Shanghai University
Article Information
- BAI Yucen (柏雨岑), ZHANG Tao (张涛), QIU Tianlong (邱天龙), GAO Yan (高燕), ZHANG Xiaofang (张晓芳)
- Nutrient effects of broodstocks on the larvae in Patinopecten yessoensis
- Chinese Journal of Oceanology and Limnology, 2015, 33(4): 988-996
- http://dx.doi.org/10.1007/s00343-015-4225-4
Article History
- Received Jul. 9, 2014;
- accepted in principle Sep. 23, 2014;
- accepted for publication Jan. 4, 2015
2 University of Chinese Academy of Sciences, Beijing 100049, China;
3 Tianjin Bohai Sea Fisheries Research Institute, Tianjin 300457, China;
4 Zhangzi Island Fishery Group, Ltd., Dalian 116001, China
Patinopecten yessoensis, which belongs to Mollusc: Lamellibranchia, is a commercial valuable species, inhabiting coastal waters of the northern isl and s of Japan, the northern part of the Korean Peninsula, and Russian Primorye, Sakhalin and Kurile Isl and s(Li et al., 2007). P. yessoensis was introduced into China from Japan in 1982, by the Marine Fishery Research Institute of Liaoning Province, the purpose being to increase scallop cultivation in the northern parts of the Chinese coast. P. yessoensisis larger and comm and s a higher market price than is the case for the Chinese native zhikong scallop Chlamys farreri and the bay scallop Argopecten irradians. The introduced species was rapidly accepted by scallop farmers in China and by 2005, the annual production of the Japanese scallop had reached about 150 000 tons(Li et al., 2007).
Many research projects on the techniques of artificial seed production and culture and nutrition were carried out, in association with the P. yessoensisaquaculture industry(Li et al., 2007). P. yessoensisseed is produced exclusively in hatcheries in China(Li et al., 2007). High quality larval nutrition is crucial for survival and growth, and important when determining yields. Most bivalve larvae are pelagic, and the larvae depend either completely on the stored nutrients till metamorphosis(lecithotrophic development), or on the planktonic food(planktotrophic development)(Honkoop et al., 1999). However, they always require stored nutrients during embryogenesis and growth, until the firstfeeding stage(Gallager and Mann, 1986; Honkoop et al., 1999). The nutrients in the eggs and the larvae during the early stages of development, which are necessary for the growth and survival of larvae, are exclusively endogenous and furnished by the broodstocks. It is therefore reasonable to deduce that the condition of the broodstocks, especially their gonads, has a strong influence on the larvae.
Carbohydrate, lipid, and protein are three main nutrients in the broodstocks and larvae. In contrast to the adult stage, when the main energy source is from carbohydrates(Navarro et al., 1989), more than half of the energy required during embryogenesis is provided by lipids(Gallager and Mann, 1986; Honkoop et al., 1999). In some species, the proportion of larvae reaching the first-feed stage was positively related to the initial concentration of lipids in eggs(Gallager et al., 1986). Many studies have illustrated that lipids are essential for bivalve larval nutrition(Thompson et al., 1996; Wikfors et al., 1996). Docosahexenoic acid(DHA)is a preferentially incorporated fatty acid(FA)that plays a role in the maintenance of structural and functional integrity of cell membranes during larval development and metamorphosis of scallops, as well as gonadal development and embryogenesis, while eicosapentaenoic acid(EPA)is mainly utilized as a source of energy(Beninger et al., 2003; Hamdani and Soltani-Mazouni, 2011).
The broodstocks, their diet, and the newly released larvae compose a nutrient chain. However, within the nutrient chain of diet-broodstock-larvae, most research has focused on the effect of diet on broodstocks, or the relationship between diet and larvae(Soudant et al., 1996; Hamdani and SoltaniMazouni, 2011; Arafa et al., 2012). Our research has focused on the intermediate link, i.e. on the broodstock-gonads(mainly ovaries) and the larvae, characterized by lipids and FAs. The objective of this research is to develop an index to evaluate the development of gonads and the quality of larvae. 2. MATERIAL AND METHOD 2.1 Collection and maintenance of broodstocks
P. yessoensisbroodstock was collected in January 2009, in the sea off Zhangzi Isl and , Dalian, Liaoning Province, China, and reared separately in three hatcheries(Hatchery 1, Hatchery 2, and Hatchery 3)in Zhangzi Isl and . The initial water temperature for rearing was 4℃. After a 2–3-day acclimation, water temperature was raised artificially at a rate of 0.5℃a day until 12℃. The diet was comprised of mixed algae: Nitzschia closterium minutissima, Phaeodactylum tricornutum, and Tetraselmis chuii. A half of the rearing water was exchanged by fresh equal-temperature seawater daily to ensure high water quality. 2.2 Experimental procedure and sample preparation
In early February 2009, 99 broodstocks in each hatchery were selected with the shell height of 12–16 cm. The broodstocks from the same hatchery were r and omly divided into three ponds(33 each, 5 m ×2 m×1.5 m), and prepared for spawning. In each pond, ten broodstocks(five female, and five male)were chosen r and omly and their gonads were sampled prior to spawning, frozen by means of liquid nitrogen, and preserved in -20℃ freezer.
After dry in the shade stimulation for half an hour, the broodstock was transferred into a water bath maintained at 16℃, and ejaculated and spawned. After spawning, another ten broodstock specimens(five female, five male)were chosen r and omly from each pond and their gonads were sampled immediately, using the same method described above. The fertilized eggs were hatched at 16℃ and subsequent embryos and larvae were cultured at 15℃.
When the larvae developed into D-larvae, five samples were taken from each pond, using a 100-mesh bolting-silk, and then frozen by liquid nitrogen and preserved at -20℃ in refrigerators. 2.3 Determination of different nutrient composition
All samples were freeze-dried, homogenized, and sent to IOCAS Research Center of Analysis and Measurement for determination of the concentrations of total protein, lipid, and carbohydrate, and different FAs.
The Kjeldahl method was used to determine total protein concentration(Li et al., 2006) and the total lipid concentration was determined using the Soxhlet extraction method(Li et al., 2006). Total carbohydrate was determined using the method described in Li et al.(2006). The values obtained were expressed as percentage weight of dry tissue. The undetermined organic matter(UOM)was calculated as 100–total carbohydrate–total protein–total lipid.
The determination of FA in ovaries followed the procedure of Cook et al.(2000), with Gas Chromatography(Agilent 7890A)using a DB-FFAP elastic quartz capillary vessel column(30 m 0.25 mm 0.25 μm), with nitrogen as carrier gas. The initial column temperature was 50℃ for 3 min, programmed to rise to 230℃ at a rate of 10℃/min. The temperature of the Flame Ionization Detector(FID)was 280℃ and the split ratio was 1:10. Values obtained were expressed as mg/g(dry tissue weight).
The nutrient concentration in ovaries prior to spawning was abbreviated as BC, and the nutrient concentration in ovaries after spawning was abbreviated as AC. Decreased nutrient in the ovaries was expressed as DC=BC–AC; LC represented the larval nutrient concentration. 2.4 Statistical analysis
All statistical analysis was carried out with the aid of SPSS V.18. One-way ANOVA was used to compare the BC and AC of total protein, total carbohydrate, total lipid, and UOM in ovaries and testes, as well as the BC, DC, and LC of different FAs. Correlation between FA LC and the corresponding BC and DC was analyzed using the Pearson method. 3 RESULT 3.1 Variations in gonadal nutrient composition
before and after spawning Results summarized in Table 1 indicated that the concentration of total carbohydrate was significantly higher(1.47 times)in ovaries than in testes(P<0.01)before spawning. The concentration of total carbohydrate decreased significantly in ovaries after spawning(P<0.05). No such differences were, however, noted in the testes. The concentration of total protein was significantly higher(1.32 times)in the testes than in the ovaries. The concentration of total protein decreased significantly in testes after spawning(P<0.05), but there was almost no variation in the ovaries. The concentration of total lipid was significantly higher(2.78 times)in ovaries than in testes(P<0.05). It decreased significantly in the ovaries after spawning, but there was no significant variation in the testes after spawning. The concentration of UOM was significantly higher in ovaries than in testes(P<0.05).
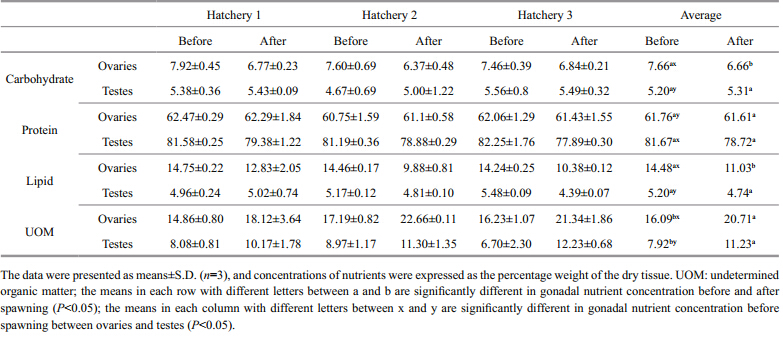 |
Table 2 presents the concentration of various FAs in the ovaries before and after spawning. A comparison of the BC, DC, and LC FAs suggested that the BC of total FA, saturated fatty acids(SFA), monounsaturated fatty acids(MUFA), 14:00, 16:00, 17:00, 18:00, 16:1n5, 16:1n7, 20:1n7, 20:1n9, 20:1n11, 20:2n6, and 20:4n6>their LC>corresponding DC. However, the sequence of EPA and DHA was BC>DC>LC.
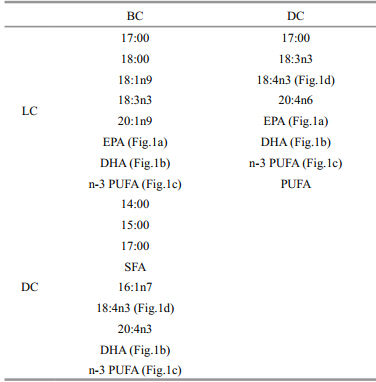
Correlations between the FA concentrations in the ovaries and those in the larvae were determined. The FAs, which had significant positive correlation among BC, DC, and LC were presented in Table 3. The LC of 17:00, 18:00, 18:1n9, 18:3n3, 20:1n9, EPA(Fig. 1a), DHA(Fig. 1b), and n-3PUFA(Fig. 1c)had significant positive correlation with BC(P<0.05). The LC of 17:00, 18:3n3, 18:4n3(Fig. 1d), 20:4n6, EPA(Fig. 1a), DHA(Fig. 1b), n-3 polyunsaturated fatty acids(PUFA)(Fig. 1c), and total PUFA had significant positive correlation with their DC(P<0.05). The BC of 14:00, 15:00, 17:00, SFA, 16:1n7, 18:4n3(Fig. 1d), 20:4n3, DHA(Fig. 1b), n-3 PUFA(Fig. 1c)had significant positive correlation with DC(P<0.05).
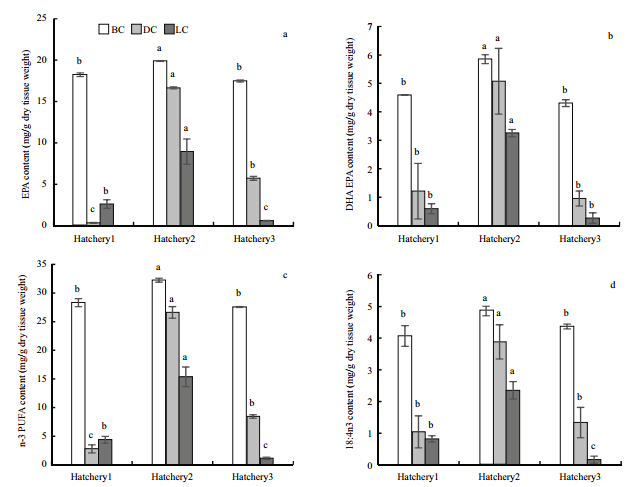 |
| Fig. 1 The BC, DC, and LC of EPA(a), DHA(b), and n-3 PUFA(c), 18:4n3(d)in three hatcheries(means ±S.D., n=3)
Values of the same index with different letters differ significantly among three hatcheries(P<0.05). |
However, the LC of 14:00, 16:00, 16:1n7, 18:2n6, SFA and MUFA were negatively correlated with DC(P<0.01). Hatchery 2 was significantly higher in LCof all PUFAs and total FA than the other two hatcheries(P<0.05), though relatively lower in other FAs. 4 DISCUSSION 4.1 Carbohydrates, proteins, and lipids in the
ovaries and testes The ovary contains significantly more carbohydrate and lipid, but less protein than testes. This may be due to the presence of different components in oocytes and spermatocytes. Hamdani and Soltani-Mazouni(2011)also concluded that in Donax trunculus the composition of biochemical components in the ovaries was higher than in the testes, due to different energy dem and s in female and male gametes(Beninger et al., 2003). In addition, the triacylglycerols and acylglycerols are the main constituents of the oocytes(Napolitano et al., 1992)whereas spermatozoids do not store lipid reserves(Soudant et al., 1996). The spermatozoa of male bivalves contain few energy reserves, while females require yolk for the developing oocytes(Beninger and Le Pennec, 1997). The histological study of P. yessoensis gonad also indicated that the nutrient was transferred from auxiliary cell to oocyte, to format yolk precursors, such as carbohydrate yolk precursor, lipid yolk granule, and protein yolk granule, which would eventually be intermingled and become yolk granules(Chung et al., 2005). Therefore, it is easy to conclude that the reason of the absence of significant variation in the total spermatic lipids after spawning and the decrease in carbohydrate and lipid in ovaries after spawning lies in the different nutrient storage in different gametes. It may thus be necessary to separate the females and the males during the breeding season, so as to design suitable diets that satisfy their different nutrient dem and s.
Carbohydrates, proteins and lipids are all important nutrients for the embryonic development of P. yessoensis, and they play different roles in metabolism. These are catabolized simultaneously and linearly in time with embryonic development. Whyte et al.(1991)found that the energy dem and s of P. yessoensisduring development are derived from nutrients in the following proportions: 47.6% from lipids, 7.5% from carbohydrates and 44.9% from proteins, and carbohydrates played a significant role in balancing the use of nutrients for synthesis, as opposed to energy production.
P. yessoensisgonads contained a higher proportion of lipids, in comparison to other species, which might be a response to the low ambient water temperatures(Whyte et al., 1991; Arafa et al., 2012). Lipids are the prime energy source during embryonic and early larval development(Whyte et al., 1991). High lipid reserves in P. yessoensismay therefore be regarded as an adjustment to the long developing period and low food availability in a cold environment, which would be necessary for supporting metabolism until temperature rises and food abundance increases(Honkoop et al., 1999).
The protein concentration in ovaries, of 61.76%, was much higher than that of 53.4% found by Whyte et al.(1991)in the eggs, which may be due to the relatively high protein concentration in the ovary tissue and differences in the culturing diet and general condition of the experimental animals. The lack of any significant variation after spawning is an indication that the protein was a major constituent of the ovary tissue, and the protein concentration in the oocytes was relatively low. 4.2 Comparison and correlation of the BC, DC, and LC of the FAs
The levels of SFAs and MUFAs in larvae(i.e. the LC)were significantly higher than the decrease of that in the ovaries after spawning(DC), indicating that the accumulation of these FAs in the embryos and D larvae. In other research, the increase of SFAs and MUFAs accompanying embryogenesis, a period of endogenous nutrition was also observed(Langdon and Waldock, 1981; Whyte et al., 1989). Although regarded as an energy source, SAFs are neither collectively nor individually essential for larval development, as SAF concentration in the larvae has a negative correlation with larval growth(Leonardos and Lucas, 2000), and the accumulation of SFA is thought to indicate a sub-optimal physiological state(Frolov and Pankov, 1992). A negative correlation between LC and DC of SFA was observed in this research, which seemed to support the theory mentioned above. Conversely, the LC of PUFAs declined, especially the 20:5n3(EPA) and 22:6n3(DHA)that were regarded as nutritionally essential for most marine species(Langdon and Waldock, 1981; Sargent et al., 1999). EPA is considered as an energy providing material and is consumed during embryogenesis, but DHA plays a structural role and its concentration is relatively constant in eggs and embryos(Labarta et al., 1999; Beninger et al., 2003; Pernet et al., 2005 ; Hamdani and Soltani-Mazouni, 2011). Consequently, the decline in DHA can be explained by either the oxidative degradation(as an energy source), or the release of other material-rich components of PUFAs, e.g. mucus, from the ovaries when spawning.
The LC of many kinds of FAs had a positive correlation with their BC or DC(Table 3). Most of them were unsaturated FAs, indicating that these may be significant nutrients(inherited from broodstocks)in the larvae. It also indicated that the nutrient composition in the larvae was influenced by the condition of the broodstocks, especially the unsaturated FAs. It has been reported that, the LC of FAs affects the viability of the larvae, larval growth and (to some extent)the settlement, spat growth, and juvenile survival in many bivalves(Helm et al., 1973; Kraeuter et al., 1981). Berntsson et al.(1997)demonstrated a significant relationship between larval growth rate and the 22:6n3 concentration of the broodstock in Ostrea edulis. Marine invertebrates are thought to have limited ability to synthesize PUFAs, such as 20:5n3 and 22:6n3(Chu and Greaves, 1991). As a result, PUFAs are not only nutritionally important(Enright et al., 1986a, b)but also indicative of the physiological state of the larvae(Langdon and Waldock, 1981). They can function as an energy source, or precursors to produce eicosanoids or prostagl and ins, which play a significant role in metabolism in marine invertebrates(Whyte et al., 1991; Labarta et al., 1999). They are also positively correlated with larval growth(Leonardos and Lucas, 2000), especially n-3 PUFAs, which is considered to be a direct index of growth for Mytilus edulis larvae(Leonardos and Lucas, 2000). In the present study, the LC of PUFAs, n-3 PUFAs(mainly EPA and DHA)had a significant correlation with their DC, indicating the influence by the broodstocks. Consequently, these FAs are significant for larval development and growth, and may be effective indexes for evaluating both the quality of broodstocks and larvae.
Nevertheless, in Gallager and Mann’s research(1986)on Mercenaria mercenariaL. and CrassostreavirginicaGmelin, the initial egg lipid concentration correlated with the larval survival rate during the early stages, rather than during the subsequent larval growth period. A minimum of 12% lipids of the ashfree dry weight was necessary for high survival, to the pediveliger stage, but relatively high lipid levels did not guarantee high larval survival. Hence, the indicative function of lipids or FAs is variable within different species, and more research is required to investigate these mechanisms. 5 ACKNOWLEDGEMENT
We would like to thank all the members of the Research Department of Dalian Zhangzi Isl and Fishery Group, Ltd. In particular, we would also like to thank all the anonymous reviewers for professional revision of the manuscript.
| Arafa S, Chouaibi M, Sadok S, El Abed A. 2012. The influence of season on the gonad index and biochemical composition of the sea urchin Paracentrotus lividus from the Golf of Tunis. Scientific World J., 2012 : Article ID PMC3354710. |
| Beninger P G, Le Pennec G, Le Pennec M. 2003. Demonstration of nutrient pathway from the digestive system to oocytes in the gonad intestinal loop of the scallop Pecten maximus L.. Biol. Bull., 205 (1): 83-92. |
| Beninger P G, Le Pennec M. 1997. Reproductive characteristics of a primitive bivalve from a deep-sea reducing environment: giant gametes and their significance in Acharax alinae (Cryptodonta: Solemyidae). Mar. Ecol. Prog. Ser., 157 : 195-206. |
| Berntsson K M, Jonsson P Ŕ, Wängberg S A, Carlsson A S. 1997. Effects of broodstock diets on fatty acid composition, survival and growth rates in larvae of the European flat oyster, Ostrea edulis. Aquaculture, 154 (2): 139-153. |
| Chu F L E, Greaves J. 1991. Metabolism of palmitic, linoleic, and linolenic acids in adult oysters, Crassostrea virginica. Mar. Biol., 110 (2): 229-236. |
| Chung E Y, Park Y J, Lee J Y, Ryu D K. 2005. Germ cell differentiation and sexual maturation of the hanging cultured female scallop Patinopecten yessoensis on the east coast of Korea. J. Shellfish Res., 24 (4): 913-921. |
| Cook E J, Bell M V, Black K D, Kelly M S. 2000. Fatty acid compositions of gonadal material and diets of the sea urchin, Psammechinus miliaris : trophic and nutritional implications. J. Exp. Mar. Biol. Ecol., 255 (2): 261-274. |
| Enright C T, Newkirk G F, Craigie J S, Castell J D. 1986a. Evaluation of phytoplankton as diets for juvenile Ostrea edulis L.. J. Exp. Mar. Biol. Ecol., 96 (1): 1-13. |
| Enright C T, Newkirk G F, Craigie J S, Castell J D. 1986b. Growth of juvenile Ostrea edulis L. fed Chaetoceros gracilis Schütt of varied chemical composition. J. Exp. Mar. Biol. Ecol., 96 (1): 15-26. |
| Frolov A V, Pankov S L. 1992. The reproduction strategy of oyster Ostrea edulis L. from the biochemical point of view. Comp. Biochem. Physiol. B, 103 (1): 161-182. |
| Gallager S M, Mann R, Sasaki G C. 1986. Lipid as an index of growth and viability in three species of bivalve larvae. Aquaculture, 56 (2): 81-103. |
| Gallager S M, Mann R. 1986. Growth and survival of larvae of Mercenaria mercenaria (L.) and Crassostrea virginica (Gmelin) relative to broodstock conditioning and lipid content of eggs. Aquaculture, 56 (2): 105-121. |
| Hamdani A, Soltani-Mazouni N. 2011. Changes in biochemical composition of the gonads of Donax trunculus l. (Mollusca, Bivalvia) from the Gulf of Annaba (Algeria) in relation to reproductive events and pollution. Jordan J. Biol. Sci., 4 (3): 149-156. |
| Helm M M, Holland D L, Stephenson R R. 1973. The effect of supplementary algal feeding of a hatchery breeding stock of Ostrea edulis L. on larval vigour. J. Mar. Biol. Assoc. UK, 53 (3): 673-684. |
| Honkoop P, Van der Meer J, Beukema J J, Kwast D. 1999. Reproductive investment in the intertidal bivalve Macoma balthica. J. Sea Res., 41 (3): 203-212. |
| Kraeuter J N, Castagna M, van Dessel R. 1981. Egg size and larval survival of Mercenaria mercenaria (L.) and Argopecten irradians (Lamarck). J. Exp. Mar. Biol. Ecol., 56 (1): 3-8. |
| Labarta U, Fernández-Reiriz M J, Pérez-Camacho A. 1999. Energy, biochemical substrates and growth in the larval development, metamorphosis and postlarvae of Ostrea edulis (L.). J. Exp. Mar. Biol. Ecol., 238 (2): 225-242. |
| Langdon C J, Waldock M J. 1981. The effect of algal and artificial diets on the growth and fatty acid composition of Crassostrea gigas spat. J. Mar. Biol. Assoc. UK, 61 (2): 431-448. |
| Leonardos N, Lucas I A N. 2000. The use of larval fatty acids as an index of growth in Mytilus edulis L. larvae. Aquaculture, 184 (1-2): 155-166. |
| Li Q, Liu W G, Shirasu K, Chen W M, Jiang S X. 2006. Reproductive cycle and biochemical composition of the Zhe oyster Crassostrea plicatula Gmelin in an eastern coastal bay of China. Aquaculture, 261 (2): 752-759. |
| Li Q, Xu K F, Yu R H. 2007. Genetic variation in Chinese hatchery populations of the Japanese scallop (Patinopecten yessoensis) inferred from microsatellite data. Aquaculture, 269 (1-4): 211-219. |
| Napolitano G E, MacDonald B A, Thompson R J, Ackman R G. 1992. Lipid composition of eggs and adductor muscle in giant scallops (Placopecten magellanicus) from different habitats. Mar. Biol., 113 (1): 71-76. |
| Navarro E, Iglesias J I P, Larrañaga A. 1989. Interannual variation in the reproductive cycle and biochemical composition of the cockle Cerastoderma edule from Mundaca Estuary (Biscay, North Spain). Mar. Biol., 101 (4): 503-511. |
| Pernet F, Bricelj V M, Parrish C C. 2005. Effect of varying dietary levels of ω6 polyunsaturated fatty acids during the early ontogeny of the sea scallop, Placopecten magellanicus. J. Exp. Mar. Biol. Ecol., 327(2): 115-133. |
| Sargent J, Bell G, McEvoy L, Tocher D, Estevez A. 1999. Recent developments in the essential fatty acid nutrition of fish. Aquaculture, 177 (1-4): 191-199. |
| Soudant P, Marty Y, Moal J, Samain J F. 1996. Fatty acids and egg quality in great scallop. Aquacult. Int., 4 (3): 191-200. |
| Thompson P A, Guo M X, Harrison P J. 1996. Nutritional value of diets that vary in fatty acid composition for larval Pacific oysters (Crassostrea gigas). Aquaculture, 143 (3- 4): 379-391. |
| Whyte J N C, Bourne N, Ginther N G. 1991. Depletion of nutrient reserves during embryogenesis in the scallop Patinopecten yessoensis (Jay). J. Exp. Mar. Biol. Ecol., 149 (1): 67-79. |
| Whyte J N C, Bourne N, Hodgson C A. 1989. Influence of algal diets on biochemical composition and energy reserves in Patinopecten yessoensis (Jay) larvae. Aquaculture, 78 (3-4): 333-347. |
| Wikfors G H, Patterson G W, Ghosh P, Lewin R A, Smith B C, Alix J H. 1996. Growth of post-set oysters, Crassostrea virginica, on high-lipid strains of algal flagellates Tetraselmis spp. Aquaculture, 143 (3-4): 411-419. |
 2015, Vol. 33
2015, Vol. 33