Shanghai University
Article Information
- REN Hai (任海), LI Jian (李健), LI Jitao (李吉涛), LIU Ping (刘萍), LIANG Zhongxiu (梁忠秀), WU Jianhua (吴建华)
- Transcript profiles of mitochondrial and cytoplasmic manganese superoxide dismutases in Exopalaemon carinicauda under ammonia stress
- Chinese Journal of Oceanology and Limnology, 2015, 33(3): 714-724
- http://dx.doi.org/10.1007/s00343-015-4143-5
Article History
- Received May 7, 2014;
- accepted in principle Jul. 22, 2014;
- accepted for publication Sep. 6, 2014
2 Key Laboratory of Sustainable Development of Marine Fisheries, Ministry of Agriculture, Yellow Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences, Qingdao 266071, China
The ridgetail white prawn Exopalaemon carinicauda is an important cultured economic shrimp species in China (Xu et al., 2010). However, intensified farming and deteriorating conditions in the aquatic environment have resulted in frequent disease outbreaks, for example, “milky shrimp” disease (Xu et al., 2010) and WSSV (Shen et al., 2013). The health of aquatic animals depends on complex interactions among the environment, the pathogen, and the host (Lightner and Redman, 1998). Environmental factors affect not only the abundance of pathogens, but also the disease-resistance of shrimps (Huang et al., 2002). Ammonia is a toxic substance that is often present in aquaculture water and negatively affects aquatic species (Pan et al., 2003). Ammonia is the main product of protein catabolism in decapoda crustaceans, and is generated mainly in the mineralization process of organic wastes such as unconsumed feed and feces (Chen et al., 2012). Elevated concentrations of ammonia in the culture environment result in ammonia accumulation in the body, inducing oxidative damage and negatively affecting the health of the organism (Maltby 1995; Pan et al., 2003; Nimptsch and Plugmacher 2007; Xu et al., 2012) .
Superoxide dismutase (SOD) plays a critical role in the antioxidant defense pathway against oxidative stress (Fridovich, 1995; Chandra et al., 2000; Areekit et al., 2011). This enzyme catalyzes a dismutation reaction in which the superoxide radical is converted to hydrogen peroxide and molecular oxygen (Pipe et al., 1993). In decapod crustaceans, there are two types of manganese SOD; the mitochondrial MnSOD and the cytosolicMnSOD (encoded by mMnSOD and cMnSOD, respectively). Mitochondrial MnSODs are found in plants, bacteria, vertebrates, and invertebrates, while the cytosolic SOD is found only in crustaceans (Li et al., 2010). Both cMnSOD and mMnSOD have been cloned in crustaceans, including Callinectes sapidus (AF264029, AF264030), Macrobrachium rosenbergii (DQ073104, DQ157765), Marsupenaeus japonicus (GQ181123, GQ478988), Fenneropenaeus chinensis (EF427949, DQ205424), Procambarus clarkii (EU254488, FJ892724), Cherax quadricarinatus (JQ040506, JQ763321), and Penaeus monodon (AY726542, KC461130) .
Previous studies have shown that mMnSOD and cMnSOD are induced in response to pathogen infection and environmental stress in marine invertebrate animals, and their expressions are significantly induced in shrimps in response to bacteria (Li et al., 2010; Lin et al., 2010), viruses (Gómez-Anduro et al., 2006; Zhang et al., 2007), and changes in pH (Wang et al., 2009) and dissolved oxygen levels (García-Triana et al., 2010). However, little is known about the roles of mMnSOD and cMnSOD in the ammonia stress response of penaeid species.
The aims of this study were to isolate and characterize the mMnSOD cDNA from E. carinicauda, and to evaluate the responses of mMnSOD and cMn SOD in various tissues of E. carinicauda to ammonia stress. 2 MATERIAL AND METHOD 2.1 Animal rearing
Healthy adult E. carinicauda with an average body weight of 3.67±0.25 g were collected from the Haifeng Aquaculture Co. Ltd. (Sh and ong, China). Before the experiment, the prawns were cultured in filtered, aerated seawater (salinity 30.18, pH 8.13) at 20.6±0.2°C for 1 week. During the acclimation period, the prawns were fed twice daily with commercial prawn pellets. One-third of the water in each group was exchanged every day. 2.2 Total RNA isolation from hepatopancreas and cDNA preparation
Total RNA was isolated from the hepatopancreas using Trizol Reagent (Invitrogen, Carlsbad, CA, USA), following the manufacturer’s instructions. Genomic DNA was removed from the RNA using DNase I (Promega, Madison, WI, USA). Singlestr and ed cDNA was synthesized using M-MLV reverse transcriptase (TaKaRa, Japan) according to the manufacturer’s instructions. The 3′- and 5′-end rapid amplification of cDNA ends (RACE) cDNA templates were obtained using a SMART cDNA Kit (Clontech, Palo Alto, CA, USA), according to the manufacturer’s instructions. 2.3 Cloning of E. carinicauda mMnSOD
The full-length mMnSOD cDNA of E. carinicauda was obtained using expressed sequence tag (EST) and RACE methods. The EST sequences were obtained from the cDNA library of E. carinicauda in our laboratory. Basic local alignment search tool (BLAST) analysis indicated that the EST sequences were similar to the sequences of mMnSODs of other shrimps. A GSP (gene-specific primer) F1 was designed for 3′ RACE, and GSP R1 was designed for 5′ RACE based on the EST sequences (Table 1). According to partial sequence data of mMnSOD, the 3′ and 5′ ends of the mMnSOD cDNA were obtained using a SMART RACE cDNA Amplification Kit (Clontech). For 5′- and 3′-RACE, PCRs were conducted using the cDNA template from hepatopancreas RNA and the primers GSP R1, GSP F1, and the anchor universal primer (UPM). The PCR reaction conditions were those described in the SMART cDNA Kit manual.
The PCR products were separated on a 1.0% agarose gel. After electrophoresis, the b and was excised and purified with a gel extraction kit (Sangon Biotech Co. Ltd., Shanghai, China). Then, the purified PCR fragments were cloned into the pMD18-T simple vector according to the manufacturer’s instructions (TaKaRa, Otsu, Japan), and transformed into Escherichia coli DH 5α. Positive clones were selected based on blue/white spot selection and validated by PCR, and then sequenced by the Sunny Biotechnology Co. Ltd. (Shanghai, China). 2.4 Sequence analysis
We conducted BLAST searches using nucleotide sequences as well as the deduced amino acid sequence of mMnSOD cDNA (http://www.ncbi.nlm.nih.gov/BLAST/). The SignalP program (www.cbs.dtu.dk/services/SignalP/tool.html) was used to identify the signal peptide, and ExPASy tools were used to search for the targeting sequence. Multiple alignment of the ridgetail white prawn mMnSOD gene was conducted using DNAMAN software (Lynnon Biosoft, Vaudreuil, Canada). A neighbor-joining phylogenetic tree of mMnSODs was constructed using MEGA 4.0 software (Tamura et al., 2007). 2.5 Expressions of mMnSOD in different tissues
The transcript levels of mMnSOD mRNA in hemocytes, stomach, hepatopancreas, gill, eyestalk, ovary, and muscle were analyzed by RT-qPCR. Total RNA and first-strand cDNA were obtained as described above. The β-actin gene of E. carinicauda (accession No. JQ 045354) was used as the endogenous reference gene. 2.6 Exposure to ammonia
After acclimation, 180 prawns (average weight, 3.67±0.25 g) were r and omly selected and distributed evenly among 12 plastic containers, each with a 40-L volume. Six containers were designated as the ammonia stress group, and six as the control group. In the ammonia stress group, the concentration of ammonia-N was set at 34.87±0.46 mg/L, based on our previous experimental results (Ren et al., 2014). The total ammonia nitrogen (TAN) levels were measured and adjusted by adding NH 4 Cl (20 g/L) solution every day. The experiment was 72 h long and no feed was supplied.
Six prawns were r and omly selected from the ammonia stress group and the control group at 1, 3, 6, 12, 24, 48, and 72 h. The hemocytes and the hepatopancreas were collected for total RNA extraction, and single-str and ed cDNA was synthesized as described above. 2.7 Analysis of transcript profiles of mMnSOD and cMnSOD after ammonia exposure
We analyzed the transcript levels of mMnSOD and cMnSOD by qRT-PCR using an ABI PRISM 7500 Sequence Detection System (Applied Biosystems, Foster City, CA, USA). The primers used to amplify mMnSOD, cMnSOD, and β-actin are shown in Table 1. mMnSOD mRNA was detected as a 144-bp PCR product using the primers F2 and R2. The cMnSOD mRNA was detected as a 174-bp product using the GSPs F3 and R3, which were designed according to the available sequence in NCBI (GenBank accession no. KC819309). The endogenous reference gene β-actin was detected as a 114-bp product using the primers actin-F and actin-R (Table 1). The RT-PCR amplifications were performed in triplicate. Each RTPCR mixture contained 3.0 μL RNA-free water, 5.0 μL TaKaRa SYBR Premix Ex Taq II (2×), 0.4 μL each primer, 0.2 μL Rox reference Dye II (50×) and 1 μL cDNA (1:3 dilution). The PCR reaction conditions were those described in the manufacturer’s protocol. A negative control was prepared by replacing the template with diethypyrocarbonate (DEPC) -treated water. The RT-qPCR data were analyzed using SDS Software V2.1 (Applied Biosystems) to estimate the transcript copy numbers in each sample. The relative transcript levels of mMnSOD and cMnSOD were determined using the ΔΔC t method (Cheng et al., 2006). 2.8 Statistical analysis
All values are mean±st and ard deviation (n =3). Data were analyzed by one-way ANOVA (González-Rodríguez et al., 2012) using SPSS 16.0 software. 3 RESULT 3.1 Sequence analysis of mMnSOD cDNA
The cDNA sequence of E. carinicauda mMnSOD has been deposited in GenBank under the accession number KF156268. The full-length mMnSOD cDNA was 1 014-bp long, and contained a 37-bp 5′-untranslated region (UTR), a 321-bp 3′-UTR, a putative polyadenylation signal (AATAAA), and a 26-bp poly (A) tail. The open reading frame (ORF) was predicted to encode a protein of 218 amino acids with a 16-amino-acid signal peptide, as predicted by SignalP (Fig. 1). The protein had a calculated molecular mass of 23.87 kDa and a theoretical isoelectric point of 6.75. The mMnSODs equence contained two theoretical N-glycosylation sites (NHT and NLS), the MnSOD signature sequence (180 DVWEHAYY187), and four putative Mn binding sites (H48, H96, D180, and H184) (Fig. 1).
 |
|
Fig. 1 Nucleotide sequence of E. carinicauda mMnSOD cDNA and deduced amino acid sequence Black boxes show start codon (ATG) and stop codon (TAG) ; double underline shows mitochondrial-targeting sequence peptide; red box: polyadenylation signal sequence (AATAAA) ; green boxes: potential N-glycosylation sites NHT and NLS; underlining shows putative mMnSODs ignature; gray highlight shows theoretical manganese binding sites (H48, H96, D180, and H184). |
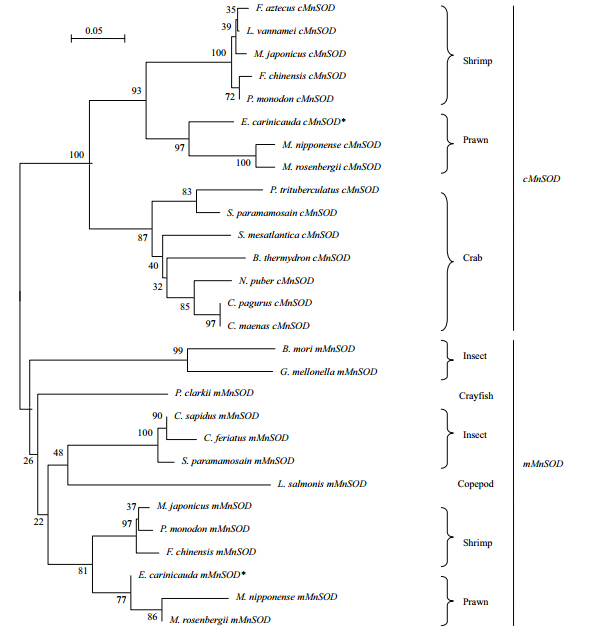
The BLASTP analysis revealed that the deduced amino acid sequence of mMnSODs howed high similarities to those of M. rosenbergii (97% identity), Macrobrachium nipponense (95% identity), F. chinensis (89% identity), C. sapidus (84% identity), P erisesarma b idens (82% identity), Danio rerio (72% identity), and Homo sapiens (69% identity). We conducted a multiple alignment comparing the E. carinicauda mMnSOD with those of other animals (Fig. 2). This analysis revealed that there was strong conservation of a potential N-glycosylation site (NLS) and the putative MnSOD signature (180 DVWEHAYY 187). A phylogenetic tree was constructed using MnSODs sequences with MEGA 4.0 software (Fig. 3). mMnSOD and cMnSOD from E. carinicauda were each clustered in subgroups, and were significantly divided. The mMnSOD of E. carinicauda grouped with mMnSODs of M. nipponense and M. rosenbergii, but was distant from the cMnSODs of M. nipponense and M. rosenbergii and other shrimps (Fig. 3).
 |
|
Fig. 2 Multiple sequence alignments for mMnSOD proteins from different species Amino acid sequences of mMnSODs from B os Taurus (AAX09005), Callinectes sapidus (AAF74770), D anio rerio (AAP34300), Fenneropenaeus chinensis (ABB05539), Homo sapiens (AAP36352), M acrobrachium. nipponense (AEK77428), Macrobrachium rosenbergii (AAZ81617), M arsupenaeus japonicus (ADB90402), P enaeus monodon (AGI99530), Perisesarma bidens (CAR82604), Procambarus clarki i (AGH30393), S cylla paramamosain (ACM61856), Sparus aurate (AFV39807), T aeniopygia guttata (ACH44745). Potential N-glycosylation sites (NHT/S and NLS) and putative signature (180 DVWEHAYY 187) are boxed. The assumed manganese binding sites, namely H48, H96, D180 and H184, are marked by black arrows. |
 |
|
Fig. 3 Phylogenetic tree for cMnSOD and mMnSOD proteins * indicates cMnSOD and mMnSOD of E. carinicauda. Abbreviations of arthropod species and GenBank accession numbers are as follows: F arfantepenaeus aztecus (AAO42752), L itopeneaus vannamei (ABC59529), M arsupenaeus japonicus (BAB85211, ADB90402), Fenneropenaeus chinensis (ACS49842, ABB05539), P enaeus monodon (AAW50395, AGI99530), M acrobrachium. nipponense (AEK77429, AEK77428), Macrobrachium rosenbergii (ABU55005, AAZ81617), P ortunus trituberculatus (ACH99175), S cylla paramamosain (ADA63848, ACM61856), S egonzacia mesatlantica (CAR85670), B ythograea thermydron (CAR85668), N ecora puber (CAR85664), C ancer pagurus (CAR85665), C arcinus maenas (CAR85674), B ombyx mori (NP_001037299), G alleria mellonella (ABR08302), Procambarus clarki i (AGH30393), C allinectes sapidus (AAF74770), C harybdis feriatus (O96347) and Lepeophtheirus salmonis (ADD24212) . |
The relative transcript levels of mMnSOD in different tissues (hemocytes, hepatopancreas, gill, stomach, eyestalk, ovary, and muscle) were measured by fluorescence RT-PCR. We used the β-actin gene was used as the normalization gene. The highest mMnSOD transcript levels were in the hepatopancreas, followed by gill, and the lowest transcript levels were in the ovary (Fig. 4).
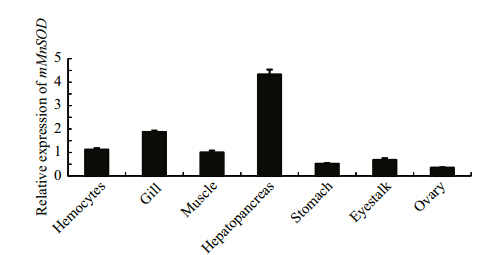 |
| Fig. 4 Relative transcript levels of mMnSOD in different tissues of E. carinicauda as determined by RT-qPCR |
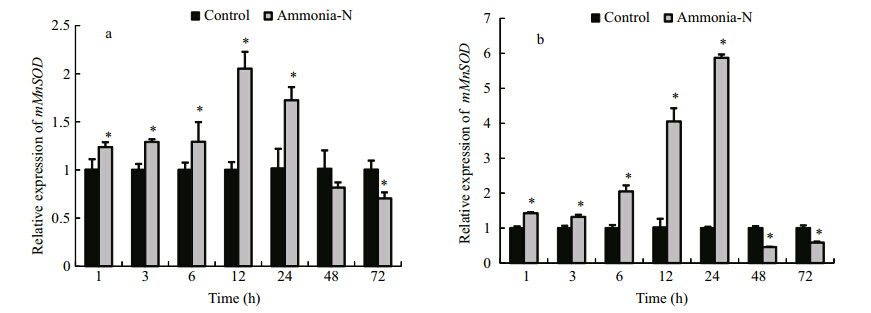
We evaluated the transcript levels of mMnSOD in hemocytes and the hepatopancreas after exposure to ammonia stress (Fig. 5). Compared with the control group, the ammonia-stressed group showed significant increases in mMnSOD transcript levels in the hemocytes at 1 h to 24 h of ammonia stress (P < 0.05), followed by a decrease to the minimum value at 72 h (0.70-fold that at 0 h;P < 0.05) (Fig. 5a). The mMnSOD transcript profile in the hepatopancreas was similar to that in the hemocytes. The mMnSOD transcript levels in the hepatopancreas significantly increased from 1 h to 24 h of ammonia stress (P < 0.05), and then decreased at 48 h and 72 h (P < 0.05) (Fig. 5b).
 |
|
Fig. 5 Transcript levels of mMnSOD in hemocytes (a) and the hepatopancreas (b) of E. carinicauda under ammonia stress as determined by fluorescence RT-PCR Relative values are shown (normalized against β-actin transcript level). Values are mean±SD (n =3). * indicates significant difference (P < 0.05) between ammonia-stressed group and control at each time point. |
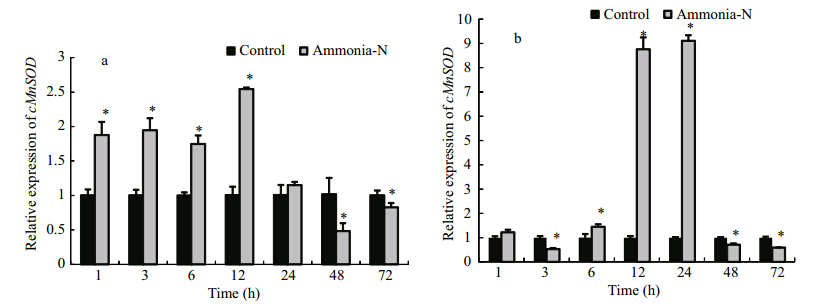
We evaluated changes in the transcript levels of cMnSOD in hemocytes and the hepatopancreas of E. carinicauda during an ammonia stress treatment (Fig. 6). The transcript levels of cMnSOD mRNA in hemocytes were significantly up-regulated at 1 h to 12 h of ammonia stress (P < 0.05), and then decreased to a level significantly lower than that in the control group at 48 h and 72 h (P < 0.05) (Fig. 6a). In the hepatopancreas, the transcript level of cMnSOD significantly decreased at 3 h of ammonia stress, increased further from 6 h to 24 h, and reached the maximum level at 24 h (9.10-fold that at 0 h;P < 0.05). Then, the transcript level sharply decreased from 48 h to 72 h to a level lower than that in the control group (P < 0.05) (Fig. 6b).
 |
|
Fig. 6 Transcript levels of cMnSOD transcript in hemocytes (a) and the hepatopancreas (b) of E. carinicauda under ammonia stress, as determined by fluorescence RT-PCR Relative values are shown (normalized against β-actin transcript level). Values are mean±SD (n =3). * indicates significant difference (P < 0.05) between ammonia-stressed group and control at each time point. |
mMnSOD genes have been cloned and characterized in M. rosenbergii (Cheng et al., 2006), F. chinensis (Zhang et al., 2007), hydrothermal Bythograeidae and littoral crabs (Marchand et al., 2009), and M. japonicus (Lin et al., 2010). However, the mMnSOD in E. carinicauda had not been described. In the present study, the full-length 1 014-bp cDNA of mMnSOD was cloned from E. carinicauda. Sequence analyses revealed conserved sequences and characteristic motifs, such as the MnSOD signature (180DVWEHAYY187) and four putative Mn binding sites (H48, H96, D180, and H184), which are consistent with other mMnSODs equences (Cheng et al., 2006; Marchand et al., 2009; Li et al., 2010; Lin et al., 2010). These structures are highly conserved among the mMnSODs of vertebrates and invertebrates. However, the putative N-glycosylation sites NHT and NLS differ among species. The multiple sequence alignment showed that the E. carinicauda mMnSOD sequence shares 82% to 97% identities with mMnSODs from other crustaceans. The presence of signature sequences and conserved Mn binding sites, and the high homology to mMnSODs equences from other species suggested that the mMnSOD from E. carinicauda encodes a member of the MnSOD family. In molecular phylogeny analyses, MnSODs group into two distinct clusters: one cluster consists of mMnSODs, and the other cluster, which is distant from the first cluster, consists of cMnSODs. The mMnSOD cluster contains mMnSODs equences from diverse organisms such as plants, bacteria, vertebrates and invertebrates, whereas the latter cluster contains sequences from crustaceans (crab, crayfish, prawn, and shrimp) (Li et al., 2010). In the present study, the mMnSOD and cMnSOD of E. carinicauda were segregated into two divergent groups. This suggested that MnSOD gene duplication event occurred at an early stage of arthropod evolution, The mMnSODs from crustaceans and other eukaryotes share a common ancient ancestor, and their sequences have been strongly conserved. The gene encoding cMnSOD probably originated from a gene duplication event that resulted in a protein with a new subcellular location (Brouwer et al., 2003; Gómez-Anduro et al., 2006; Li et al., 2010) .
We detected transcripts of mMnSOD in all of the tested tissues of E. carinicaud a. This result is consistent with fact that SODs participate in many biological processes (Meng et al., 2013). Similar results were have been reported for the kuruma shrimp M. japonicus (Lin et al., 2010) and the giant freshwater prawn M. rosenbergii (Cheng et al., 2006). The highest transcript levels of mMnSOD were in the hepatopancreas, which is the metabolic center for exogenous material in crustaceans. This result suggested that mMnSOD plays an important role in the antioxidant defense system in the hepatopancreas.
Ammonia is a major pollutant in aquatic environments. It originates from industrial waste, agricultural run-off, and the mineralization process of organic wastes such as feces and unconsumed feed (Randall et al., 2002; Chen et al., 2012). High concentrations of ammonia in water lead to the accumulation of ammonia in the tissues of aquatic organisms, triggering the release of reactive oxygen species (ROS). The oxidative stress imposed by ammonia can increase the susceptibility of marine organisms to microbial attack (Liu et al., 2004), and increase the mortality of organisms (Chen et al., 1990). SODs are metalloenzymes that catalyze the dismutation of superoxide anions. These enzymes play a critical role in antioxidant defenses (Li et al., 2010; Lin et al., 2010). Previous studies have shown that ammonia stress significantly affects SOD activity. For example, when the white shrimp L. vannamei was exposed was 21.60 mg/L ammonia, SOD activity markedly decreased. This caused a negative immune response, which increased mortality due to infection by Vibrio alginolyticus (Liu et al., 2004). Also, a short-term increase in SOD activity was observed in gill, hepatopancreas, and muscle tissues of Scylla paramamosain after exposure to a low concentration of ammonia (Zeng et al., 2011) .
Several previous studies have shown that in crustaceans, mMnSOD genes are induced rapidly by WSSV, β-glucan, V. alginolyticus, and lipopolysaccharide (LPS) (Zhang et al., 2007; Lin et al., 2010; Sook Chung et al., 2012). In the present study, ammonia rapidly and significantly induced mMnSOD in hemocytes and the hepatopancreas of E. carinicauda. Compared with the control group, the ammonia-stressed group showed significant increases in mMnSOD transcript levels in hemocytes and the hepatopancreas from 1 h to 24 h of the ammonia stress treatment, with maximum levels at 12 h and 24 h, respectively. A significant increase in the mRNA levels of mMnSOD observed in ammonia stress group could be due to the ROS stimulation produced by ammonia stress, the result may indicate that mMnSOD plays a vital role in the oxidative stress response in E. carinicauda. As the duration of the ammonia stress treatment extended, the transcript levels of mMnSOD in hemocytes and the hepatopancreas decreased to low levels at 48 h to 72 h. This reduction was probably because mMnSOD expression was inhibited by excess ROS that accumulated in response to ammonia. An excess of ROS can result in damage to the normal functions of cells and tissues. Similar results have been reported in F. chinensis, in which mMnSOD plays an essential role in removing excess superoxide radicals (Zhang et al., 2007). The transcript profile analysis showed that mMnSOD is inducible by ammonia, suggesting that mMnSOD plays a role in the antioxidant defense response of E. carinicauda.
The expression of cMnSODs in crustaceans can be significantly induced by changes in pH (Wang et al., 2009), WSSV (Gómez-Anduro et al., 2006), hypoxia, and reoxygenation (García-Triana et al., 2010). These patterns of induction suggest that cMnSOD plays a vital role in regulating the redox system in response to abiotic and biotic stresses. In this study, cMnSOD transcript levels in ammonia-stressed E. carinicauda reached maximum levels at 12 h in hemocytes and at 24 h in the hepatopancreas. This may indicate that the hemocytes and the hepatopancreas have different roles in the antioxidant defense system. The hepatopancreas is the main metabolic center for ROS production in crustaceans (Duan et al., 2013). Excess ROS in hepatopancreas may have inhibited the normal function of cells, resulting in decreased levels of cMnSOD transcripts at 3 h of the stress treatment. The results suggested that cMnSOD might be involved in the antioxidant response to environmental stress. The relative expression level of cMnSOD in ammoniastressed E. carinicauda significantly decreased at 48 h to 72 h in both hemocytes and the hepatopancreas. This result was consistent with those of previous studies, which reported that antioxidant enzyme mRNA expression depends on the duration and the dose of the stress treatment, and is related to the metabolic capacity of the organism (Zhang et al., 2004; Jo et al., 2008; Park et al., 2009) .
Superoxide dismutases are very important enzymes in antioxidant defense pathways (Fridovich, 1995). In the present study, both cMnSOD and mMnSOD mRNA levels increased in hemocytes and the hepatopancreas of E. carinicauda in response to ammonia stress. In another study, cMnSOD and mMnSOD transcript levels greatly increased in hemocytes of M. japonicus after challenges with V. alginolyticus or β-glucan (Lin et al., 2010). In P. clarkii, the mRNA levels of three SODs (Cu-ZnSOD, mMnSOD, and cMnSOD) in the hepatopancreas and hemocytes increased in response to challenges by Spiroplasma eriocheiris and Ameromonas hydrophila (Marchand et al., 2009). Similarly, mMnSOD and cMnSOD transcript levels markedly increased in hemocytes of C. sapidus after challenge with LPS (Zeng et al., 2011). Those findings showed that abiotic factors and biotic stressors can cause a variety of disorders, and result in the production of ROS (Tang et al., 2012). As the main antioxidant enzymes, MnSODs eliminate superoxide anions, thereby avoiding damage to physiological metabolism and the redox balance when the host encounters abiotic or biotic stresses (Fridovich, 1975; Zhang et al., 2007; Park et al., 2009). More research is needed to explore the mechanism by which ammonia stress affects MnSODs in E. carinicauda.
In summary, a 1 014-bp mMnSOD cDNA was cloned from the hepatopancreas of E. carinicauda. Sequence analyses confirmed that it encodes a member of the SOD family. We detected mMnSOD mRNAs in the hemocytes, stomach, hepatopancreas, gill, ovary, eyestalk, and muscle. Both mMnSOD and cMnSOD were rapidly induced in hemocytes and the hepatopancreas in response to ammonia stress. These findings suggest that both mMnSOD and cMnSOD are involved in the antioxidant defense response against environmental stress in E. carinicauda.
| Areekit S, Kanjanavas P, Khawsak P, Pakpitchareon A,Potivejkul K, Chansiri G, Chansiri k. 2011. Cloning, expression, and characterization of thermotolerant manganese superoxide dismutase from Bacillus sp.MHS47. International Journal of Molecular Sciences, 12 (1): 844-856, http://dx.doi.org/10.3390/ijms12010844. |
| Brouwer M, Hoexum Brouwer T, Grater W, Brown-Peterson N. 2003. Replacement of a cytosolic copper/zinc superoxide dismutase by a novel cytosolic manganese superoxide dismutase in crustaceans that use copper (haemocyanin) for oxygen transport. Biochem ical J ournal, 374 (Pt 1): 219-228, http://dx.doi.org/10.1042/BJ20030272. |
| Chandra J, Samali A, Orrenius S. 2000. Triggering and modulation of apoptosis by oxidative stress. Free Radical Biology and Medicine, 29 (3-4): 323-333, http://dx.doi. org/10.1016/S0891-5849(00)00302-6. |
| Chen J C, Liu P C, Lei S C. 1990. Toxicity of ammonia and nitrite to Penaeus monodon adolescents. Aquaculture, 89 (2): 127-137, http://dx.doi.org/10.1016/0044-8486(90) 90305-7. |
| Chen Y Y, Sim S S, Chiew S L, Yeh S T, Liou C H, Chen J C. 2012. Dietary administration of a Gracilaria tenuistipitata extract produces protective immunity of white shrimp Litopenaeus vannamei in response to ammonia stress.Aquaculture, 370-371 : 26-31, http://dx.doi.org/10.1016/j. aquaculture.2012.09.031. |
| Cheng W, Tung Y H, Chiou T T, Chen J C. 2006. Cloning and characterisation of mitochondrial manganese superoxide dismutase (mtMnSOD) from the giant freshwater prawn Macrobrachium rosenbergii. Fish & Shellfish Immunology, 21 (4): 453-466, http://dx.doi.org/10.1016/j. fsi.2006.02.005. |
| Cheng W T, Tung Y H, Liu C H, Chen J C. 2006. Molecular cloning and characterisation of cytosolic manganese superoxide dismutase (cytMn-SOD) from the giant freshwater prawn Macrobrachium rosenbergii. Fish &Shellfish Immunology, 20 (4): 438-449, http://dx.doi. org/10.1016/j.fsi.2005.05.016. |
| Duan Y F, Liu P, Li J T, Li J, Chen P. 2013. Expression profiles of selenium dependent glutathione peroxidase and glutathione S-transferase from Exopalaemon carinicauda in response to Vibrio anguillarum and WSSV challenge.Fish & Shellfish Immunology, 35 (3): 661-670, http:// dx.doi.org/10.1016/j.fsi.2013.05.016. |
| Fridovich I. 1975. Superoxide dismutases. Annual Review of Biochemistry, 44 : 147-159, http://dx.doi.org/10.1146/ annurev.bi.44.070175.001051. |
| Fridovich I. 1995. Superoxide radical and superoxide dismutases. Annual Review of Biochemistry, 64 : 97-112, http://dx.doi.org/10.1146/annurev.bi.64.070195.000525. |
| García-Triana A, Zenteno-Savín T, Peregrino-Uriarte AB,Yepiz-Plascencia G. 2010. Hypoxia, reoxygenation and cytosolic manganese superoxide dismutase (cMnSOD) silencing in Litopenaeus vannamei : effects on cMnSOD transcripts, superoxide dismutase activity and superoxide anion production capacity. Developmental & Comparative Immunology, 34 (11): 1 230-1 235, http://dx.doi.org/10. 1016/j.dci.2010.06.018. |
| Gómez-Anduro G A, Barillas-Mury C V, Peregrino-Uriarte A B, Gupta L, Gollas-GalvÁn T, HernÁndez-López J, Yepiz-Plascenciaa G. 2006. The cytosolic manganese superoxide dismutase from the white shrimp Litopenaeus vannamei : molecular cloning and expression. Developmental &Comparative Immunology, 30 (10): 893-900, http://dx.doi. org/10.1016/j.dci.2006.01.002. |
| GonzÁlez-Rodríguez G, Colubi A, Gil M A. 2012. Fuzzy data treated as functional data: a one-way ANOVA test approach. Computational Statistics & Data Analysis, 56 (4): 943-955, http://dx.doi.org/10.1016/j.csda.2010.06. 013. |
| Huang X H, Li C L, Liu C W, Zheng L, He J. 2002. Studies on two microalgae improving environment of shrimp pond and strengthening anti-disease ability of Penaeus vannamei. Acta Hydrobiologica Sinica, 26 (4): 342-347. (in Chinese with English abstract) |
| Jo P G, Choi Y K, Choi C Y. 2008. Cloning and mRNA expression of antioxidant enzymes in the Pacific oyster,Crassostrea gigas in response to cadmium exposure.Comparative Biochemistry and Physiology Part C :Toxicology & Pharmacology, 147 (4): 460-469, http://dx. doi.org/10.1016/j.cbpc.2008.02.001. |
| Li J T, Chen P, Liu P, Gao B Q, Wang Q Y, Li J. 2010. The cytosolic manganese superoxide dismutase cDNA in swimming crab Portunus trituberculatus : molecular cloning, characterization and expression. Aquaculture, 309 (1-4): 31-37, http://dx.doi.org/10.1016/j.aquaculture. 2010.09.008. |
| Lightner DV, Redman R M. 1998, Shrimp disease and current diagnostic methods. Aquaculture, 164 (1-4): 201-220, http://dx.doi.org/10.1016/S0044-8486(98)00187-2. |
| Lin Y C, Lee F F, Wu C L, Chen J C. 2010. Molecular cloning and characterization of a cytosolic manganese superoxide dismutase (cytMnSOD) and mitochondrial manganese superoxide dismutase (mtMnSOD) from the kuruma shrimp Marsupenaeus japonicus. Fish & Shellfish Immunology, 28 (1): 143-150, http://dx.doi.org/10.1016/j. fsi.2009.10.012. |
| Liu C H, Chen J C. 2004. Effect of ammonia on the immune response of white shrimp Litopenaeus vannamei and its susceptibility to Vibrio alginolyticus. Fish & Shellfish Immunology, 16 (3): 321-334, http://dx.doi.org/10.1016/S1050-4648(03)00113-X. |
| Maltby L. 1995. Sensitivity of the crustaceans Gammarus pulex (L.) and Astllus aquaticus (L.) to short-term exposure to hyposia and unionized ammonia: observations and possible mechanism. Water Res earch, 29 (3): 781-787, http://dx.doi.org/10.1016/0043-1354(94)00231-U. |
| Marchand J, Leignel V, Moreau B, Chénais B. 2009.Characterization and sequence analysis of manganese superoxide dismutases from Brachyura (Crustacea:Decapoda): hydrothermal Bythograeidae versus littoral crabs. Comparative Biochemistry and Physiology Part B :Biochemistry and Molecular Biology, 153 (2): 191-199, http://dx.doi.org/10.1016/j.cbpb.2009.02.019. |
| Meng Q G, Chen J, Xu C C, Huang Y Q, Wang Y, Wang T T,Zhai X T, Gu W, Wang W. 2013. The characterization, expression and activity analysis of superoxide dismutases (SODs) from Procambarus clarki i. Aquaculture, 406-407 : 131-140, http://dx.doi.org/10.1016/j.aquaculture. 2013.05.008. |
| Nimptsch J. Pflugmacher S. 2007. Ammonia triggers the promotion of oxidative stress in the aquatic macrophyte,Myriophyllum mattogrossense. Chemosphere, 66 (4): 708-714, http://dx.doi.org/10.1016/j.chemosphere.2006.07.064. |
| Pan C H, Chien Y H, Hunter B. 2003. The resistance to ammonia stress of Penaeus monodon Fabricius juvenile fed diets supplemented with astaxanthin. Journal of Experimental Marine Biology and Ecology, 297 (1): 107-118, http://dx.doi.org/10.1016/j.jembe.2003.07.002. |
| Park H, Ahn I Y, Lee J K, Shin S C, Lee J, Choy E J. 2009.Molecular cloning, characterization, and the response of manganese superoxide dismutase from the Antarctic bivalve Laternula elliptica to PCB exposure. Fish &Shellfish Immunology, 27 (3): 522-528, http://dx.doi.org/ 10.1016/j.fsi.2009.07.008. |
| Pipe R K, Porte C, Livingstone D R. 1993. Antioxidant enzymes associated with the blood cells and haemolymph of the mussel Mytilus edulis. Fish & Shellfish Immunology, 3 (3): 221-333, http://dx.doi.org/10.1006/fsim.1993.1022. |
| Randall D J, Tsui T K N. 2002. Ammonia toxicity in fish.Marine Pollution Bulletin, 45 (1-12): 17-23, http://dx.doi. org/10.1016/S0025-326X(02)00227-8. |
| Ren H, Li J, Li J T, Liang Z X, Liang J P, Ge Q Q, Liu P. 2014.Effects of acute ammonia stresses on antioxidant system enzyme activities and GPx gene expression in Exopalaemon carinicauda. Journal of Agro-Environment Science, 33 (4): 647-655. (in Chinese with English abstract) |
| Shen H, Wan X H, Wang L B, Fei R M, Li S, Bai L, Ling Y, Li H, Gao B. 2013. Study on experimental infection of Exopalaemon carinicauda Holehuis with white spot syndrome virus. Marine Sciences, 37 (5): 55-60. (in Chinese with English abstract) |
| Sook Chung J, Bachvaroff T, Trant J, Place A. 2012. A second copper zinc superoxide dismutase (CuZnSOD) in the blue crab Callinectes sapidus : cloning and up-regulated expression in the hemocytes after immune challenge. Fish & Shellfish Immunology, 32 (1): 16-25, http://dx.doi.org/ 10.1016/j.fsi.2011.08.023. |
| Tamura K, Dudley J, Nei M, Kumar S. 2007. MEGA 4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Molecular Biology and Evolution, 24 (8): 1 596-1 599, http://dx.doi.org/10.1093/molbev/msm092. |
| Tang T, Huang D W, Zhou C Q, Li X, Xie Q J, Liu F S. 2012,Molecular cloning and expression patterns of copper/zinc superoxide dismutase and manganese superoxide dismutase in Musca domestica. Gene, 505 (2): 211-220, http://dx.doi.org/10.1016/j.gene.2012.06.025. |
| Wang W N, Zhou J, Wang P, Tian T T, Zheng Y, Liu Y, Mai W J, Wang A L. 2009. Oxidative stress, DNA damage and antioxidant enzyme gene expression in the Pacific white shrimp Litopenaeus vannamei when exposed to acute pH stress. Comparative Biochemistry and Physiology Part C :Toxicology & Pharmacology, 150 (4): 428-435, http:// dx.doi.org/10.1016/j.cbpc.2009.06.010. |
| Xu D D, Liu X H, Cao J M Du Z Y, Huang Y H, Zhao H X, Xie C X. 2012. Dietary glutathione as an antioxidant improves resistance to ammonia exposure in Litopenaeus vannamei.Aquaculture Research, 43 (2): 311-316, http://dx.doi.org/ 10.1111/j.1365-2109.2011.02820.x. |
| Xu W J, Xie J J, Shi H, Li C W. 2010. Hematodinium infections in cultured ridgetail white prawns, Exopalaemon carinicauda, in eastern China. Aquaculture, 300 (1): 25-31, http://dx.doi.org/10.1016/j.aquaculture.2009.12.024. |
| Zeng Y Y, Jiang Y X, Ai C X. 2011. Effects of ammonia-N stress on the activities of superoxide dismutase and glutathione peroxidase in different tissues and organs of Scylla paramamosain. Journal of Oceanography in Taiwan Strait, 30 (2): 210-215. (in Chinese with English abstract) |
| Zhang J F, Wang X R, Guo H Y, Wu J C, Xue Y Q. 2004.Effects of water-soluble fractions of diesel oil on the antioxidant defenses of the goldfish, Carassius auratus.Ecotoxicology and Environmental Safety, 58 (1): 110-116, http://dx.doi.org/10.1016/j.ecoenv.2003.08.025. |
| Zhang Q L, Li F H, Wang B, Zhang J Q, Liu Y C, Zhou Q,Xiang J H. 2007. The mitochondrial manganese superoxide dismutase gene in Chinese shrimp Fenneropenaeus chinensis : cloning, distribution and expression. Developmental & Comparative Immunology, 31 (5): 429-440, http://dx.doi.org/10.1016/j.dci.2006.08. 005. |
 2015, Vol. 33
2015, Vol. 33



