Shanghai University
Article Information
- WANG Hui (王辉), YANG Hongshuai (杨洪帅), LIU Jiahui (刘加慧) , Li Yanhong (李艳红), LIU Zhigang (刘志刚)
- Combined effects of water temperature and copper ion concentration on catalase activity in Crassostrea ariakensis
- Chinese Journal of Oceanology and Limnology, 2015, 33(4): 905-912
- http://dx.doi.org/10.1007/s00343-015-4212-9
Article History
- Received Sep. 15, 2014;
- accepted in principle Jan. 4, 2015;
- accepted for publication Jan. 7, 2015
2 Fisheries College of Guangdong Ocean University, Zhanjiang 524088, China
Crassostrea ariakensis,which belongs to the genus Crassostrea,family Ostreidae,class Lamellibranchia,phylum Mollusca,is a eurythermic and euryhaline bivalve. Owing to its delicious taste and high nutritional value,this species has become one of the principal economic shellfish harvested along the coast of the southern China seas(Wang et al., 2014).
It is well known that aquatic animals are profoundly affected by the environments they inhabit, and that changes in the water environment can markedly affect the ability of shellfish to resist stress(Littlewood and Ford, 1990; Lacoste et al., 2001). Temperature strongly influences many shellfish through modulation of biological and biochemical reactions such as growth(Bayne,1965),metabolism(Bougrier et al., 1995),reproduction(Fearman and Moltschaniwskyj, 2010) and immunity(Lesser and Kruse, 2004). In addition to the physiological reactions of stress induced upon exposure of aquatic animals to changes in temperature,excess reactive oxygen species(ROS)(inclusive of oxygen free radicals)are produced. These compounds can denature proteins,lipids and nucleic acids,causing further damage to the health of organisms(Lushchak,2011).
Anthropogenic activities have led to severe heavy metals pollution in offshore sea areas. Since many shellfish of economic importance are distributed in these areas,they commonly take up heavy metals. Copper ion(Cu2+),which is most toxic to C. ariakensis(Jiang and Niu, 2006),impedes the embryonic and larval development of C. ariakensis(Coglianese and Martin, 1981) and contributes to the production of excess oxygen free radicals and oxidative stress,which affects the expression of antioxidase genes and enzymatic activities(Regoli et al., 1998; Pipe et al., 1999; Bebianno et al., 2004; Maria and Bebianno, 2011). These findings indicate that heavy metal copper ions may affect the oxidation-reduction equilibrium in shellfish.
Under normal conditions,the level of oxygen free radicals is maintained through a dynamic equilibrium mechanism in aquatic animals. Changes in temperature and copper ion concentration can lead to increased oxygen free radicals. Clearance of oxygen free radicals from aquatic animals mainly depends on some small reductive molecules(such as glutathione and ascorbic acid) and a train of antioxidases(such as superoxide dismutase and catalase)(Livingstone,2001). Catalase,which has a molecular architecture that includes an iron porphyrin ring with four ferrous atoms,is a key enzyme in the defense system established during the process of evolution of organisms. Catalase catalyzes H2O2into H2O2 and O2,preventing the H2O2from being converted into noxious -OH under the action of Fe-chelate. In conjunction with superoxide dismutase,a doubleenzyme system is formed to remove oxygen free radicals(Drążkiewicz et al., 2004; Guo et al., 2011). Catalase is present in various tissues of organisms,notably the liver. To date,the effects of only single environmental factors on the catalase activity of shellfish have been reported(Fan et al., 2004; Myung and Cheol, 2010; Li et al., 2012; Zhang et al., 2011),while between-factor interactions have not been examined and no reliable or predictive models have been established. Because multiple environmental factors can simultaneously act on catalase activity,it is necessary to examine the combined effects of environmental factors. In the present study,the combined effects of temperature and copper ion concentration on catalase activity were investigated using a central composite design and response surface method. The results revealed how the two factors affected the response examined and the mathematical model of the response toward the factors of interest was constructed. 2 MATERIAL AND METHOD 2.1 Experimental material
Experimental subjects were provided by an oyster culture farm in Potou District,Zhanjiang,Guangdong Province. All specimens were healthy adults of the same age(1.5 years old)with a mean shell length of 6.4(±0.52)cm. After removing the exterior biofoulings,samples were acclimated for 14 d in s and -filtered sea water with a heavy metal mass concentration that met the Grade I sea water quality st and ard. The temperature and salinity for the acclimation were 25(±0.1)°C and 25(±0.2),respectively. Mixed algae of Platymonassubcordiformis and Chlorella(1:1)were fed to the shellfish daily, and water was exchanged once per day and aeration was given continuously over the courses of the acclimation and experiment. 2.2 Experimental design
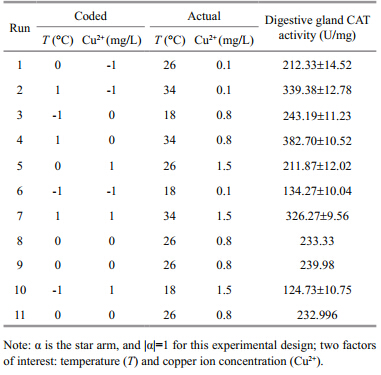
In the present study,the central composite design(Face-centered)was employed to examine the combined effects of temperature and copper ion concentration on the digestive gl and CAT activity in C. ariakensis. Based on the results of preliminary trials,the temperature was set to range from 18℃ to 34℃,while the copper ion concentration was set to range from 0.1 to 1.5 mg/L. When this form of central composite design is used,each factor assumes 3 coded levels in factor space,-1,0,+1, and the star arm |α|=1. To check the model adequacy,the number of central points was set at 3. There were 11 experimental runs(combination of the two factors), and each factorial and axial point was replicated once. The order of these experimental runs was arranged in a r and om fashion to remove systematic errors. 2.3 Experimental management
According to the design(Table 1),20 oysters were cultured for each replicate of the factorial and axial runs,which were carried out in plastic buckets containing 50 L of sea water. Temperature was automatically controlled by electronic heaters(EHEIM), and salinity and pH were held at 25(±0.2) and 7.8(±0.1),respectively. Each day,water exchange was conducted once,with the two factor regimes consistent with the setups corresponded to the experimental runs in Table 1. The entire experiment lasted for 30 d.
After the experiment ended,five oysters were taken from each replicate and the shells were cleared. The soft tissues were then rinsed several times using distilled water. The digestive gl and s were separated and put in a tissue homogenizer,then 9 volumes of precooled 0.9% physiological saline water(mL/v)were added and the samples were homogenized on ice. The homogenized tissue fluid was then decanted into a centrifuge tube and centrifuged for 10 min at 10℃ and 3 000 r/min(655 × g). The supernatant was then transferred into a new centrifuge tube and maintained at 4℃. Analysis was performed within 6 h. 2.5 Enzymatic activity measurement
The Coomassie Brilliant Blue method was used to measure the protein contents contained in the above enzyme fluid. Enzyme activity was gauged using a CAT visible light reagent kit(Nanjing Jiancheng SciTech Co. Ltd.,Nanjing). One tissue CAT enzyme unit was defined as the quantity of 1 moL H2O2 resolved by tissue protein per milligram per second. 2.6 Data analysis
The following quadratic regression model of digestive gl and CAT activity in C. ariakensis was assumed,with temperature and copper ion concentration as the two explanatory variables:
Y= β0+ β1T+ β2Cu2++ β3T×Cu2++ β4T2+ β5(Cu2+)2+ ε,
where,Y is the response,i.e.,CAT activity,or its transformation,β0is the intercept,β1 and β2are linear effects of temperature and copper ion concentration,respectively,β3is the synergistic effect between temperature and copper ion concentration,β4 and β5are quadratic effects of temperature and copper ion concentration,respectively and εis the r and om error,which is assumed to conform to the normal distribution with a mean of zero. All of the above effects were estimated using the least squares method and were tested by the Fstatistic. The model adequacy was evaluated using the lack-of-fit test(Ftest). Statistical processing and analysis of experimental data were conducted utilizing SAS(V9.13), and response surface and corresponding contour plots were provided to show how the two factors,temperature and copper ion concentration affected the response of interest. 3 RESULTAt the end of the experiment,oyster death had only occurred in runs 4 and 7,which had mortality rates of 10% and 5%,respectively. The experimental data describing the CAT activity of the digestive gl and in C. ariakensis are listed in the right column of Table 1. Since the factorial and axial runs were replicated,their st and ard deviations were provided. To evaluate the model adequacy by the lack-of-fit test,three center runs were listed separately. 3.1 Model adequacy
The ANOVA outcomes for the model adequacy are listed in Table 2. The P value of the model of catalase activity in the digestive gl and was 0.003 8,indicating that it was highly significant. Although the P value for the model lack-of-fit test was 0.013<0.05,this was caused by the very small pure experimental error(mean square=15.53), and does not indicate that the model was inadequate.
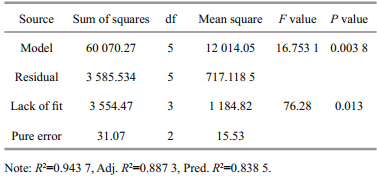 |
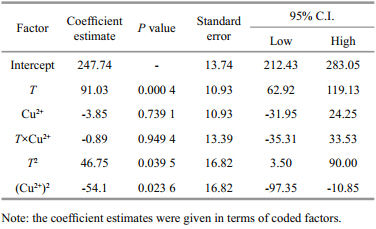
Coded model coefficients are given in Table 3 to directly compare the magnitude of the effects between factors. The linear effect of temperature on the digestive gl and CAT activity in C. ariakensis was highly significant(P<0.01),while the linear effect of copper ion concentration and the interaction between the two factors were not significant(P>0.05) and the quadratic effects of temperature and copper ion concentration were significant(P<0.05). Furthermore,since the unit of two factors was removed by factorial coding,the effects of temperature were found to be far greater than those of the copper ion concentration. The following model equation of the digestive gl and CAT activity toward temperature and copper ion concentration was obtained:
 |
Y=0.1562–0.0143 T+0.0561Cu2+–8.93×10 -4T×Cu2++3.51×10 -4T20.0235(Cu2+)2.
The unadjusted,adjusted and predictive coefficients of determination of the equation were 0.943 7,0.887 3 and 0.838 5,respectively. Additionally,the orthogonality of the composite design used in this study enables the nonsignificant terms to be deleted directly from the equation. 3.3 Response surface for the combined effects of temperature and copper ion concentration on the digestive gl and CAT activity
Visualizations of how the two factors affect the digestive gl and CAT activity in C. ariakensis are given in Fig. 1(a: response surface diagram; b: contour plot). CAT activity varied with temperature and copper ion concentration in a curvilinear manner. When temperature was held at a fixed level,CAT activity increased and then decreased as copper ion concentration increased,with the inflection point occurring at approximately 0.8 mg/L. When the copper ion concentration was fixed at a certain level,CAT activity increased with increasing temperature, and it was found to further increase as the temperature increased above 34°C,which was the upper-limit temperature set in this study.
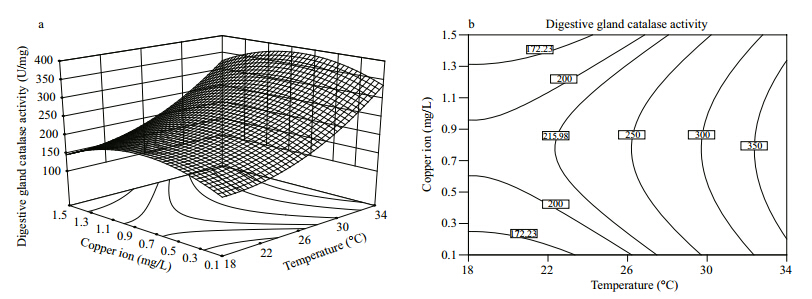 |
| Fig. 1 Response surface(a) and contour plot(b)of the combined effects of temperature and copper ion concentration on the CAT activity in the digestive gl and of C. a riakensis |
activity As shown in Table 3,the linear effects of temperature on the digestive gl and CAT activity in C. ariakensiswere highly significant(P<0.01). The CAT activity gradually increased with increasing temperature over the range investigated in this study(Fig. 1). There have been no reports of the effects of temperature on CAT activity in C. ariakensis, and only a few analogous studies of other bivalves are available. For example,Sun et al.(2008)found that CAT activity in Hiatula chinensis increased as temperature increased from 20°C to 40°C,which is similar to the results of the present study. Within the suitable range of temperature,aquatic organisms adapt themselves to changes in ambient temperature through autogenous physiological and biochemical mechanisms(Hochachka and Somero, 1984).
Increased temperature results in enhanced respiration and intensified production of ROS and oxidative stress,thereby stimulating alterations in antioxidase activity(Lushchak and Bagnyukova, 2006; Verlecar et al., 2007). In an investigation of the effects of temperature on the digestive gl and CAT activity in Scapharca broughtonii,Myung and Cheol(2010)found that the CAT activity increased as temperature changed from 20°C to 30°C at 3 h,6 h,24 h and 48 h,which is analogous to the results of the present study. Viarengo et al.(1991) and Cancio et al.(1999)studied the seasonal changes in CAT activity in Mytilusgalloprovincialis and found that the CAT activity increased with increasing temperature,whereas its antioxidase activity decreased during winter. Viarengo et al.(1991),Cancio et al.(1999) and Donaghy and Volety(2011)investigated changes in CAT activity at even lower temperatures and found that the CAT activity was even weaker at around 10°C. In the present study,the CAT activity was not evaluated at 10°C; therefore,changes in CAT activity at low temperatures are not discussed.
In this study,when temperature increased above 34℃,the digestive gl and CAT activity still increased(Fig. 1). These findings demonstrated that the CAT activity in C. ariakensisis stronger at 34℃. As shown in Table 3,the quadratic effect of temperature on the digestive gl and CAT activity was significant(P<0.05),indicating that the influence of temperature on CAT activity peaked. Xiao et al.(2003)found that the CAT activity in Tegillarca granosa increased as temperature changed from 20°C to 40°C,but that when temperature surpassed 50℃ the CAT activity decreased remarkably. Sun et al.(2008)also reported that the CAT activity in H. chinensis began to decline at temperatures higher than 40°C. Within suitable temperature ranges for aquatic organisms,the CAT activity usually increases with temperature to a certain point,then begins to decrease. At high temperatures,aquatic organisms eliminate excess reactive oxygen species by increasing antioxidase activity,but the increase is temporary, and its activity gradually declines with continued exposure to stress(Parihar et al., 1997). Lushchak and Bagnyukova(2006)found that short-term stress caused by high temperatures could promote increased CAT activity in golden fish,but that tissue CAT activity markedly declined after the high-temperature stress lasted for more than 4 h. 4.2 Effect of copper ion concentration on digestive gl and CAT activity
Most studies of the effects of copper ion on shellfish CAT activity conducted to date have investigated changes in CAT activity over time in response to copper ions held at specific concentrations(Isani et al., 2003; de Almeida,2004; Jing et al., 2006; Vlahogianni and Valavanidis, 2007 ; Gomes et al., 2012). As a result,little is known about changes in shellfish CAT activity with varying copper ion concentrations. In the present study,the linear effects of copper ion concentration on the digestive gl and CAT activity in C. ariakensiswere found to be insignificant(P>0.05),but the quadratic effect was significant(P<0.05)(Table 3). These findings indicate that,in the range of copper ion concentration investigated in the present study,the digestive gl and CAT activity varied with copper ion concentration in a curvilinear fashion(Fig. 1). The peak CAT activity was observed when the copper ion concentration was maintained at approximately 0.8 mg/L,regardless of temperature. However,it should be noted that this optimal copper ion concentration hinges on many factors that vary with species,tissue, and phase of growth. Although the impact of copper ion concentration on the CAT activity in C. ariakensis has not previously been reported,there have been similar reports for other bivalves including Chlamys farreri(Fan et al., 2004; Wu et al., 2005),Mytilus coruscus(Li et al., 2012) and Sinonovacula constricta(Liu et al., 2012). Low concentrations of heavy metal ions in water can induce the production of high levels of reactive oxygen species in shellfish, and the oxidative stress associated with these ROS will be eliminated by increased CAT activity(Stebbing,1982; Verlecar et al., 2008). At higher concentrations,heavy metal ions in water may cause damage to the structure of DNA in impacted organisms via excess reactive oxygen species,resulting in decreased CAT activity(Chakraborty et al., 2010). Geret et al.(2002)found that,although copper ion concentration could significantly influence CAT activity in Ruditapes decussatu(P<0.05),the CAT activity was stronger at lower concentrations. This discrepancy between the present study and their findings may be due to the hormesis effect or differences in the species and tissues investigated. 4.3 Synergistic effect of temperature and copper ion concentration on digestive gl and CAT activity
Although the effects of individual factors on the CAT activity in shellfish have been examined by Fan et al.(2004),Myung and Cheol(2010),Li et al.(2012) and Zhang et al.(2011),these studies did not evaluate the synergistic effects between multiple factors or construct reliable and predictable models. Temperature impacts the absorption of heavy metals into shellfish by altering the chemical and dynamic characteristics of the water body(Mubiana and Blust, 2007). Shellfish CAT activity varies with season and salinity in heavy metals-polluted areas of the sea(Vlahogianni et al., 2007). In the present study,the synergistic effects of temperature and copper ion concentration on CAT activity were found to be insignificant(P>0.05)(Table 3),indicating that the two factors affect the digestive gl and CAT activity of C. ariakensisindependently. However,Maria and Bebianno(2011)found that copper ion concentration and benzo(a)pyrene(BaP)had a significant interactive effect on antioxidase activity in M. galloprovincialis. Although Geret et al.(2002),Jing et al.(2007),Zanette et al.(2011) and Banni et al.(2014)investigated the effects of two factors on the CAT activity in shellfish,no interactions were reported,nor were models given in these studies. In the present study,a model of digestive gl and CAT activity in C. ariakensiswas established with unadjusted and predictive coefficients of determination of 0.943 7 and 0.838 5,respectively. These findings indicate that the model has excellent goodness of fit to the experimental data, and that it can be used for practical application under the conditions investigated in this study. It is obvious that the model built in this study lends itself to testing environmental pollution by utilizing digestive gl and CAT activity as an indicator; accordingly,the model will enable the healthy culture of C. ariakensis.
In a natural water environment,some complicated actions such as antagonism or synergism among heavy metal ions may occur(Kargin and Cogun, 1999). For example,Solé et al.(1995)found that seasonal changes in oxidative enzymes in M. galloprovincialis were influenced by pollutants in the sea,as well as ambient parameters including salinity,DO and suspended materials. Therefore,the CAT activity in C. ariakensismay simultaneously be affected by ambient factors other than temperature and copper ion concentration in the culture environment of this species. Accordingly,further studies investigating the combined effects of more factors on antioxidase activity in C. ariakensis are warranted. 5 CONCLUSION
In this study,we examined the combined effects of water temperature and copper ion concentration on catalase activity in the digestive gl and of C. ariakensisusing a central composite experimental design and response surface method. The results showed that the linear and quadratic effects of temperature were significant,as were the quadratic effects of copper ion concentration. However,the linear effects of copper ion concentration and the synergistic effects of temperature and copper ion concentration were not significant. Moreover,the effects of temperature were greater than those of copper ion concentration. The model of catalase activity in the digestive gl and of C. ariakensisin response to the two factors of interest was reliable and predictive under the study conditions. Overall,the results suggest that simultaneous variation of temperature and copper ion concentration alters the activity of antioxidant enzyme catalase by modulating active oxygen species metabolism.
| Banni M, Hajer A, Sforzini S, Oliveri C, Boussetta H, Viarengo A. 2014. Transcriptional expression levels and biochemical markers of oxidative stress in Mytilus galloprovincialis exposed to nickel and heat stress. Comparative Biochemistry and Physiology Part C : Toxicology & Pharmacology, 160 : 23-29. |
| Bayne B L. 1965. Growth and the delay of metamorphosis of the larvae of Mytilus edulis (L.). Ophelia, 2 (1): 1-47. |
| Bebianno M J, Géret F, Hoarau P, Serafim M A, Coelho M R, Gnassia-Barelli M, Roméo M. 2004. Biomarkers in Ruditapes decussatus : a potential bioindicator species. Biomarkers, 9 (4-5): 305-330. |
| Bougrier S, Geairon P, Deslous-Paoli J M, Bacher C, Jonquières G. 1995. Allometric relationships and effects of temperature on clearance and oxygen consumption rates of Crassostrea gigas (Thunberg). Aquaculture, 134 (1-2): 143-154. |
| Cancio I, Ibabe A, Cajaraville M P. 1999. Seasonal variation of peroxisomal enzyme activities and peroxisomal structure in mussels Mytilus galloprovincialis and its relationship with the lipid content. Comparative Biochemistry and Physiology Part C : Pharmacology, Toxicology and Endocrinology, 123 (2): 135-144. |
| Chakraborty S, Ray M, Ray S. 2010. Toxicity of sodium arsenite in the gill of an economically important mollusc of India. Fish & Shellfish Immunology, 29 (1): 136-148. |
| Coglianese M P, Martin M. 1981. Individual and interactive effects of environmental stress on the embryonic development of the Pacific oyster, Crassostrea gigas. I. The toxicity of copper and silver. Marine Environmental Research, 5 (1): 13-27. |
| de Almeida E A, Miyamoto S, Bainy A C D, de Medeiros M H G, Di Mascio P. 2004. Protective effect of phospholipid hydroperoxide glutathione peroxidase (PHGPx) against lipid peroxidation in mussels Perna perna exposed to different metals. Marine Pollution Bulletin, 49 (5-6): 386- 392. |
| Donaghy L, Volety A K. 2011. Functional and metabolic characterization of hemocytes of the green mussel, Perna viridis : in vitro impacts of temperature. Fish & Shellfish Immunology, 31 (6): 808-814. |
| Drążkiewicz M, Skórzyńska-Polit E, Krupa Z. 2004. Copperinduced oxidative stress and antioxidant defence in Arabidopsis thaliana. BioMetals, 17 (4): 379-387. |
| Fan Z J, Yang A G, Liu Z H. 2004. Effects of Cu2+on immune factor Chlamys farreri. Journal of Fishery Sciences of China, 11 (6): 576-579. (in Chinese with English abstract) |
| Fearman J, Moltschaniwskyj N A. 2010. Warmer temperatures reduce rates of gametogenesis in temperate mussels, Mytilus galloprovincialis. Aquaculture, 305 (1-4): 20-25. |
| Geret F, Serafim A, Barreira L, João Bebianno M. 2002. Response of antioxidant systems to copper in the gills of the clam Ruditapes decussatus. Marine Environmental Research, 54 (3-5): 413-417. |
| Gomes T, Pereira C G, Cardoso C, Pinheiro J P, Cancio I, Bebianno M J. 2012. Accumulation and toxicity of copper oxide nanoparticles in the digestive gland of Mytilus galloprovincialis. Aquatic Toxicology, 118 -119 : 72-79. |
| Guo H Y, Zhang D C, Cui S, Chen M Q, Wu K C, Li Y, Su T F, Jiang S G. 2011. Molecular characterization and mRNA expression of catalase from pearl oyster Pinctada fucata. Marine Genomics, 4 (4): 245-251. |
| Hochachka P W, Somero G N. 1984. Biochemical Adaptation. Princeton University Press, Princeton. p.356-449. |
| Isani G, Monari M, Andreani G, Fabbri M, Carpene E. 2003. Effect of copper exposure on the antioxidant enzymes in bivalve mollusc Scapharca inaequivalvis. Veterinary Research Communications, 27 (1): 691-693. |
| Jiang T J, Niu T. 2006. Effects of heavy metals on superoxide dismutase (SOD) of Crassostrea rivularis. Ecology and Environment, 15 (2): 289-294. (in Chinese with English abstract) |
| Jing G, Li Y, Xie L P, Zhang R Q. 2006. Metal accumulation and enzyme activities in gills and digestive gland of pearl oyster (Pinctada fucata) exposed to copper. Comparative Biochemistry and Physiology Part C : Toxicology & Pharmacology, 144 (2): 184-190. |
| Jing G, Li Y, Xie L P, Zhang R Q. 2007. Different effects of Pb2+and Cu2+on immune and antioxidant enzyme activities in the mantle of Pinctada fucata. Environmental Toxicology and Pharmacology, 24 (2): 122-128. |
| Kargin F, Cogun H Y. 1999. Metal interactions during accumulation and elimination of zinc and cadmium in tissues of the freshwater fish Tilapia nilotica. Bulletin of Environmental Contamination and Toxicology, 63 (4): 511-519. |
| Lacoste A, Jalabert F, Malham S, Cueff A, Gélébart F, Cordevant C, Lange M, Poulet S A. 2001. A Vibrio splendidus strain is associated with summer mortality of juvenile oysters Crassostrea gigas in the Bay of Morlaix (North Brittany, France). Diseases of Aquatic Organisms, 46 (2): 139-145. |
| Lesser M P, Kruse V A. 2004. Seasonal temperature compensation in the horse mussel, Modiolus modiolus : Metabolic enzymes, oxidative stress and heat shock proteins. Comparative Biochemistry and Physiology Part A : Molecular & Integrative Physiology, 137 (3): 495-504. |
| Li Y F, Gu Z Q, Liu H, Shen H D, Yang J L. 2012. Biochemical response of the mussel Mytilus coruscus (Mytiloida: Mytilidae) exposed to in vivo sub-lethal copper concentrations. Chinese Journal of Oceanology and Limnology, 30 (5): 738-745. |
| Littlewood D T J, Ford S E. 1990. Physiological responses to acute temperature elevation in oysters, Crassostrea virginica, parasitized by Haplosporidium nelsoni (MSX). Journal of Shellfish Research, 8 : 159-163. |
| Liu H M, Dong Y H, Huo L H, Lin Z H, Wang Z P. 2012. Acute toxicity of Cu2+and its effects on antioxidant enzymes in Sinonovacula constricta juveniles. Journal of Fishery Sciences of China, 1 9 (1): 182-187. (in Chinese with English abstract) |
| Livingstone D R. 2001. Contaminant-stimulated reactive oxygen species production and oxidative damage in aquatic organisms. Marine Pollution Bulletin, 42 (8): 656- 666. |
| Lushchak V I, Bagnyukova T V. 2006. Temperature increase results in oxidative stress in goldfish tissues. 2. Antioxidant and associated enzymes. Comparative Biochemistry and Physiology Part C : Toxicology & Pharmacology, 143 (1): 36-41. |
| Lushchak V I. 2011. Environmentally induced oxidative stress in aquatic animals. Aquatic Toxicology, 101 (1): 13-30. |
| Maria V L, Bebianno M J. 2011. Antioxidant and lipid peroxidation responses in Mytilus galloprovincialis exposed to mixtures of benzo(a)pyrene and copper. Comparative Biochemistry and Physiology Part C : Toxicology & Pharmacology, 1 54 (1): 56-63. |
| Mubiana V K, Blust R. 2007. Effects of temperature on scope for growth and accumulation of Cd, Co, Cu and Pb by the marine bivalve Mytilus edulis. Marine Environmental Research, 63 (3): 219-235. |
| Myung I A, Cheol Y C. 2010. Activity of antioxidant enzymes and physiological responses in ark shell, Scapharca broughtonii, exposed to thermal and osmotic stress: effects on hemolymph and biochemical parameters. Comparative Biochemistry and Physiology Part B : Biochemistry and Molecular Biology, 155 (1): 34-42. |
| Parihar M S, Javeri T, Hemnani T, Dubey A K, Prakash P. 1997. Responses of superoxide dismutase, glutathione peroxidase and reduced glutathione antioxidant defenses in gills of the freshwater catfish (Heteropneustes fossilis) |
| to short-term elevated temperature. Journal of Thermal Biology, 22 (2): 151-156. |
| Pipe R K, Coles J A, Carissan F M M, Ramanathan K. 1999. Copper induced immunomodulation in the marine mussel, Mytilus edulis. Aquatic Toxicology, 46 (1): 43-54. |
| Regoli F, Nigro M, Orlando E. 1998. Lysosomal and antioxidant responses to metals in the Antarctic scallop Adamussium colbecki. Aquatic Toxicology, 40 (4): 375- 392. |
| Solé M, Porte C, Albaiges J. 1995. Seasonal variation in the mixed-function oxygenase system and antioxidant enzymes of the mussel Mytilus galloprovincialis. Environmental Toxicology and Chemistry, 14 (1): 157- 164. |
| Stebbing A R D. 1982. Hormesis-the stimulation of growth by low levels of inhibitors. Science of the Total Environment, 22 (3): 213-234. |
| Sun Y Y, Gao R C, Wen Y M, Chen N, Wang S. 2008. Effects of Temperature on hydrolase and antioxidase activities in liver of clam Hiatula chinensis and H. diphos. Fisheries Science, 27 (10): 543-544. (in Chinese with English abstract) |
| Verlecar X N, Jena K B, Chainy G B N. 2007. Biochemical markers of oxidative stress in Perna viridis exposed to mercury and temperature. Chemico - Biological Interactions, 167 (3): 219-226. |
| Verlecar X N, Jena K B, Chainy G B N. 2008. Modulation of antioxidant defences in digestive gland of Perna viridis (L.), on mercury exposures. Chemosphere, 71 (10): 1 977- 1 985. |
| Viarengo A, Canesi L, Pertica M, Livingstone D R. 1991. Seasonal variations in the antioxidant defence systems and lipid peroxidation of the digestive gland of mussels. Comparative Biochemistry and Physiology Part C : Comparative Pharmacology, 100 (1-2): 187-190. |
| Vlahogianni T, Dassenakis M, Scoullos M J, Valavanidis A. 2007. Integrated use of biomarkers (superoxide dismutase, catalase and lipid peroxidation) in mussels Mytilus galloprovincialis for assessing heavy metals’ pollution in coastal areas from the Saronikos Gulf of Greece. Marine Pollution Bulletin, 54 (9): 1 361-1 371. |
| Vlahogianni T H, Valavanidis A. 2007. Heavy-metal effects on lipid peroxidation and antioxidant defence enzymes in mussels Mytilus galloprovincialis. Chemistry and Ecology, 23 (5): 361-371. |
| Wang H, Liu J H, Yang H S, Liu Z G. 2014. Effect of simultaneous variation in temperature and ammonia concentration on percent fertilization and hatching in Crassostrea ariakensis. Journal of Thermal Biology, 41 : 43-49. |
| Wu Y C, Lv X, Wang F, Zhao Y F, Liu C F. 2005. Accumulation of Copper in Chlamys farreri tissues and its effect on catalase activity. Chinese Journal of Applied & Environmental Biology, 11 (5): 559-562. (in Chinese with English abstract) |
| Xiao X, Deng R P, Chen Z L, Han Y L. 2003. The comparison of the characteristics of catalase in oyster and Tegillarca granosa. Food Science, 24 (9): 32-34. (in Chinese) |
| Zanette J, de Almeida E A, da Silva A Z, Guzenski J, Ferreira J F, Mascio P D, Marques M R F, Bainy A C D. 2011. Salinity infl uences glutathione S -transferase activity and lipid peroxidation responses in the Crassostrea gigas oyster exposed to diesel oil. Science of the Total Environment, 409 (10): 1 976-1 983. |
| Zhang Y, Fu D K, Yu F, Liu Q Y, Yu Z N. 2011. Two catalase homologs are involved in host protection against bacterial infection and oxidative stress in Crassostrea hongkongensis. Fish & Shellfish Immunology, 31 (6): 894- 903. |
 2015, Vol. 33
2015, Vol. 33