506-508. |
| Romera E, GonzÁlez F, Ballester A, BlÁzquez M, Munoz J. 2007. Comparative study of biosorption of heavy metals using different types of algae. Bioresource Technol., 98 (17): 3 344-3 353. |
| Tahri Joutey N, Bahafid W, Sayel H, Ananou S, El Ghachtouli N. 2014. Hexavalent chromium removal by a novel Serratia proteamaculans isolated from the bank of Sebou River (Morocco). Environ. Sci. Pollut. Res. Int., 21 (4): 3 060-3 072. |
| Thacker U, Parikh R, Shouche Y, Madamwar D. 2006.Hexavalent chromium reduction by Providencia sp.Process Biochem., 41 (6): 1 332-1 337. |
| Zahoor A, Rehman A. 2009. Isolation of Cr (VI) reducing bacteria from industrial effluents and their potential use in bioremediation of chromium containing wastewater. J.Environ. Sci., 21 (6): 814-820. |
| Zayed A M, Terry N. 2003. Chromium in the environment: factors affecting biological remediation. Plant Soil, 249 (1): 139-156. |
| Zhang K, Li F. 2011. Isolation and characterization of a chromium-resistant bacterium Serratia sp. Cr-10 from a chromate-contaminated site. Appl. Microbiol. Biotechnol., 90 (3): 1 163-1 169. |
 2015, Vol. 33
2015, Vol. 33


