Shanghai University
Article Information
- LI Qiang (李强) , ZHAO Yue (赵越) , ZHANG Xu (张旭) , WEI Yuquan (魏雨泉) , QIU Linlin (邱琳琳) , WEI Zimin (魏自民) , LI Fuheng (李富恒)
- Spatial heterogeneity in a deep artificial lake plankton community revealed by PCR-DGGE fingerprinting
- Chinese Journal of Oceanology and Limnology, 2015, 33 (3) : 624-635
- http://dx.doi.org/10.1007/s00343-015-4184-9
Article History
- Received Jul. 19, 2014;
- accepted in principle Oct. 5, 2014;
- accepted for publication Nov. 5, 2014
Plankton,generally comprised of prokaryotic and eukaryotic planktonic organisms,are important components in various water bodies (e.g.,lakes,reservoirs, and rivers) , and play a key role in the functioning of aquatic ecosystems and biogeochemical cycles (Yan and Yu, 2011) . Moreover,they can be a good indicator of water quality and trophic status because different plankton taxa have specific preferences and tolerances to certain environmental conditions (Bianchi et al., 2003; Yan et al., 2008) .
However,because of their small size and lack of distinguishing features,ecological studies on plankton through traditional morphological methods are difficult. With the development of molecular biology,phylogenetically meaningful macromolecules (e.g.,16S and 18S rRNA genes) obtained from environmental samples are now widely used for such purposes. However,the classical cloning-sequencing method is time-consuming and unsuitable for the simultaneous analysis of many different samples. Recently,the application of fingerprinting and NGS (next generation sequencing) technologies has made it possible to uncover plankton diversity and dynamics at the metagenomic level. Among these technologies,denaturing gradient gel electrophoresis (DGGE) has become a popular method for plankton community composition (PCC) characterization because it is reliable,reproducible,rapid, and inexpensive (Muyzer,1999) . Since Muyzer et al. (1993) introduced the DGGE method into microbial ecology,it has been widely applied to different types of lake ecosystems for the study of bacterioplankton (Ovreås et al., 1997; Lindström,2000) ,cyanobacteria (Boutte et al., 2006; Tan et al., 2009) ,small eukaryotes (Tarbe et al., 2011; Zhao et al., 2011) ,fungi (Xuan et al., 2011; Kagami et al., 2012) ,viruses (Zhong et al., 2013) , and archaea (Berdjeb et al., 2013) . To date,most studies in lakes have focused on a single plankton group (either prokaryotic or eukaryotic) . Therefore,lake PCC in its entirety remains poorly understood,making it difficult to reach higher-order ecological conclusions about general planktonic trends and patterns (Yan and Yu, 2011) .
Numerous studies have shown that temporal and /or spatial changes in environmental factors are the driving forces behind shifts in PCC. In terms of temporal plankton distribution,water temperature is the decisive factor influencing the seasonal changes in PCC. Sommer et al. (1986) , (Sommer,1996) developed a Plankton Ecology Group (PEG) model to explain the relationship between seasonal changes and plankton succession. Regarding spatial plankton distribution,however,the driving forces behind shifts in PCC vary among lakes. pH,transparency,alkalinity,concentrations of total nitrogen,total phosphorus,nitrate,silicate,dissolved oxygen and chemical oxygen dem and , and rotifer and crustacean density have been reported as being the main factors related to plankton community spatial distribution (Yan et al., 2008; Yu et al., 2008; Wu et al., 2009a; Li et al., 2012) . Moreover,most studies have focused on shallow lakes; only latitudinal gradients in PCC differences have been explored. Whether or not a similar relationship exists on a vertical gradient is unknown at present.
In this study,we applied DGGE analysis based on partial 16S and 18S rRNA gene fragments to give a comprehensive picture of horizontal and vertical heterogeneity in both prokaryotic and eukaryotic PCC in a deep artificial lake (Songhua Lake) in China. The major determinants of PCC in deep freshwater lakes were explored by multivariate statistics. Additionally,the taxonomic information of some putative dominant members of the community was determined by sequencing dominant DGGE b and s. 2 MATERIAL AND METHOD 2.1 Study sites,sample collection, and physicochemical analysis
Songhua Lake (42°58′E,126°41′N) ,located in Jilin province,is the largest river-type reservoir in the middle part of Northeast China. The lake has a surface area of 554 km2 and a capacity of 1.08×1010m3. It is a mesotrophic deep freshwater artificial lake,with a maximum depth of 77.5 m. The lake constitutes an important water reservoir for energy production,irrigation,flood control,recreation,fisheries, and navigation.
Sampling was conducted at seven stations (S1 to S7) following a downstream/upstream gradient on May 7th,2011. Water samples were collected at three depths (0.5,6, and 16 m) at each station in a 2.5-L organic glass water sampler. Approximately 1 L of each water sample was used for PCR-DGGE analysis,samples were first filtered through 400-mesh boltingsilk, and then through a 0.22-μm filter (Millipore,USA) using an electric pump; the filters were then stored at -80°C until DNA extraction. Another 1 L of each water sample was used for physicochemical analysis. Temperature,pH, and conductivity were measured with thermometer,pH meter, and conductivity meter respectively in the field during sampling. Chlorophyll a (chl a) ,total nitrogen (TN) ,total phosphorus (TP) ,ammonia,nitrate, and nitrite concentrations,as well as chemical oxygen dem and (CODMn) were measured according to st and ard methods (Zhang and Huang, 1991; Huang et al., 2000) . 2.2 DNA extraction and PCR-DGGE analysis
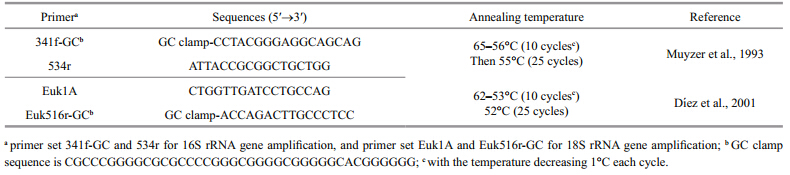
DNA was extracted from biomass collected on filters according to Tan et al. (2009) . To analyze the DNA of the entire plankton community,both 16S rRNA and 18S rRNA genes were amplified with prokaryotic and eukaryotic primers (Table 1) . Polymerase chain reaction (PCR) conditions for each 50-μL reaction mixture were: 0.5 mmol/L of each primer,1×PCR buffer (1.5 mmol/L Mg2+ plus) ,250 mmol/L dNTPs,2.5 U of Taq™ DNA polymerase (TaKaRa,Japan) , and 40 ng of extracted DNA. A touch down PCR protocol was performed: an initial denaturation step at 94°C for 5 min,then each cycle was carried out at 94°C for 0.5 min,at the annealing temperature (Table 1) for 0.5 min, and extended at 72°C for 1 min, and finally extended at 72°C for 10 min. After each PCR,the product sizes were verified on 1.0% agarose gel with ethidium bromide staining.
DGGE was performed on a Dcode™ Universal Mutation Detection System (Bio-Rad,USA) . Electrophoresis was performed in 6% (m/ v) polyacrylamide gels (ratio of acrylamide to bisacrylamide,37.5:1) submerged in 1×TAE buffer at 60°C. PCR products containing approximately equal amounts of DNA samples with similar sizes wereseparated on the gel containing a linear denaturant gradient of urea and deionized formamide. For the separation of 16S rRNA gene fragments from prokaryotic organisms,the electrophoresis conditions were 4 h at 150 V in a linear 35%–60% denaturant agent gradient (100% denaturant agent was defined as 7 mol/L urea and 40% (v/ v) deionized formamide) (Li et al., 2013b) ; while for the separation of 18S rRNA gene fragments from eukaryotic organisms,the conditions were 16 h at 120 V in a linear 25%–45% denaturant agent gradient (Li et al., 2013a) . After electrophoresis,gels were stained in 3×GelRed™ nuclear acid gel stain (Biotium,USA) and photographed with a UVP Imaging System (UVP Inc.,USA) . 2.3 DGGE b and sequencing and phylogenetic analysis
Prominent DGGE b and s were selected,excised, and transferred to 30-μL Milli-Q water (Millipore,USA) ,incubated overnight for elution of DNA at 4°C. Then,2 μL of the supernatant was used as a template for reamplification with the original primer pair (without GC clamp) . PCR products were purified with the QIAquick ®PCR-Purification Kit (Qiagen,Germany) . The 16S rRNA gene fragment PCR products were sequenced with the 534r primer, and the 18S rRNA fragment PCR products were sequenced with the Euk516r primer. All sequencing reactions were done by Invitrogen (Shanghai,China) on an ABI 3730 automated sequencer (Applied Biosystems,USA) .
The sequences obtained,100–200 bases for 534r and 500–600 bases for Euk516r,were compared with public DNA databases (GenBank,NCBI) by BLAST (http://blast.ncbi.nlm.nih.gov/) to find closely related sequences. All sequences were imported into MEGA 5.2 (Tamura et al., 2011) for phylogenetic analysis. The initial tree was constructed using the maximumlikelihood method,the tree topology robustness was confirmed by the bootstrap method with 1 000 replications, and evolutionary distances were computed using the maximum composite likelihood method. 2.4 Data analysis
The DGGE images were analyzed with Gel-Pro v4.0 image software (Media Cybermetics,USA) . The b and s that co-migrated to the same position were detected, and a binary matrix was constructed based on the presence (1) or absence (0) of co-migrating b and s in each lane.
For cluster analysis,Jaccard’s coefficient of similarity was analyzed by the unweighted pair group method with arithmetic averages (UPGMA) using the software MVSP v3.13 (Kovach Computing Services,UK) .
Ordination techniques based on DGGE fingerprints were performed to elucidate the factors affecting community composition. The data were first logarithmically transformed to eliminate the influence of extreme values on ordination scores. Detrended correspondence analysis (DCA) was then performed with the software Canoco for Windows v4.54 (Biometris,the Netherl and s) to test if weighted averaging or linear methods were appropriate. The longest gradient length from DCA was 1.596,this value indicated a clear linear relationship (Lepš and Šmilauer,2003) , and redundancy analysis (RDA) was performed to investigate species-environment correlations. Manual forward selection and Monte Carlo testing (499 permutations) were used to select the environmental factors that significantly affected (P<05) plankton distribution. 2.5 Nucleotide sequences
Nucleotide sequences no less than 150 bases in length obtained from DGGE b and s have been deposited in GenBank under accession numbers KF536589–KF536605. 3 RESULT 3.1 Lake physicochemical characteristics
Environmental factors corresponding to 21 samples from Songhua Lake (Table S1) were somewhat similar,indicating that water quality in the lake was generally stable. Briefly,temperature ranged from 4.2–6.9°C and decreased with depth; while conductivity ranged from 260.9–295.0 μS/cm and increased with depth. TP ranged from 0.018–0.126 mg/L and COD Mnranged from 3.64–4.48 mg/L. High nitrogen concentration was a characteristic of Songhua Lake water quality,of which,nitrate accounted for 83%–91% of total nitrogen. The TN and nitrate concentrations were high in downstream sites (S1–3) and low in upstream sites (S5–7) ,while nitrite,ammonia, and COD Mnconcentrations exhibited the opposite pattern.
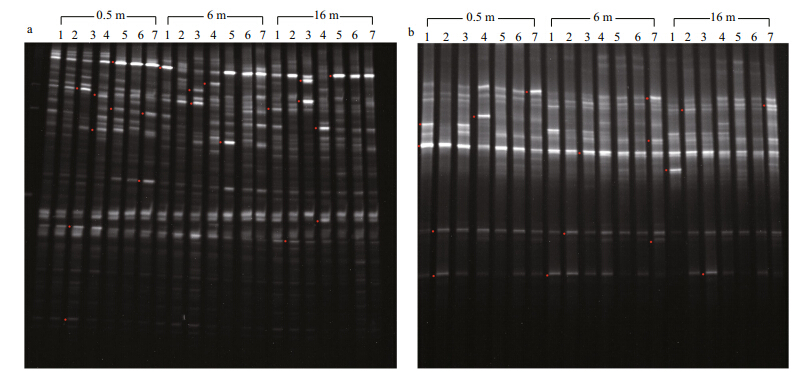 |
| Fig. 1 16S (a) and 18S (b) rRNA gene fragment DGGE profiles amplified from plankton community DNA
Numbers 1–7 above each lane indicate the sampling sites S1–S7; red dots on the left indicate b and s that were excised,reamplified, and sequenced. |
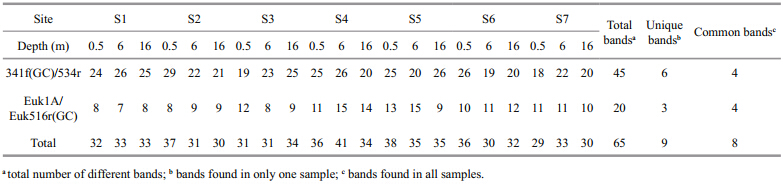
Figure 1 shows the prokaryotic and eukaryotic DGGE profiles of samples from Songhua Lake in May. Sixty-five independent DGGE b and s (45 16S rRNA gene fragments and 20 18S rRNA gene fragments) were detected (Table 2) . The number of b and s in each sample ranged from 18–29 (mean=22.9) with the prokaryotic primer set and 7–15 (mean=10.5) with the eukaryotic primer set. In all DGGE b and s,14% of the b and s (9/65) were unique (found only in a single sample) , and 12% (8/65) were common (found in all samples) . Additionally,53.3% of the prokaryotic b and s and 45% of the eukaryotic ones appeared in at least half of the samples.
UPGMA clustering of both DGGE profiles (Fig. 2) indicated that the 21 samples grouped into 2 distinct clusters. Cluster I contained all three depth samples from S1–3, and the 0.5 m depth sample from S4; while Cluster II contained samples from S4,S5,S6, and S7. Each cluster was subdivided into two subclusters,clusters I-a and II-a were dominated by the 0.5 m and 6 m samples,while clusters I-b and II-b contained only 16 m samples.
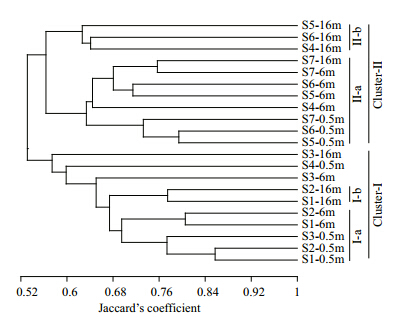 |
| Fig. 2 UPGMA dendrograms showing the similarity of plankton communities with Jaccard’s coefficient based on 16S and 18S rRNA gene DGGE b and s |
No significant autocorrelation was detected among the eight environmental factors. Forward selection and Monte Carlo testing revealed that temperature,COD Mn,TN,ammonia,nitrate, and nitrite were significantly (P<05) correlated with plankton community variability. The results of the RDA ordination carried out on 21 samples,based on sixenvironmental factors are illustrated in Fig. 3. The first three axes explained 77.1% of the cumulative percentage variance in b and s-environment,indicating a high correlation between DGGE b and s and environmental factors. The first three axes eigenvalues were 0.200,0.092, and 0.055,indicating that the first axis explained a higher proportion of the plankton community variation, and the three axes combined explained 34.7% of the observed variation in the plankton community. The correlations between DGGE b and s and environmental factors were high,especially for the first (R=0.933) and the third axes (R=0.930) . Among the six environmental factors,TN (R=0.912 8) ,nitrite (R=-0.886 2) ,nitrate (R=0.8739) ,ammonia (R=-0.856 5) , and CODMn (R=-0.613 3) exhibited the highest correlations with the first axis (Fig. 3a) ,while temperature had higher correlations with the third (R=0.596 4) and second axes (R= -0.475 1) than the first (Fig. 3b) .
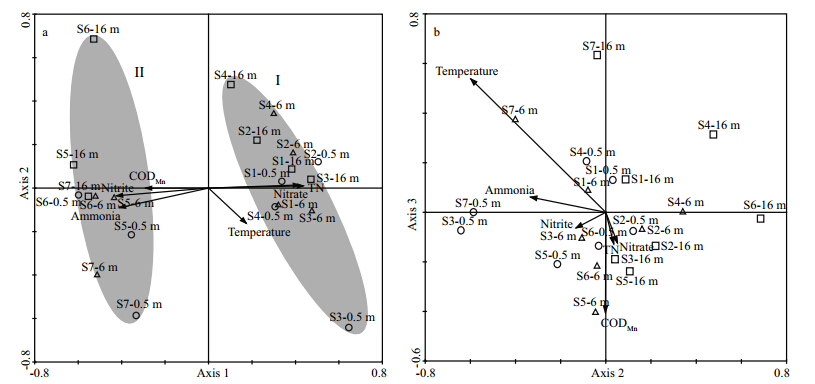 |
| Fig. 3 Results from RDA ordination of PCC,as revealed by DGGE fingerprinting
Circles indicate samples from 0.5 m depth,triangles indicate samples from 6 m depth, and squares indicate samples from 16 m depth. Arrows indicate the direction of increasing values of the respective environmental factors; arrow lengths indicate the degree of correlation of theenvironmental factors with plankton community data. Groups I and II of communities are indicated by a gray background. a. axes 1 and 2 of the RDA biplot; b. axes 2 and 3 of the RDA biplot. |
A total of 37 dominant DGGE b and s (21 16S and 16 18S rRNA gene b and s) were excised and directly sequenced,representing 25 different phylotypes (Table S2 and Fig. 4) . Among these phylotypes,84% exhibited no less than 97% similarity to sequences in GenBank,indicating that many lineages in the present study were affiliated with different freshwater planktonphyla. The phylotypes based on the 16S rRNA gene were members of four prokaryotic phyla,includingProteobacteria,Actinobacteria,Bacteroidetes, and Cyanobacteria. Within the Proteobacteria,the Classes Alfaproteobacteria,Betaproteobacteria, and Gammaproteobacteriawere found in the sequence data. While the phylotypes based on the 18S rRNA gene were members of the eukaryotic phyla Bacillariophyta,Pyrrophyta,Ciliophora,Rotifera, and Stramenopiles. Moreover,two phylotypes based on the 16S rRNA gene were similar to eukaryotic phytoplankton plastid DNA (Bacillariophyta and Cryptophyta chloroplast sequences) .
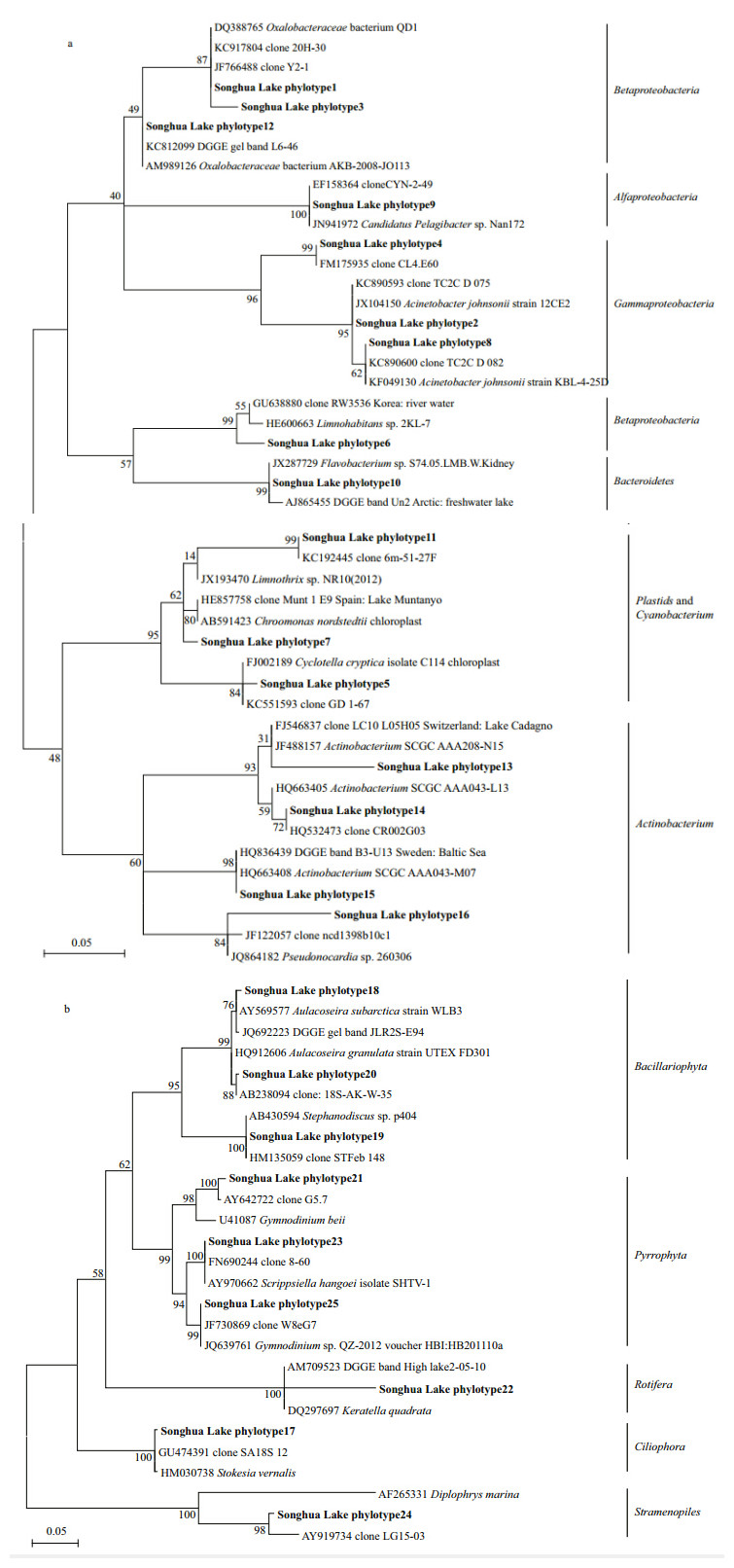 |
| Fig. 4 Maximum-likelihood trees showing the phylogenetic relationships between phylotypes and their closest matches froma BLAST search
Bootstrap values are based on 1 000 runs. a. phylotypes based on the 16S rRNA gene sequences; b. phylotypes based on the 18S rRNA gene sequences. |
Plankton community composition plays an important role in the functioning of aquatic ecosystems and is an ideal indicator for monitoring water quality,but has seldom been studied as an integrated system because of divergence in morphology,huge numbers of taxa, and differences in analytical techniques. DGGE technology provides a cultivation-independent method to analyze multiple samples simultaneously with st and ardized sets of procedures. However,as a PCR-based method,many uncertainties may exist when DGGE is applied in ecological studies. Some of these may be because of differences in DNA extraction efficiencies because different planktonic organisms differ in cell lysis efficiency,genome size, and /or rRNA gene copy numbers. Differences in PCR reactions may also be problematic. Preferential priming,differences in elongation rates, and number of cycles to reach equimolarity among different amplicons may cause biases (Dıez et al., 2001) . Moreover,some uncertainties may arise from DGGE itself. For instance,the DGGE method typically reveals only the most abundant phylotypes (≥1%) (Muyzer et al., 1993; MacNaughton et al., 1999) ,the actual existence of plankton diversity would be much higher than that of the DGGE profiles displayed. Additionally,500–600 bp fragments are the maximum suitable for DGGE,which may limit its application for phylogeny analysis. Although it has many problems,DGGE technology is still an acceptable and powerful method for plankton diversity studies when combined with b and sequencing and statistical approaches. 4.2 Plankton diversity in Songhua Lake
As previously described by other studies (Kowalchuk et al., 1997; Lyautey et al., 2005) ,DGGE b and s co-migrating to the same position in a gel always reveals identical sequences (defined as “phylotypes”) . The phylotypes retrieved in this study covered producers,consumers, and decomposers in a typical freshwater ecosystem. Moreover,prokaryotic and eukaryotic plankton exhibit a cosmopolitan distribution,since most of the phylotypes found in Songhua Lake have also been detected in other freshwater bodies located elsewhere in the world (Finlay,2002; Newton et al., 2011) .
In the lacustrine system,eukaryotic phytoplankton is the major producer and plays an important role in the carbon flow of lakes. In this study,three eukaryotic phytoplankton phyla (Bacillariophyta,Pyrrophyta, and Cryptophyta) were identified in Songhua Lake samples. Among them,Bacillariophytawas the most abundant; five phylotypes (from both 18S rRNA and 16S plastid sequences) were associated with this phylum. The results were in agreement with microscopic phytoplankton identification by us (data not published) ,which showed that Cryptophyta and Bacillariophyta were predominant in spring samples from Songhua Lake. Although there were three phylotypes related to Pyrrophyta,the abundance was quite low when identified by microscopy. This discrepancy may result from the potential biases of PCR amplification as has been discussed (De Wever et al., 2005; Sapp et al., 2007) .
Zooplankton primarily preying on bacteria,small algae,or other protozoa,usually act as consumers in freshwater ecosystems. In the present study,three zooplankton phylotypes were recorded. The first was highly homologous (99%) to the Stokesiavernalissequence,a member of Ciliophora,which are major consumers of bacteria and other protists and play an essential role in the microbial food web (Zhao et al., 2011) . The second was related to a Rotifera sequence,which was also obtained from a high altitude freshwater lake in China (Wu et al., 2009b) . The third was related to a sequence from the uncultured freshwater eukaryote clone LG15-03,a member of the Heterokontafound in an oligotrophic freshwater lake in the USA (Richards et al., 2005) .
Aquatic bacteria are the principle degraders and mineralizers of organic compounds to their inorganic constituents in freshwater systems. Newton et al. (2011) identified 21 putative aquatic bacteria phyla occurring in different lake ecosystems,in which,the sequences of the four most common phyla ( Proteobacteria,Actinobacteria,Bacteroidetes, and Cyanobacteria ) in freshwater lakes were retrieved in this study. The phylum Proteobacteriawas the most studied of the bacterial phyla, and the phylum with the most phylotypes (up to eight) in our study. The eight Proteobacteriaphylotypes were from three classes: Alfaproteobacteria,Betaproteobacteria, and Gammaproteobacteria. In our study,four phylotypes were related to the Actinobacteria. Phylogenetic analysis showed that two phylotypes belonged to the acI lineage,one belonged to the acII lineage, and the last belonged to the acTH2 lineage. Within the phylum Bacteroidetes,one phylotype clustered with the Flavobacteria,which might have a specialized role in DOM (dissolved organic material) uptake and degradation (Kirchman,2002) . One phylotype was classified as Cyanobacteria,like phototrophic freshwater organisms such as eukaryotic phytoplankton members of the Cyanobacteriaplay a key role in nutrient cycling in lakes. 4.3 Influence of environmental factors on PCC
Both the 16S and 18S rRNA gene DGGE patterns exhibited variation in b and number,position, and intensity,which suggested the existence of spatial heterogeneity in prokaryotic and eukaryotic PCC in Songhua Lake. RDA was applied to explore the correlation between PCC and environmental factors. The biplots showed that the inorganic nutrients (especially different inorganic nitrogen forms) ,COD Mn, and water temperature significantly affected PCC in Songhua Lake. Nitrogen is one of the key elements associated with lake eutrophication,its biogeochemical cycling is an important part of the material and energy circulation in the biosphere, and it plays an important role in nutrient cycling in lakes (Zeng et al., 2007) . Nitrate,nitrite, and ammonia are the three most common forms of inorganic nitrogen in freshwater systems. In Songhua Lake,nitrate concentration is the main part of total nitrogen as shown in Fig. 3. The distribution of nitrate,nitrite, and ammonia at different sampling sites reflect obvious horizontal gradients,which was in agreement with the plankton community distribution,as shown by both the RDA biplots and the UPGMA cluster analysis. COD Mnis an indicator of water quality. A high CODMnvalue often indicates bad water quality as a result of organic and /or chemical pollution. In recent years,Songhua Lake has suffered from agriculture nonpoint source pollution. There were 110 000 hectares of farml and around the lake area, and every year large quantities of chemical fertilizers and pesticides were added to the lake from upstream rivers,causing rises in total nitrogen concentrations and CODMn values. The changes significantly affected the plankton community distribution, and consequently impaired the functions of the aquatic ecosystem.
Numerous studies have shown that water temperature is one of the deterministic environmental factors influencing PCC in aquatic ecosystems (Lindström,2001; Sapp et al., 2007; Tan et al., 2009; Wu et al., 2009a; Li et al., 2012) . Songhua Lake is located in the northeast region of China,which is the coldest region in the country, and the annual mean temperature has been 2.5–5.7°C in recent years (He et al., 2013) . During the sampling period (May 7th,2011) ,the average water temperature of Songhua Lake was 5.7°C. Such a low temperature may explain why we obtained the least independent DGGE b and s compared to other studies (Yan et al., 2007,2008; Li et al., 2012) . However,in the present study water temperature was not highly relevant to the first RDA axis. It was noted that water temperature differences among both sampling sites and depths were minute in Songhua Lake (no more than 3°C) ; it is unreasonable to explain the PCC horizontal and vertical heterogeneity with such small differences. This finding was supported by De Wever et al. (2005) ,when they studied the factors affecting horizontal and vertical differences in the bacterial community composition (BCC) in a meromictic,permanently temperature stratified lake (Lake Tanganyika) in East Africa. Sapp et al. (2007) also suggested that the limitation of bacterioplankton communities because of inorganic nutrients occurs in periods when temperature is not limiting,which was in agreement with the present study. However,although it was not the most important factor,water temperature did influence the plankton community in Songhua Lake to some degree as shown in the biplot scaling of RDA axes 2 and 3 (Fig. 3b) .
With the universal primer pairs for the 16S and 18S rRNA genes,many uncultivated groups of planktonic organisms were identified in Songhua Lake. However,it did not permit more precise identification of plankton to the species or sub-species levels,which would be helpful for a more comprehensive underst and ing of the interaction of different plankton categories and how they function in the lacustrine ecosystems (Kormas et al., 2010; Oikonomou et al., 2012) . The development of species or sub-species specific primers and acquisition of more sequence information with DGGE and other molecular technologies,such as NGS,should be considered in future studies. 5 CONCLUSION
This study demonstrates the presence of horizontal and vertical heterogeneity in Songhua Lake PCC. The RDA results suggested that inorganic nutrient concentrations (especially different inorganic nitrogen forms) ,CODMn, and water temperature constitute the driving forces behind PCC variation. Sequence information based on dominant DGGE b and s revealed that Songhua Lake plankton comprised ten common freshwater phyla ( Proteobacteria,Actinobacteria,Bacteroidetes,Cyanobacteria,Bacillariophyta,Pyrrophyta,Cryptophyta,Ciliophora,Stramenopiles, and (Rotifera () ,which covered producers,consumers, and decomposers in a typical freshwater ecosystem.
| "Berdjeb L, Pollet T, Chardon C, Jacquet S. 2013. Spatiotemporal changes in the structure of archaeal communities in two deep freshwater lakes. FEMS Microbiol. Ecol., 86 (2) : 215-230. |
| Bianchi F, Acri F, Aubry F B, Berton A, Boldrin A, Camatti E,Cassin D, Comaschi A. 2003. Can plankton communities be considered as bio-indicators of water quality in the Lagoon of Venice? Mar. Pollut. Bull., 46 (8) : 964-971. |
| Boutte C, Grubisic S, Balthasart P, Wilmotte A. 2006. Testing of primers for the study of cyanobacterial molecular diversity by DGGE. J. Microbiol. Methods, 65 (3) : 542-550. |
| De Wever A, Muylaert K, Van der Gucht K, Pirlot S, Cocquyt C, Descy J-P, Plisnier P-D, Vyverman W. 2005. Bacterial community composition in Lake Tanganyika: vertical and horizontal heterogeneity. Appl. Environ. Microbiol., 71 (9) : 5 029-5 037. |
| Dıéz B, Pedrós-Alió C, Marsh T L, Massana R. 2001.Application of denaturing gradient gel electrophoresis (DGGE) to study the diversity of marine picoeukaryotic assemblages and comparison of DGGE with other molecular techniques. Appl. Environ. Microbiol., 67 (7) : 2 942-2 951. |
| Finlay B J. 2002. Global dispersal of free-living microbial eukaryote species. Science, 296 (5570) : 1 061-1 063. |
| He W, Bu R C, Xiong Z P, Hu Y M. 2013. Characteristics of temperature and precipitation in Northeastern China from 1961 to 2005. Acta. Ecol. Sin., 33 (2) : 519-531. (in Chinese with English abstract) |
| Huang X F, Chen W M, Cai Q M. 2000. Survey, Observation and Analysis of Lake Ecology. China Standard Press,Beijing, China. 247p. (in Chinese) |
| Kagami M, Amano Y, Ishii N. 2012. Community structure of planktonic fungi and the impact of parasitic chytrids on phytoplankton in Lake Inba, Japan. Microb. Ecol., 63 (2) : 358-368. |
| Kirchman D L. 2002. The ecology of Cytophaga-Flavobacteria in aquatic environments. FEMS Microbiol. Ecol., 39 (2) : 91-100. |
| Kormas K A, Vardaka E, Moustaka-Gouni M, Kontoyanni V,Petridou E, Gkelis S, Neofitou C. 2010. Molecular detection of potentially toxic cyanobacteria and their associated bacteria in lake water column and sediment.World J. Micorb. Biot., 26 (8) : 1 473-1 482. |
| Kowalchuk G A, Stephen J R, De Boer W, Prosser J I, Embley T M, Woldendorp J W. 1997. Analysis of ammoniaoxidizing bacteria of the beta subdivision of the class Proteobacteria in coastal sand dunes by denaturing gradient gel electrophoresis and sequencing of PCRamplified 16S ribosomal DNA fragments. Appl. Environ.Microbiol., 63 (4) : 1 489-1 497. |
| Lepš J, Šmilauer P. 2003. Multivariate Analysis of Ecological Data Using CANOCO. Cambridge University Press,Cambridge, United Kingdom. 282p. |
| Li Q, Zhao Y, Li Y H, Wei Z M. 2013a. DGGE analysis of picoeukaryotes genetic diversity and relationship with environmental factors. J. Northeast Agri. Univ., 44 (8) : 70-75. (in Chinese with English abstract) |
| Li X M, Yu Y H, Zhang T L, Feng W S, Ao H Y, Yan Q Y. 2012.Seasonal variation of plankton communities influenced by environmental factors in an artificial lake. Chin. J.Oceanol. Limnol., 30 (3) : 397-403. |
| Li Y H, Xu Q G, Zhao Y, Li Q, Wei Z M, Zhao X Y. 2013b.Bacterial community structure in different spatial distribution of Songhua Lake. J. Agro-Environ. Sci., 32 (4) : 764-770. (in Chinese with English abstract) |
| Lindström E S. 2000. Bacterioplankton community composition in five lakes differing in trophic status and humic content. Microb. Ecol., 40 (2) : 104-113. |
| Lindström E S. 2001. Investigating influential factors on bacterioplankton community composition: results from a field study of five mesotrophic lakes. Microb. Ecol., 42 (4) : 598-605. |
| Lyautey E, Lacoste B, Ten-Hage L, Rols J L, Garabetian F. 2005. Analysis of bacterial diversity in river biofilms using 16S rDNA PCR-DGGE: methodological settings and fingerprints interpretation. Water Res., 39 (2-3) : 380-388. |
| MacNaughton S J, Stephen J R, Venosa A D, Davis G A, Chang Y J, White D C. 1999. Microbial population changes during bioremediation of an experimental oil spill. Appl.Environ. Microbiol., 65 (8) : 3 566-3 574. |
| Muyzer G. 1999. DGGE/TGGE a method for identifying genes from natural ecosystems. Curr. Opin. Microbiol., 2 (3) : 317-322. |
| Muyzer G, de Waal E C, Uitterlinden A G. 1993. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reactionamplified genes coding for 16S rRNA. Appl. Environ.Microbiol., 59 (3) : 695-700. |
| Newton R J, Jones S E, Eiler A, McMahon K D, Bertilsson S. 2011. A guide to the natural history of freshwater lake bacteria. Microbiol. Mol. Biol. Rev., 75 (1) : 14-49. |
| Oikonomou A, Katsiapi M, Karayanni H, Moustaka-Gouni M,Kormas K A. 2012. Plankton microorganisms coinciding with two consecutive mass fish kills in a newly reconstructed lake. ,Sci. World J. 2012 : Article ID 504135. |
| OvreÅs L, Forney L, Daae F L, Torsvik V. 1997. Distribution of bacterioplankton in meromictic Lake Saelenvannet, as determined by denaturing gradient gel electrophoresis of PCR-amplified gene fragments coding for 16S rRNA.Appl. Environ. Microbiol., 63 (9) : 3 367-3 373. |
| Richards T A, Vepritskiy A A, Gouliamova D E, Nierzwicki-Bauer S A. 2005. The molecular diversity of freshwater picoeukaryotes from an oligotrophic lake reveals diverse, distinctive and globally dispersed lineages. Environ.Microbiol., 7 (9) : 1 413-1 425. |
| Sapp M, Wichels A, Wiltshire K H, Gerdts G. 2007. Bacterial community dynamics during the winter-spring transition in the North Sea. FEMS Microbiol. Ecol., 59 (3) : 622-637. |
| Sommer U, Gliwicz Z M, Lampert W, Duncan A. 1986. The PEG-model of seasonal succession of planktonic event in fresh waters. Arch. Hydrobiol., 106 (4) : 433-471. |
| Sommer U. 1996. Plankton ecology: The past two decades of progress. Naturwiss enschaften, 8 3 (7) : 293-301. |
| Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol.Evol., 28 (10) : 2 731-2 739. |
| Tan X, Kong F X, Zeng Q F, Cao H S, Qian S Q, Zhang M. 2009. Seasonal variation of Microcystis in Lake Taihu and its relationships with environmental factors. J. Environ.Sci. China, 21 (7) : 892-899. |
| Tarbe A L, Stenuite S, Balagué V, Sinyinza D, Descy J P,Massana R. 2011. Molecular characterisation of the smalleukaryote community in a tropical Great Lake (Lake Tanganyika, East Africa) . Aquat. Microb. Ecol., 62 (2) : 177-190. |
| Wu L, Yu Y H, Zhang T L, Feng W S, Zhang X, Li W. 2009a.PCR-DGGE fingerprinting analysis of plankton communities and its relationship to lake trophic status.Int. Rev. Hydrobiol., 94 (5) : 528-541. |
| Wu Q L, Chatzinotas A, Wang J J, Boenigk J. 2009b. Genetic diversity of eukaryotic plankton assemblages in eastern Tibetan lakes differing by their salinity and altitude.Microb. Ecol., 58 (3) : 569-581. |
| Xuan H X, An S Q, Sun Q Y, Zhou C F. 2011. Diversity of aquatic fungi in different areas of Lake Taihu. J. Lake Sci., 23 (3) : 469-478. (in Chinese with English abstract) |
| Yan Q Y, Yu Y H. 2011. Metagenome-based analysis: a promising direction for plankton ecological studies. ,Sci.China Life. Sci. 54 (1) : 75-81. |
| Yan Q Y, Yu Y H, Feng W S, Deng W N, Song X H. 2007.Genetic diversity of plankton community as depicted by PCR-DGGE fingerprinting and its relation to morphological composition and environmental factors in Lake Donghu. Microb. Ecol., 54 (2) : 290-297. |
| Yan Q Y, Yu Y H, Feng W S, Yu Z G, Chen H T. 2008. Plankton community composition in the Three Gorges Reservoir Region revealed by PCR-DGGE and its relationships with environmental factors. J. Environ. Sci. (China) , 20 (6) : 732-738. |
| Yu Y H, Yan Q Y, Feng W S. 2008. Spatiotemporal heterogeneity of plankton communities in Lake Donghu, China, as revealed by PCR-denaturing gradient gel electrophoresis and its relation to biotic and abiotic factors. FEMS Microbiol. Ecol., 63 (3) : 328-337. |
| Zeng J, Yang L Y, Xiao L, Yin D Q, Qin B Q. 2007.Biogeochemical cycling of nitrogen in lakes and the role of microorganisms in conversion of nitrogen compounds.J. Lake Sci., 19 (4) : 382-389. (in Chinese with English abstract) |
| Zhang Z S, Huang X F. 1991. Research Methods of Freshwater Plankton. Science Press, Beijing, China. p.333-347. (in Chinese) |
| Zhao B Y, Chen M J, Sun Y, Yang J X, Chen F Z. 2011. Genetic diversity of picoeukaryotes in eight lakes differing in trophic status. Can. J. Microbiol., 57 (2) : 115-126. |
| Zhong X, Berdjeb L, Jacquet S. 2013. Temporal dynamics and structure of picocyanobacteria and cyanomyoviruses in two large and deep peri-alpine lakes. "FEMS Microbiol.Ecol., 86 (2) : 312-326. |
 2015, Vol. 33
2015, Vol. 33