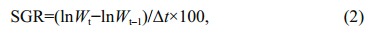Shanghai University
Article Information
- JIANG Senhao (姜森颢) , DONG Shuanglin (董双林) , GAO Qinfeng (高勤峰) , REN Yichao (任贻超) , WANG Fang (王芳)
- Effects of water depth and substrate color on the growth and body color of the red sea cucumber, Apostichopus japonicus
- Chinese Journal of Oceanology and Limnology, 2015, 33 (3) : 616-623
- http://dx.doi.org/10.1007/s00343-015-4178-7
Article History
- Received Jun. 20, 2014;
- accepted in principle Aug. 25, 2014;
- accepted for publication Oct. 24, 2014
2 Jiangsu Provincial Key Laboratory of Coastal Wetland Bioresources and Environmental Protection, Yancheng Normal University, Yancheng 224051, China;
3 Marine Science and Engineering College, Qingdao Agricultural University, Qingdao 266109, China
In Japan,three forms of the sea cucumber Apostichopus japonicusare recognized: red,green and black based on color variations (Choe and Ohshima, 1961) . Red A. japonicushave a distinctive bright red pigment (Kanno and Kijima, 2002) . In their natural range,red A. japonicushave usually found on gravel beds,offshore,whereas green and black color morphs inhabit inshore areas with a s and -mud bottom (Nishimura,1995) . In China,green A. japonicushave are an important aquaculture species,with production at 100 000 tons in 2010 (Jiang et al., 2013) . However as red is favored by traditional Chinese customs,the commercial value of red A.japonicushave nearly twice that of the green variant.
Red A. japonicushave introduced to China from Japan for pond culture in recent years. However,the red body-color of the introduced animal became celadon after around 6 months of culturing in northern China. There is little information in the literature to explain this phenomenon. There are significant differences in water depth between the culture ponds in China and the native habitat of red A. japonicushave. Water depth influences light intensity and spectral composition. Light is an important ecological factor,it can influence organisms by its intensity,quality and photoperiod (Odum and Barrett, 2005) . Many studies have demonstrated that light intensity and color have multifarious influences on the feeding,growth,behavior,physiology and ecology of aquatic organisms (Kawamura et al., 1996; Wang et al., 2003; Juell and Fosseidengen, 2004; Sheng et al., 2006; Barcellos et al., 2009; Mei et al., 2009) . Zhang et al. (2009) found that A. japonicushavecould select the artificial substrates with distinct colors. Laboratory studies indicated that light affects oxygen consumption,ammonia excretion,growth and body color of A. japonicushave (Xue et al., 2007; Sui et al., 2010) . However,there is still little information on the effect of light on the growth of A. japonicushavein the field.
The effects of water depth and substrate color on the growth and body color of red A. japonicushavein an earthen pond were investigated. 2 MATERIAL AND METHOD 2.1 Experimental animals
First generation,juvenile,red A postichopusjaponicusintroduced from Kyushu,Japan were used in the experiment. Initial mean body wet weights of the experimental animals were 1.48±0.13 g and 1.91±0.19 g,in the water depth trial and substrate color trial,respectively. The sea cucumbers fed on natural foods throughout the experiment,with no added supplements. 2.2 Experimental design 2.2.1 Water depth trial
The experiment was carried out in a 30 hectare cofferdam at the Homey Group,Sh and ong Province (36°55′–56′N,122°08′–09′E) from April to December 2009. The water depth in the cofferdam was around 3.0 m. Five depth treatments were set up: 20,50,100,150 and 200 cm from the surface. Suspended cages made of polyethylene materials (60 cm×30 cm×12 cm) were used to house the experimental animals at each depth. There were five sea cucumber juveniles in each cage. Five cages were vertically suspended as a group (one cage at each experimental depth) what were joined by a nylon rope and anchored in place using cement and floats. There were five replicate groups.
Once a month,three sea cucumbers were r and omly collected from each cage and their body weights (wet weight) were measured. Survival rates were also calculated monthly. Each sample was photographed and the color was compared with a Pantone st and ard color card. The animals were gently returned to their cages after measurements. Light intensity,water temperature,dissolved oxygen (DO) ,salinity and pH were measured in situ,at each depth. 2.2.2 Substrate color trial
The substrate color trial was conducted in a culture pond for sea cucumbers at the Homey Group,Sh and ong Province (36°55′N,122°10′E) from May to November 2009. The pond area was 0.4 hectares and the water depth was 1.5–1.7 m. The experimental sea cucumbers were stocked in cages suspended at a water depth of 1.2 m, and five experimental animals were held in each cage. The cages were made of a stainless steel frame covered by a polyethylene net with a mesh size of 0.5 cm. Each cage had a base and sides made of colored PVC: red,orange,yellow,green,blue,white or black. There were four replicates for each color treatment. Sampling of sea cucumbers and data collection methods were the same as those used in the water depth experiment. 2.2.3 Color grading and water quality parameters
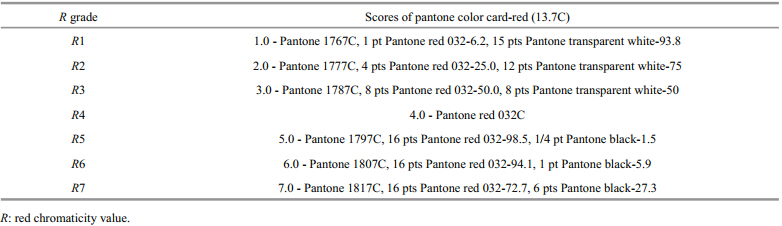
Red chromaticity grades were developed based on the dorsal color of red A. japonicushave, and a Pantone St and ard Color Card-red (PSCC-red) (Table 1) . The application of color cards such as the Roche Color Card and SalmoFan (used in the evaluation of salmon flesh color) were used as a guide to help develop the seven red chromaticity grades (Christiansen et al., 1995; Forsberg and Guttormsen, 2006) . The red chromaticity grades (Rvalues) ranged from pale red (1) to intense red (7) . The color of each sea cucumber used in the experiments was scored by comparison with the red chromaticity score card (PSCC-red) . The color comparisons were all carried out by a single person (the first author) under natural light.
Whole wet body weights of red A. japonicushave were measured using an electronic balance (U.S. Mettler Toledo,Model: PL202-5) . Light intensity at each water depth was analyzed using an illumination photometer (Shanghai Jiadingxuelian,Model: ZDS-10W-2D) . Water temperature,pH,salinity and DO levels in the culture ponds were measured using a multi-parameter water quality analyzer (U.S. YSI,Model: 556 MPS) . 2.3 Calculations and data analysis
Production (P) ,specific growth rate (SGR) and body color change rate (BCCR) of Apostichopusjaponicuswere calculated as follows:
where n is the sampling time,Ntis the number of sea cucumbers surviving at sampling time t,ΔWis the mean weight gain of an individual between two sampling times (g) and NtΔWis the change in biomass between two sampling times (g) . where Wt and i>Wt-1are wet body weights at sampling time t and t–1,respectively. Δtis the time interval between two sampling events (days) where Rt and Rt-1are red chromaticity values of red A. japonicushaveat sampling time t and t–1,respectively.One-way analysis of variance (ANOVA) was usedto evaluate differences in body weights,SGR,BCCR and Rvalues of red A. japonicushaveamong treatments. Prior to analysis,data were checked for normality using a Kolmogorov-Smirnov test, and homogeneity of variances were tested using Levene’s test,Duncan’s multiple range test was used to determine the significant differences at P<05. Regression analysis was used to evaluate the correlations among light intensity,water temperature and SGR, and the analyses had a critical probability level of 0.05 (or 0.01) . The software package SPSS Statistics 20.0 for Windows was used to conduct all statistical tests. 3 RESULT 3.1 Effects of water depth on growth and body color
The body weights (wet weight) ,specific growth rates (SGR) and production values of red A postichopusjaponicusat different water depths are shown in Table 2. There was a general trend for the final weights and SGRs of sea cucumbers held at deeper water layers to be higher than those of animals held at shallowers. The maximum survival rate for A. japonicushavein the depth trial (93.3%) was for sea cucumbers suspended at a depth of 200 cm.
The relationship between water depth and light intensity is shown in Fig. 1. There was a significant positive correlation between light intensity and growth (P<01) . The regression equation is: SGR=9E–08 L2–0.0004 L+1.0758 (n=40,R=0.60,P<01,F=10.13) ,where SGR is specific growth rate (%/day) and Lis light intensity (lx) .
 |
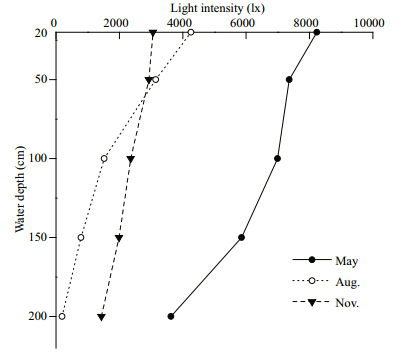 |
| Fig. 1 Light intensity at different times and water depths
Water depths (ordinate) : depths of the suspended cages (suspended at 20,50,100,150 and 200 cm below the surface) ; light intensity (abscissa) : natural light intensity at different times (May,August and November) during the experimental period (lx) . |
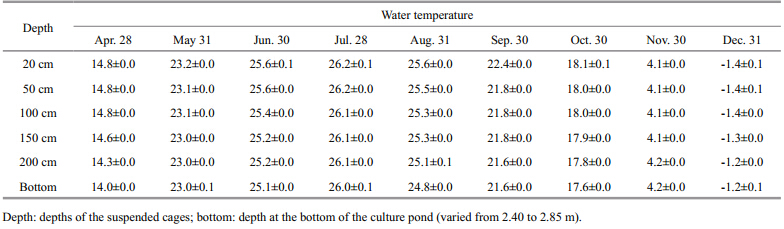
During the experimental period,water temperature in the cofferdam varied seasonally from -1.4 to 26.2°C (Table 3) . The variations of water temperatures with depths in the cofferdam were not significant,especially during spring and autumn. Regression analysis showed that there was a significant positive correlation between water temperature and the growth of sea cucumbers (P<05) . The regression equation is: SGR=-0.000 4 T3+ 0.015 1 T2–0.063 9 T–0.0322 (n=40,R=0.48,P<05,F=4.61) ,where Tis water temperature (°C) .
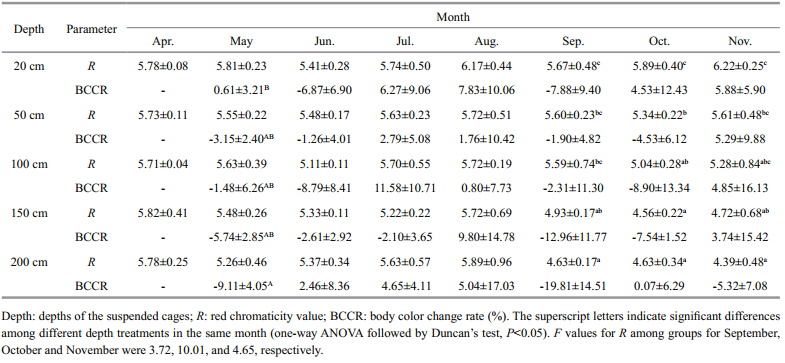
The amount of change in body color of red A. japonicusduring the experiment is shown in Table 4. Red A. japonicushavesuspended at different water depths all maintained a red body color,however there were remarkable changes in color from September to November (P<05) . The initial average red intensity (Rvalue 5.76) was recognized as the best body color value. At the end of the experiment,Rvalues for red A. japonicushavewere highest in sea cucumbers held closest to the surface (20 cm) and lowest in animals suspended near the bottom (200 cm) . Sea cucumbers suspended near the bottom (200 cm) showed the greatest degree of color change,having the maximum BCCR value.
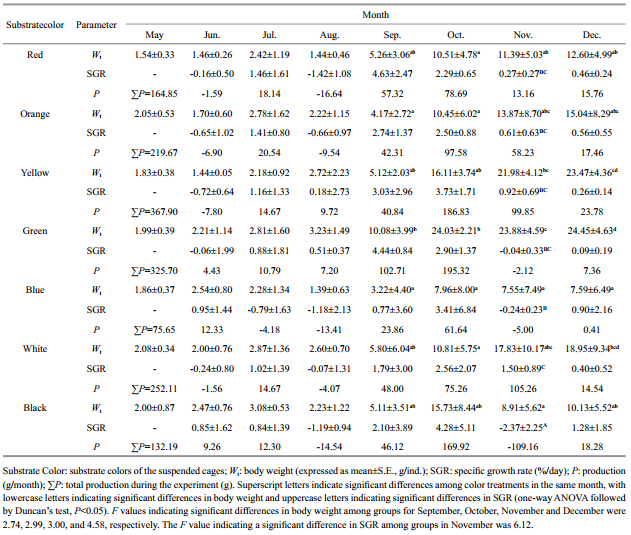
The body weights (wet weight) ,specific growth rates (SGR) and production values of red Apostichopusjaponicusgrown on different colored substrates are shown in Table 5. There was a significant effect of substrate color on the growth of red A. japonicushave (P<05) from September to December. The maximum total production value (367.90 g) was found for animals held on a yellow substrate and the minimum total production value (75.65 g) was found for animals grown on a blue substrate.
 |
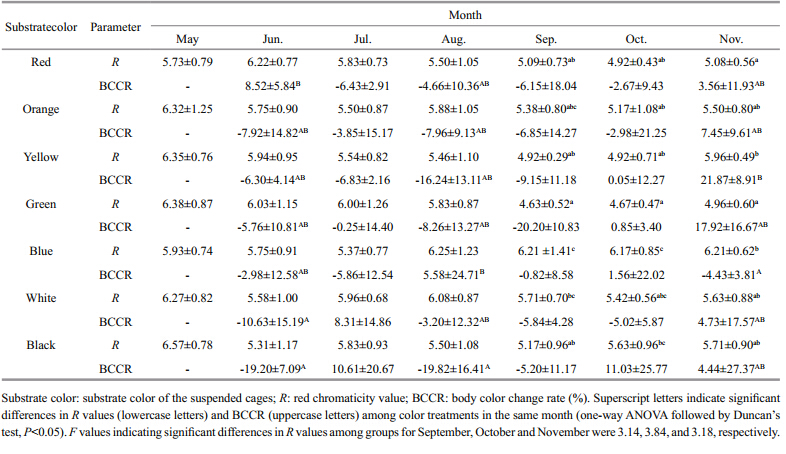
Changes in body color of red A. japonicushave grown on different colored substrates are shown in Table 6. Throughout the experiment,all animals retained a red color. However,there were significant changes in the body color of sea cucumbers held on different colored substrates from September to November (P<05) . At the end of the experiment,the red intensity (R value) was highest in sea cucumbers grown on the blue substrate, and lowest for sea cucumbers grown on the green substrate. Color change (BCCR) was greatest for sea cucumbers from the green treatment followed by black > orange > red > white > yellow and blue.
 |
Water temperature is considered to be responsible for a change in the feeding behavior of some species of holothurians. A water temperature range of 2–26°C is suitable for the aquaculture of A postichopusjaponicus (Chen,2004) . When the temperature exceeds 20–24°C in summer,A. japonicushavewill go into aestivation and stop feeding (Yang et al., 2005) . Red A. japonicushavehave a very low growth rate during winter when the seawater temperature ranges from -1.3°C to 2.1°C (Jiang et al., 2013) . Although there were significant seasonal variations in water temperature in the cofferdam used in this experiment (Table 3) ,there was little change in water temperature with depth. The growth rate of red A. japonicushave was low during the hottest months of July to August, and during the cold winter months of October to December (Table 2) . The growth rate of red A. japonicushave was greatly influenced by water temperature.
Light intensity can affect the growth of some fishes and crustaceans. Wallace et al. (1988) found that the arctic charr,Salvelinus alpinus grew best and had the lowest mortality at a light intensity of 50 lx,whereas the growth of salmon fry,Salmo salar was greater at a higher light intensity. Han et al. (2005) demonstrated that the growth rate of the Chinese longsnout catfish,Leiocassis longirostrisGünther significantly decreased when they were released under extremely high or low light intensities. Solea soleaL. larvae showed increased growth and food intake at a light intensity of 4 000 lx compared with 100 lx (Lund et al., 2010) . The shrimp,Fenneropenaeus chinensishad a maximum growth rate and food conversion efficiency under a light intensity of 300 lx (Wang et al., 2004) . Research conducted at our laboratory indicates that the optimal light intensity for the growth of red A. japonicushaveis 50 lx, and that growth rate decreases with increasing light intensity once levels exceed 300 lx (Personal communication) . In the present study,light intensities exceeded 300 lx at most of the depths tested, and light intensities in the upper water layers were always stronger than those at the bottom. Therefore,the general trend of slower growth in red A. japonicushavesuspended close to the surface (i.e.,at 20 and 50 cm) ,may be due to high light intensities (see Fig. 1 and Table 2) .
Light intensity can influence the body color of A. japonicushave (Zhang,1986) . The body color of red A. japonicushaveis mainly determined by the carotenoid content of its body wall (No and Storebakken, 1991; Pavlidis et al., 2006; Tume et al., 2009) , and currently there is no evidence to indicate that red A. japonicushavecan synthesize carotenoids. This suggests that these biochemicals are acquired from the food for red A. japonicushave,mainly from benthic diatoms. Light intensity can affect carotenoid synthesis in algae (Dong et al., 2007) ,therefore it has an indirect impact on the body color of red A. japonicushavein our study. This relationship between light intensity and body color has been shown in the shrimp,Litopenaeusvannamei (You et al., 2006) . In addition,Light intensity can also affect pigment cells,leading to morphological variations and a change in the body colors of some aquatic animals (Suqimoto,2002) . This process may influence body color in red A. japonicushave,too. Differences in water depth and therefore light intensity between the shallow culture ponds in China and their native habitat in Japan,may result in a change in the body color of red A. japonicushave.
Based on growth and body color comprehensive evaluations,we conclude that 100 cm is the optimum depth for the aquaculture of red A. japonicushavein the depth range tested in this study. 4.2 Effects of substrate color on growth and body color
Previous studies have indicated that the color of the substrate can affect the growth and body color of aquatic animals (Duray et al., 1996; Han et al., 2005; Dong et al., 2006; Zhang et al., 2012) . Str and et al. (2007) found that perch,Perca fluviatilisL. had a higher food intake and gained more weight in white tanks compared with black tanks. When cultured on substrates of mixed colors (e.g.,green and orange,green and red) ,the juvenile seahorse,HippocampuserectusPerry,had greater color change rates than animals cultured on a substrate of a single color (Lin et al., 2009) . Tume et al. (2009) found that the color grade scores of prawns,Penaeus monodon,increased significantly within 1 hour,when they were moved from a white tank to a black one. Experimental evidence indicated that the specific growth rate (SGR) and body color of the shrimp,Litopenaeus vannamei,were influenced by different light sources and illumination methods. When illuminated by a metal halide lamp,the shrimp gained a vivid body color and grew rapidly (You et al., 2006) . In our study,different substrate colors had a significant effect on both the growth and body color of red A. japonicushaveafter 6 months (Tables 5,6) . Sea cucumbers cultured on a yellow substrate yielded the maximum production and were a suitable red color (Rvalue) . Production values for red A. japonicushavecultured on a blue and black substrate were low,meanwhile,the Rvalue of a green substrate was small and other colors were not suitble,too. Therefore,yellow was the optimum substrate color for the culture of red A. japonicushave,among the seven colors tested.
The body color of juvenile red A. japonicushavecultured in suspended cages at different depths or with different colored substrates all remained red in the experimental period of 7–9 months. At the same time red A. japonicushavecultured on the muddy bottom of the same pond,changed color from red to graygreen after 3–6 months. This result indicates that cultivating red A. japonicushavein suspended cages (bottom mud isolation) is a feasible method for retaining the red body color.
Ecological factors,especially water depth and therefore light intensity, and substrate color,significantly affected the growth and body color of red A. japonicushave. Other factors such as nutrient availability may also be important. Our future research will focus on the influence of various ecological factors and nutrition on the physiology and ecology of red A. japonicushave.
| Barcellos L J G, Kreutz L C, Quevedo R M, da Rosa J G S,Koakoski G, Centenaro L, Pottker E. 2009. Influence of color background and shelter availability on jundiÁ (Rhamdia quelen) stress response. Aquaculture, 288 (1-2) : 51-56. |
| Chen J X. 2004. Present status and prospects of sea cucumber industry in China. In : Lovatelli A, Conand C, Purcell S,Uthicke S, Hamel J-F, Mercier A eds. Advances in Sea Cucumber Aquaculture and Management. FAO, Rome. p.25-38. |
| Choe S, Ohshima Y. 1961. On the morphological and ecological differences between two commercial forms, “Green” and “Red”, of the Japanese common sea cucumber, Stichopus japonicus Selenka. Bull. Jpn. Soc. Sci. Fish., 27 (2) : 97-106. (in Japanese with English abstract) |
| Christiansen R, Struksnæs G, Estermann R, Torrissen O J. 1995. Assessment of flesh colour in Atlantic salmon,Salmo salar L. Aquac. Res., 26 (5) : 311-321. |
| Dong Q L, Xing X Y, Zhao X M. 2007. Effect of light intensity on astaxanthin synthesis in Haematococcus Pluvialis.Acta Hydrob. Sinica, 31 (3) : 445-447. (in Chinese with English abstract) |
| Dong Y W, Dong S L, Tian X L, Wang F, Zhang M Z. 2006.Effects of diel temperature fluctuations on growth, oxygen consumption and proximate body composition in the sea cucumber Apostichopus japonicus Selenka. Aquaculture, 255 (1-4) : 514-521. |
| Duray M N, Estudillo C B, Alpasan L G. 1996. The effect of background color and rotifer density on rotifer intake, growth and survival of the grouper (Epinephelus s uillus) larvae. Aquaculture, 146 (3-4) : 217-224. |
| Forsberg O I, Guttormsen A G. 2006. A pigmentation model for farmed Atlantic salmon: nonlinear regression analysis of published experimental data. Aquaculture, 253 (1-4) : 415-420. |
| Han D, Xie S Q, Lei W, Zhu X M, Yang Y X. 2005. Effect of light intensity on growth, survival and skin color of juvenile Chinese longsnout catfish (Leiocassis longirostris Günther) . Aquaculture, 248 (1-4) : 299-306. |
| Jiang S H, Dong S L, Gao Q F, Wang F, Tian X L. 2013.Comparative study on nutrient composition and growth of green and red sea cucumber, Apostichopus japonicus (Selenka, 1867) , under the same culture conditions.Aquac. Res., 44 (2) : 317-320. |
| Juell J E, Fosseidengen J E. 2004. Use of artificial light to control swimming depth and fish density of Atlantic salmon (Salmo salar) in production cages. Aquaculture, 233 (1-4) : 269-282. |
| Kanno M, Kijima A. 2002. Quantitative and qualitative evaluation on the color variation of the Japanese sea cucumber Stichopus japonicus. Aquacult. Sci., 50 (1) : 63-69. (in Japanese with English abstract) |
| Kawamura G, Matsushita T, Nishitai M, Matsuoka T. 1996.Blue and green fish aggregation devices are more attractive to fish. Fish. Res., 28 (1) : 99-108. |
| Lin Q, Lin J D, Huang L M. 2009. Effects of substrate color, light intensity and temperature on survival and skin color change of juvenile seahorses, Hippocampus erectus Perry, 1810. Aquaculture, 298 (1-2) : 157-161. |
| Lund I, Steenfeldt S J, Hansen B W. 2010. Influence of dietary arachidonic acid combined with light intensity and tank colour on pigmentation of common sole (Solea solea L.) larvae. Aquaculture, 308 (3-4) : 159-165. |
| Mei Z P, Finkel Z V, Irwin A J. 2009. Light and nutrient availability affect the size-scaling of growth in phytoplankton. J. Theor. Biol., 259 (3) : 582-588. |
| Nishimura S. 1995. Guide to Seashore Animals of Japan with Color Pictures and Keys. Vol. 2. Hoikusha Publishing Co.Ltd., Osaka, Japan. |
| No H K, Storebakken T. 1991. Pigmentation of rainbow trout with astaxanthin at different water temperatures.Aquaculture, 97 (2-3) : 203-216. |
| Odum E P, Barrett G W. 2005. Fundamentals of Ecology. 5 th edn. Thomson Brooks/Cole, Belmont, USA. p.598.. |
| Pavlidis M, Papandroulakis N, Divanach P. 2006. A method for the comparison of chromaticity parameters in fish skin: preliminary results for coloration pattern of red skin Sparidae. Aquaculture, 258 (1-4) : 211-219. |
| Sheng J Q, Lin Q, Chen Q X, Gao Y L, Shen L, Lu J Y. 2006.Effects of food, temperature and light intensity on the feeding behavior of three-spot juvenile seahorses,Hippocampus trimaculatus Leach. Aquaculture, 256 (1-4) : 596-607. |
| Strand Å, Alanärä A, Staffan F, Magnhagen C. 2007. Effects of tank colour and light intensity on feed intake, growth rate and energy expenditure of juvenile Eurasian perch, Perca fluviatilis L. Aquaculture, 272 (1-4) : 312-318. |
| Sui J J, Dong S L, Tian X L, Wang F, Dong Y W. 2010. The effect of light color on respiration and excretion of sea cucumber, Apostichopus japonicus. Period. Ocean Uni.China, 40 (3) : 61-64. (in Chinese with English abstract) |
| Suqimoto M. 2002. Morphological colour changes in fish regulation of pigment cell density and morphology.Microsc. Res. Techniq., 58 (6) : 496-503. |
| Tume R K, Sikes A L, Tabrett S, Smith D M. 2009. Effect of background colour on the distribution of astaxanthin in black tiger prawn (Penaeus monodon) : effective method for improvement of cooked colour. Aquaculture, 296 (1-2) : 129-135. |
| Wallace J C, Kolbeinshavn A, Aasjord D. 1988. Observations on the effect of light intensity on the growth of Arctic charr fingerlings (Salvelinus alpinus) and salmon fry (Salmo salar) . Aquaculture, 72 (1-2) : 81-84. |
| Wang F, Dong S L, Dong S S et al. 2004. The effect of light intensity on the growth of Chinese shrimp Fenneropenaeus chinensis. Aquaculture, 234 (1-4) : 475-483. |
| Wang F, Dong S L, Huang G Q, Wu L X, Tian X L, Ma S. 2003. The effect of light color on the growth of Chinese shrimp Fenneropenaeus chinensis. Aquaculture, 228 (1-4) : 351-360. |
| Xue S Y, Fang J G, Mao Y Z, Zhou J, Zhang J H, Zhang Y. 2007. The influence of different light intensity on growth of juvenile sea cucumber Apostichopus japonicus. Mar.Fish. Res., 28 (6) : 13-18. (in Chinese with English abstract) |
| Yang H S, Yuan X T, Zhou Y, Mao Y Z, Zhang T, Liu Y. 2005.Effects of body size and water temperature on food consumption and growth in the sea cucumber Apostichopus japonicus (Selenka) with special reference to aestivation.Aquac. Res., 36 (11) : 1 085-1 092. |
| You K, Yang H S, Liu Y, Liu S L, Zhou Y, Zhang T. 2006.Effects of different light sources and illumination methods on growth and body color of shrimp Litopenaeus v annamei. Aquaculture, 2 52 (2-4) : 557-565. |
| Zhang H, Wang Y G, Rong X J, Cao S M, Chen X. 2009.Behavioral responses of sea cucumber (Apostichopus japonicus) to different light intensity and settlement substratum color. Chin. J. Ecol., 28 (3) : 477-482. (in Chinese with English abstract) |
| Zhang P, Dong S L, Wang F, Wang H, Gao W, Yan Y. 2012.Effect of salinity on growth and energy budget of red and green colour variant sea cucumber Apostichopus japonicus (Selenca) . Aquac. Res., 43 (11) : 1 611-1 619. |
| Zhang X Y. 1986. Experiment on the influence of light to pigment of juvenile sea cucumber Apostichopus japonicus. Mariculture, 1 (1) : 37-38. (in Chinese) |
 2015, Vol. 33
2015, Vol. 33