Shanghai University
Article Information
- LU Guangyuan (卢光远), SONG Xiuxian (宋秀贤), YU Zhiming (俞志明), CAO Xihua (曹西华), YUAN Yongquan (袁涌铨)
- Effects of modified clay flocculation on major nutrients and diatom aggregation during Skeletonema costatum blooms in the laboratory
- Chinese Journal of Oceanology and Limnology, 2015, 33(4): 1007-1019
- http://dx.doi.org/10.1007/s00343-015-4162-2
Article History
- Received Jun. 5, 2014;
- accepted in principle Aug. 28, 2014;
- accepted for publication Sep. 23, 2014
2 University of Chinese Academy of Sciences, Beijing 100049, China
Harmful algal blooms(HABs)have become more frequent and longer duration in coastal waters over the past three decades(Anderson et al., 2012). HABs can elicit several negative effects on aquatic environment(such as depleting the oxygen, blocking the sunlight, destroying the habitats of organisms) and life health(including poisoning/killing marine mammals, birds and human)(Anderson, 2009; Certner et al., 2011). Among the various control strategies for HABs(physical manipulation needs lots of manpower and expensive equipment, chemicals treatment has toxic effect of some byproduct and high residual for marine organisms, microbial agents only has limited in laboratory research), the coagulationflocculation of algae cells using modified clay has been applied in field as a recommended and practical method because of low cost, high efficiency, less residual(Anderson, 1997; Pan et al., 2011; Chen and Pan, 2012), particularly in Japan, China, and North America(Pierce et al., 2004; Yu et al., 2004; Anderson, 2009; Certner et al., 2011; Anderson et al., 2012).
Most previous studies estimated the algal growth potential in response to different nutrient levels without considering the impacts of harmful algae decaying. After the HABs, particularly diatoms, it generates massive concentrations of algal organic matters(Puskaric and Mortain-Bertr and , 2003; Villacorte et al., 2013) and a large vertical flux of materials transported from the upper euphotic water columns to deeper aphotic zones, that called “the biological nutrient pump” of the ocean(Iversen and Ploug, 2013; Kemp and Villareal, 2013). Through this mechanism, a substantial fraction of major nutrients assimilated by phytoplankton was exported from the upper mixed surface zones into the deeper waters and sediments(Turner, 2002; Ploug et al., 2008). However, some studies demonstrated the increasing nutrient flux at the water-sediment interface after one HABs event(Conley and Johnstone, 1995). A key determining factor for the potential vertical fluxes of major nutrients in the water column is the degradation rate of organic matter relative to its sinking velocity(Ploug et al., 2008). One important effect of MC treatment is that increasing the sinking velocity of these aggregations from the surface waters(Chen and Pan, 2012). Armstrong et al.(2002)highlighted the importance of mineral ballasts in providing the excess density required, thereby promoting the sinking of aggregation(Ingalls et al., 2003; Lee et al., 2008; Ploug et al., 2008; Iversen and Ploug, 2010). The gravitational effect strongly increases the sedimentation rate of phytoplankton aggregations(Gehlen et al., 2006; Moriceau et al., 2007; Moriceau et al., 2009). Simultaneously, biogenic minerals, including calcite and opal are important factors controlling particle sinking velocity and increasing vertical nutrient fluxes in the ocean(Ploug et al., 2008). When MC flocculated with algae cells, it may influence the benthic ecosystem and the nutrient cycle. However, the role of modified clay in nutrient transport is more complex than a simple effect as adsorption, and it remains poorly understood. Thus, the potential of the long-term effects of HABs mitigation by MC treatment should be of particular concern before large-scale application.
Some researchers investigated the self-influences of only MC particles including the impacts on the growth of aquatic organisms(fishes and clams), and the adsorption of dissolved elements, algal toxins, or organic pollutant(Yu et al., 1995, 1999, 2004; Archambault et al., 2004; Pierce et al., 2004; Pan et al., 2012; Ahammed Shabeer et al., 2014a, b). Few studies have the concern with the MC treatment on the long-term effects of diatom degradation and the conversion of the inorganic major nutrients(Ingalls et al., 2003, 2006). Diatoms, which produce opal, are the dominant species of phytoplankton in aquatic ecosystems(Nelson et al., 1995; Moriceau et al., 2007, 2009). Skeletonema costa tum(Grev.)Cleve(S. costatum)is a common, high biomass HAB diatom in coastal estuaries(Curl and Mcleod, 1961; Blanchemain and Grizeau, 1996; Yamamoto et al., 2004; Kooistra et al., 2008; Desai et al., 2010) and also a dominant HAB species along the China’s coasts that often blooms during the early spring and summer(Zhu et al., 2009; Ji et al., 2011).
Thus, the present study investigated the alternations of S. costatum seawater by comparing the control of S. costatum at decaying phase(A1), the MC treatment(A2) and the MC treatment in sediment condition(A3). The aim of the study was to clarify two points: whether MC treatment increased the degradation of S. costatumdetritus; whether MC changed the release of the major nutrients(including nitrogen, phosphorus, and silicon)from algal cells into the overlying seawater during the incubation period. 2 MATERIAL AND METHOD 2.1 Algal culture and preparation
Skeletonema costatum(Grev.)Cleve(CCMM 201005)was provided by the Key Laboratory of Marine Ecology and Environmental Sciences. Prior to the incubation experiments, all of the glassware was pre-rinsed with 10% v/v HCl to remove possible contaminants for 24 h and then thoroughly washed with ultrapure water. The 0.45-μm membrane-filtered seawater(salinity 30±1, pH 8.20±0.05) and all glassware in experiments were sterilized at 121°C for 20 min. Monocultures at the exponential phase were inoculated in 2 L of sterilized seawater supplemented with f/4 medium(containing silicon)in 5-L flasks in quadruplicate(Guillard and Ryther, 1962; Guillard et al., 1973). The algae culture was maintained at a temperature of 20±1°C throughout the experiments. The culture flasks were shaken once every two days at 11:30–12:00 am. The initial cell density of theS. costatum cultures was 7×106cells/L. The irradiance intensity was approximately 60–65 μmol photons/(m2·s)under a 12 h:12 h light: dark cycle(Ji et al., 2011). The algal self-deposition, the modified clay flocculation, and the sediment incubation experiments were conducted under the same condition as detailed above.
This study used analytical reagent polyaluminum chloride(PACl)to modify Kaolin(1:5 in hyperpure water)as previously described(Yu et al., 1999; Lin et al., 2013)in slurry of 25.0 g/L. The slurry was not mixed until required for the algae removal experiments.
Freeze-dried sediment samples were obtained from the Yellow Sea, China, 121.50°–121.60°E, 33.00°–36.00°N, where S. costatum blooms often occur(Tang et al., 2006). The TON and TOC concentrations of the freeze-dried samples were(0.021 7±0.004 7)% and (0.190 2±0.050 8)%, respectively. 2.2 Cell removal experiment
Prior to the incubation comparing experiments, 500 mL of algal culture, at a cell density of 3.72×108cells/L was thoroughly stirred and divided equally among seven test tubes, each containing 50 mL for testing and optimization of the amounts of MC slurry to be used. The algae removal experiments were all performed on day 13th. After culturing the tubes of algae, 0, 0.05, 0.10, 0.50, 1.02, 2.08, and 4.35 mL aliquots of the MC mixture were added into the 50-mL algal cultures to final concentrations of 0, 0.025, 0.05, 0.25, 0.50, 1.00 and 2.00 g/L MC, respectively. The tube contents were thoroughly mixed and then allowed to settle for 3.5 h(Yu et al., 2004). Samples were collected from 3 cm below the surface seawater. The cell density and F a of the algal cultures were simultaneously monitored(See Section 2.4). Then the study calculated the removal efficiency of the cells for selecting the suitable dosage applied in the subsequent incubation experiment. 2.3 Comparing incubation experiments
The same light and temperature conditions in incubation experiments were consistent with the above cultural stage 2 . 1 . After culturing for 21 days, the algae cultures was equally divided into 75 test tubes in order to establish three comparing groups in triplicate at 8 moments sampling(24 tubes for A1, 21 tubes for A2, 12 tubes for A3, the other 18 tubes for B group) and each tube containing 100 mL culture(Fig. 1). A1 group was to determine the decaying of S. costatum detritus; A2 group was to investigate the effects of MC flocculation and incubation from day 23 to day 52; A3 group was to investigate the effects of MC flocculation under sediment condition from day 23 to day 52.
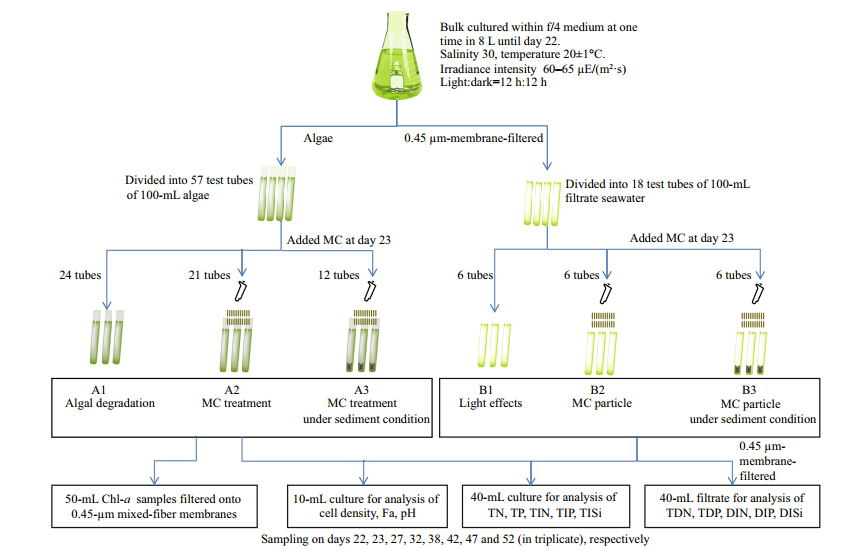 |
| Fig. 1 Experimental design and sampling workflow of S. costatumin comparing incubation experiment
A1 represents the natural death phase; A2 represents only modified clay applied in the algal culture; A3 represents modified clay flocculation with algal cells under the water-sediment systems; B1 represents the light effects on degradation of dissolved organic nutrient; B2 represents the MC flocculation effects on degradation of dissolved organic nutrient; B3 represents the MC flocculation effects under sediment environment; the B groups are the background blanks for A groups; hereafter the same. |
At the same time, the filtrate through 0.45-μm mixed-fiber of the algal culture on day 22 was divided into treatments B1, B2, and B3, which were then treated on day 23 to estimate the effects of light, modified clay, and sediment, respectively, on dissolved nutrient in seawater. The B groups were the background blanks for A groups.
Before day 22, 2.0 g of dry sediment was added into A3 and B3. On day 23, certain volume of the MC mixture based on the removal experiment 2.2 were carefully added by automatic pipettes into the 100-mL algal cultures of A2, A3, B2, and B3. During the incubation period, the samples were carefully collected to minimize contamination on days 22, 23, 27, 32, 38, 42, 47 and 52(in triplicate), respectively. The experiment was conducted under the same condition as algal culture detailed. 2.4 Sampling and parameter analysis
The cell density, pH and in vivo chlorophyll fluorescence, Fa(fsu; Fluorescence Turner Designs, TD700, Sunnyvale, CA, USA)of the algal cultures were simultaneously monitored during the cultivation and flocculation periods. The cell densities were counted in triplicate by using optical microscope(OLYMPUS IX71, Japan)after fixation with 5% v/v glutaraldehyde solution(Churro et al., 2010; Kimura et al., 2012; Maruyama and Kim, 2013).
At each specific sampling moment, each group of the three comparing groups was collected in triplicate of three tube tests. Each seawater sample was divided into three parts(Fig. 1): approximately 10 mL original seawater was collected for the analysis of cell density, Fa, and pH; 40 mL original seawater was collected for the analysis of total nitrogen(TN), total phosphorus(TP), total inorganic nitrogen(TIN), total inorganic phosphorus(TIP), and total inorganic silicon(TISi), respectively; 40 mL filtered seawater was collected for total dissolved nitrogen(TDN), total dissolved phosphorus(TDP), dissolved inorganic nitrogen(DIN), dissolved inorganic phosphorus(DIP), and dissolved inorganic silicon(DISi), respectively. All the nutrient samples were stored at -20°C for further analysis. The TN/TP and TDN/TDP samples were pre-digested with alkaline potassium persulfate prior to nutrient analysis. Nitrate was reduced by a realtime reducing agent of cadmium and then characterized with nitrite using the hydrochloride acid ethylene diamine colorimetric method(detection limit of 0.07 μmol/L). Ammonium concentrations were determined by using the indophenol-blue colorimetric method(detection limit of 0.04 μmol/L). Phosphate concentrations were determined by using the acidic molybdate-ascorbic acid spectrophotometric method with a detection limit of 0.03 μmol/L. Dissolved inorganic nutrients were determined spectrophotometrically using a Skalar TM 5-channel ContinuousFlow-Analyzer(Skalar San ++, Breda, the Netherl and s). The Chl-asample of bottom sweater or sediment was filtered onto 0.45-μm mixed-fiber membrane and then frozen at -20°C until analysis. These chlorophyll-asamples were treated in 10 mL of 100% N, N -dimethylformamide(DMF)for 24 h extraction(Inskeep and Bloom, 1985; Wang et al., 2007; Qin et al., 2013). 2.5 Data analysis
Growth rates were calculated as the differences between two sampling intervals of cell numbers(Guillard et al., 1973; Frampton et al., 2013), μ=[ln(Nt)–ln(N0)]/ t, units of /d. The algae removal efficiency(RE)was calculated as declining proportions of cell numbers, RE=(1– Nt/N22)×100%. The degradation rates were calculated using the equation p=[ln(Ct)–ln(C22)]/(t–22), and the units were /d. Organic components were estimated as the differences between the total and inorganic components. In the above data analyses, 0 and trepresent the initial time day 0 and incubation time day t, respectively; whereas N0, N22, and Nt represent the cell numbers per liter on days 0, 22, and t, respectively, and C22 and Ctrepresent the Chl- aconcentrations on days 22 and t, respectively.
The values were all averaged for triplicate treatments to produce mean concentrations/values± one st and ard deviation(Sd). Significant differences between the comparing treatments were identified by Paired-Sample T Test analysis(SPSS 16.0). The differences were considered significant when P<0.05. 3 RESULT 3.1 Algal removal and changes in incubation experiments
S. costatumcells, with a bloom level of 3.72×108 cells/L, were removed using modified clay at 0, 0.025, 0.05, 0.25, 0.50, 1.00, and 2.00 g/L concentrations. The RE value increased with increasing MC concentrations and the highest RE was up to 98.79% at a concentration of 1.00 g/L of MC. High concentration of clay application could seriously enhance the turbidity of seawater and impact the growth of filter-feeding organisms like fishes and clams in the field(Archambault et al., 2004; Anderson, 2009). Thus, we selected 0.25 g/L as the representative concentration for S. costatumthree comparing experiment(>97% cells removal)based on removal experiment and previous field studies <0.5 g/L(Fig. 2a).
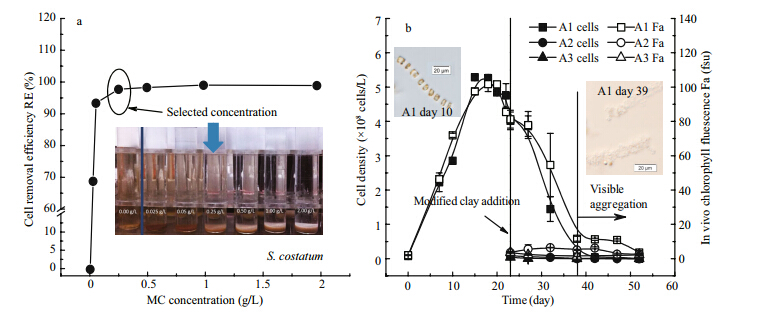 |
| Fig. 2 The RE(in y-axis)of S. costatumin different modified clay concentrations(in x-axis)(a); the cell density(in left y-axis) and in vivo chlorophyll fluorescence Fa(in right y-axis)changes in the comparing systems of S. costatum(b)
The arrows indicate the modified clay addition time on day 23; the photograph in b showed the healthy cells at day 10 and visible aggregations of diatom detritus from day 39 in A1 under microscope. |
MC treatment had reduced the high cell density of S. costatum . In the comparing incubation experiments, the cell density of A1 remained high cell density of(5.29±0.11)×108cells/L(Fig. 2b)in five days and then slowly decreased. When MC was added in A2 and A3 on day 23, the cell densities dropped sharply from(4.01±0.26)×108cells/L to(0.09±0.02)×108 cells/L and (0.04±0.01)×108 cells/L, respectively. Subsequently, the cell densities of A2 and A3 continued to decrease until below the undetectable level on day 52. The Fa value of S. costatum exhibited synchronous changes of cell density(Fig. 2b). The Fa in A1 seawater reached a maximum of 101.7±4.3 fsu on day 18 and subsequently declined to a minimum of 3.6±1.1 fsu on day 52. After the addition of MC, the F a of A2 and A3 seawater suddenly dropped from 81.3±5.2 fsu to 3.6±0.3 and 3.6±0.2, respectively, on day 23, and sustained at a lower level than that in A1 during the subsequent period. 3.2 Characteristics of total major nutrients in seawater
The total major nutrients were efficiently eliminated from seawater by MC flocculation. The TP concentration in A1 remained at 14.31±1.22 μmol/L from day 22 to day 47 but decreased to 7.58±3.19 μmol/L on day 52(Fig. 3a). The TN concentration in A1 dropped gradually from 410.62± 35.44 μmol/L on day 22 to 319.03±62.66 μmol/L on day 52(Fig. 3b). After the addition of MC, the TP concentrations in A2 and A3 dropped to <1.73 μmol/L and 1.57 μmol/L, respectively(Fig. 3). The TN concentrations in A2 and A3 abruptly decreased from 433.11±35.44 μmol/L to 236.76±40.98 μmol/L and to 252.26±8.01 μmol/L on day 23, respectively. And then they gradually decreased to lower levels of 189.89±21.54 μmol/L and 115.17±6.90 μmol/L on day 52, respectively. The TISi(including dissolved inorganic silicon and particulate inorganic silicon)exhibited continuous increases from 244.54± 29.35 μmol/L on day 22 to a maximum concentration of 363.57±11.47 μmol/L on day 47 in A1 culture. The TISi declined to a minimum concentration of 63.34±7.60 μmol/L in A2 on day 42 and 104.79± 3.31 μmol/L in A3 on day 38(Fig. 3d). Regarding MC flocculation, the TISi concentration increased by 24.12% in A1 but decreased substantially in A2(69.28%) and A3(42.62%)at day 52 compared to the primary concentration of A1 on day 22, respectively.
 |
| Fig. 3 The concentration changes of total nutrients and dissolved inorganic nutrients in three comparing incubation systems
a. total phosphorus(TP) and dissolved inorganic phosphorus or phosphate(DIP); b. total nitrogen(TN) and dissolved inorganicnitrogen(DIN); c. nitrate-N, nitrite-N and ammonium-N; this study also analyzed the concentration of total dissolved nitrogen(TDN) and phosphorus(TDP), total inorganic nitrogen(TIN) and phosphorus(TIP), not shown; d. total inorganic silicon(TISi), and dissolved inorganic silicon(DISi). The green symbols represent the natural degradation; the red symbols represent the MC flocculation; the blue symbols represent the MC-algae-sediment matrix system; the other nutrient concentrations in B1 and B2 have no changes from day 23 to 52 and thus they were removed from the graph because of space constraints. |
The dissolved inorganic nutrients in the seawater analysis generally consisted of NO3--N, NO2--N, NH4+-N, DIP and DISi. The original NH4+- N concentration in A1 on day 22 was only 2.71± 2.44 μmol/L and then gradually increased to a maximum concentration of 39.34±1.12 μmol/L on day 52(Fig. 3c). NO2--N concentration in A1 increased from 3.60±0.96 μmol/L on day 22 to 18.18± 2.31 μmol/L on day 32. In contrast, NO3--N concentration in A1 exhibited subtle changes from 163.66±40.64 μmol/L to 108.00±22.46 μmol/L. The DIP concentration in A1 dropped from 2.08± 0.34 μmol/L on day 22 to 0.19±0.06 μmol/L on day 23 and remained <0.18 μmol/L. The DISi concentration in A1 exhibited a similar trend as TISi, which increased from 219.53±4.24 μmol/L to 375.12± 51.55 μmol/L. After the addition of MC, the NH4+-N concentration in A2 decreased from 2.36±1.01 to 1.60±0.63 μmol/L on day 23 and remained <7.03±2.52 μmol/L before day 52, which was significantly decreased compared to A1(P=0.038<0.05). The performance of NH4+-N in A3 exhibited differences, reaching 4.52±1.31 μmol/L on day 23, and increasing to 30.27±2.21 μmol/L onday 52. NH4+-N concentrations in B1 and B2 were stable in low levels, but the situation in B3 was different. The increasing NH4+-N in B3 indicated the ammonium released from the original sediment(Fig. 3c in the small graph). The NO2--N concentration in A2 remained <15 μmol/L, whereas the NO2--N of A3 initially increased to 13.46±2.03 μmol/L, and then decreased to 4.10±0.73 μmol/L. However, the NO3--N concentrations in A1 and A2 exhibited similar(same result in Ayyasamy et al.(2007)), whereas that observed in A3 was consistently less than that observed in A1 and A2, declining to 33.21± 11.30 μmol/L on day 52(Fig. 3c). The DIP concentrations remained <0.20 μmol/L in A2 and <0.45 μmol/L in A3. Meanwhile, DISi concentrations in all treatments exhibited the similar changes as TISi, while the DISi kept increasing to high concentration in B1 and low concentration in B2 and B3. 3.4 Changes in the chlorophyll- acontent of aggregation and sediment
To detect the degradation of algal cells, we measured the Chl-aconcentration in the 50 mL bottom water, MC-algae aggregation or MC-algaesediment samples. MC treatment had significantly influenced the Chl-aconcentration. The Chl-aconcentration in A1 gradually decreased from 0.481±0.051 μg/mL on day 22 to 0.023±0.001 μg/mL on day 52(Fig. 4). Upon the addition of MC, the Chl-aconcentration in A2 increased from 0.430±0.014 μg/mL on day 23 to 0.785±0.017 μg/mL on day 27. Ultimately, the Chl-aconcentration declined to 0.114±0.031 μg/mL on day 52, which was still higher than that observed in A1(Fig. 4). The Chl-aconcentration in A3 initially decreased to 0.333± 0.073 μg/mL and remained at approximately 0.315 μg/mL, suggesting that the MC-sediment condition influenced the characteristics of Chl-awithin the surface sediment(Fig. 4). In addition, the degradation rates of Chl-a(ρ)were calculated. The ρvalue in A1 remained negative and declined to a minimum of -0.101/d. However, the ρvalues in A2 and A3 were -0.048/d and -0.013/d, respectively. In comparison with A1, the ρvalues of A2 and A3 decreased 47.54% and 86.76%, respectively, at the end of the incubation period. These results demonstrated that the MC-sediment matrix indeed delayed the degradation process and reduced the degradation rates of Chl-awithin the sediment condition.
 |
| Fig. 4 The chlorophyll- aconcentration(in y-axis)changes of S. costatum in three comparing incubation systems
The square symbol represents the A1 group, the cycle symbol represents the A2 group, the triangle symbol represents the A3 group; the vertical line is the addition time of modified clay. |
The present study shows that low dosages of MC could efficiently remove S. costatum cells. At day 23, after the addition of 0.25 g/L MC, the RE of the S. costatumcells at death phase were approximately 97.81% in A2 and 98.98% in A3(Fig. 2b), respectively, which were slightly higher than the RE at the growth phase(97.49%)(Fig. 2a). Meanwhile, MC treatment seriously influences the growth of the diatom S. costatum—both the residual populations left in water or the cells trapped in MC-algae matrix and MCalgae-sediment matrix. The μvalue reached its maximum level of 0.486/d and decreased during the subsequent period, particularly strong negative values in A2 and A3. Although some researchers worried about the cells might escape from the trapped MCalgae matrix into seawater, there was no simultaneous quick re-blooming of the S. costatum cells after the addition of MC in A2 and A3(Fig. 2b).
In addition, MC treatment significantly influences the microhabitat of S. costatum in seawater. The Chlain bottom water of A1 reflected the rapid decomposition of S. costatum detritus(Fig. 4; massive visible and transparent aggregation could be observed from day 39 in Fig. 2b). With algal degradation, the pH value in A1 decreased from(9.05±0.08)at day 27 to(8.07±0.02)at day 52(the same result as Balzano et al.(2011)). However, it exhibited a slow degradation of Chl-a(Fig. 4) and more stable pH values(average of 8.58)in A2 and A3, which suggested that the buffering system of the amorphous aluminum produced by MC hydrolysis(Rao et al., 2011)might stabilize the in situ changes of the seawater during MC treatment. Therefore, MC might create an organic-clay co-matrix that combines clay and algal matter(Hamm, 2002). Under the sediment condition, it easily formed the hypoxic and relative-acidic(pH<8.0)environment, which delayed the death and decomposition of the algal matters(Loucaides et al., 2012). One important effect of MC is caused by the core role of aluminum speciation in PACl–Al 13that is very effective in charge neutralizing with humic acid molecules(Zarchi et al., 2013), and its adsorption and sweep coagulation effect are significant at pH>6.0(Liu et al., 2009). In this study, the pH values of seawater in all three groups were about >7.77, which provided a suitable condition for high efficient coagulation. These changes in microhabitat significantly influence the growth of S. costatum cells. Thus, there was no second blooming observed during the incubation experiment. 4.2 Influences of MC treatment on the concentrations of major nutrient
In the present study, MC treatment could efficiently eliminate nutrient from seawater. During the comparing incubation period, the quick decreasing trends of TN and TP in A2 and A3(TN decreased to <200 μmol/L, P=0.001<0.05; TP decreased to <1.8 μmol/L, P=0.006<0.05)were attributed to MC effects(Fig. 3). Compared to the TN and TP in A1 at day 52, 53.75% of TN and 93.36% of TP in A2, as well as, 71.95% of TN and 93.60% of TP in A3 were removed by MC. The TISi concentration increased 24.21% in A1 with decreasing of cell density; however, 69.28% and 42.62% of TISi concentrations in A2 and A3 were removed by MC treatment.
In the freshwater treatment, PACl was used to as the flocculant aid to increase the efficiency of coagulation(Ahammed Shabeer et al., 2014b). Previous researchers reported the high phosphorus removal capacity of the flocculant PACl in water(Reddy et al., 2011), and lithogenic particles have a similarly strong adsorption capacity for phosphorus(Swartzen-Allen and Matijevic, 1974; Yu et al., 1995; Schroth and Sposito, 1998; Matilainen et al., 2010; Unuabonah et al., 2010). Reddy et al.(2011)reported that the mechanism of phosphorus removal by PACl was caused by the incorporation of the phosphates into suspended solids, which reduced the P content, that phosphate ions directly adsorbed onto the hydrolytic products formed by PACl and to form the phosphate precipitates with aluminum or other ions charge exchange released from clay particles(Spilling and Lindström, 2008; Zarchi et al., 2013). Upon the addition of MC, a similar phenomenon was observed for the diatom aggregation, including algal organic matter, algal cells, mineral particles, flocculants combined with each other, small-size flocs aggregated to large sizes and high sinking-velocity aggregations(Hamm, 2002; Sañudo-Wilhelmy et al., 2004). Some studies have demonstrated that as a direct result of clay suspensions, fewer metal elements remain in water after low doses of clay treatment(Schroth and Sposito, 1998; Matilainen et al., 2010; Wu et al., 2011). In addition, the cation exchange at the interface between the clay particles and seawater might change the chemical composition dissolved in the water(Swartzen-Allen and Matijevic, 1974; Spilling and Lindström, 2008; Unuabonah et al., 2010; Miao et al., 2014). Tallberg et al.(2013)reported that the combined effect of algae and s and decreased both ammonium efflux and denitrification activity, related to the antimicrobial effects of s and particles(Iversen and Ploug, 2010; Larraza et al., 2011; Salter et al., 2011).
After one dinoflagellate blooming, there was a quick increasing of DIP concentration in water because of the decomposition of algal matters(Officer and Ryther, 1980; Spilling and Lindström, 2008). However, the diatom detritus in this study did not exhibit a similar rapid transformation from org-P to DIP(phosphate)as observed in the degradation of dinoflagellates(Officer and Ryther, 1980). In this study, the DIP concentrations of all three treatments decreased to <0.5 μmol/L and did not increase during the one-month incubation. The DOP of A1, A2 and A3 remained at decreased levels of <1.0 μmol/L, indicating that no additional organic phosphorus released from the cells and dissolved into the seawater. The reduced level of dissolved phosphorus was predominantly attributed to the adsorption of biogenic silica released from death diatom detritus(Krause et al., 2011; Loucaides et al., 2012; Kemp and Villareal, 2013; Siipola et al., 2013). This suggests that both diatom self-detritus and MC particles could remove phosphorus. If the incubation time is long enough, the part of nutrient combined with biogenic silica in A1 might release into the overlying seawater, which means the diatom detritus decomposes more slowly than that in dinoflagellate.
The variability of silicon in seawater was complex because of the specific characteristics of biogenic silica(Tallberg et al., 2012). There might be an undesirable effect on the nutrient recycling process with the increased deposition of organic matter to the sediment(Conley and Johnstone, 1995; Tallberg et al., 2013). Conley and Johnstone(1995)indicated that the decreased rate of biogenic silicon remineralization with increased deposition might explain the long-term declines of DISi in the Baltic Sea, which might alter the structure of phytoplankton communities. They demonstrated that the algal deposition during spring blooms might elicit a significant impact on nutrient biogeochemical cycles between the seawater-sediment interfaces(Conley and Johnstone, 1995). In this study, the DISi concentration in A1 significantly increased 70.88%(P<0.001) and the Chl-acontent in A1 significantly decreased 95%(P<0.001)during the incubation time(Figs.3d, 4). Simultaneously, there are visible and transparent aggregations from S. costatum detritus in A1, particularly from day 39(Fig. 2b), which included mucus-rich empty frustules with polysaccharides, hydrocarbons, fatty acids or alkanes(Hamm, 2002; Ehrenhauss et al., 2004; Vidoudez and Pohnert, 2012; Zarchi et al., 2013). These results demonstrated the quick decomposition of the biogenic silica from S. costatumcells re-mineralized and released DISi. That was the reason for less intact cells of S. costatum than other diatoms observed in the sediment of previous studies. Meanwhile, it showed the quicker released rate of DISi than DIN and DIP decomposed from degradation of S. costatum(Fig. 3). After MC addition, more than 90% of the diatom cells(Fig. 2b)were flocculated and settled by MC in A2 and A3 with visible brown aggregation at day 52(Fig. 3a). Tallberg et al.(2013)indicated that as Si bound into amorphous biogenic silicon, it released much more slowly than the predominantly organically bound P and N. Thus, in the present study, we did not observe a release of dissolved silicon in A2 and A3(Fig. 3d). Consequently, comparing to the DISi in A1, the DISi in A2 and A3 decreased 64.43% and 43.61%, respectively, on day 52. Simultaneously, no excess DISi released into water in B2 and B3. We strongly believe that MC treatment can effectively eliminate the silicon that is essential element for the re-blooming of S. costatum . 4.3 Influences of MC treatment on the chemical stoichiometry of seawater
MC also significantly changed the chemical stoichiometry of seawater that related to the survival of phytoplankton species(Sañudo-Wilhelmy et al., 2004). Upon the addition of MC, the TN/TP ratios in A2 and A3 increased 3–6 times higher than that in A1(Fig. 5). Furthermore, the TON/TOP ratios in A2 and A3 were up to 90.83 and 106.57, respectively, which were also 3–5 times higher than that observed in A1 on day 52. Compared to that in A1, the DIN/DIP ratio in A3 decreased 83.9%, the DISi/DIN ratio in A2 significantly decreased 79.2%(P<0.001), and the DISi/DIP ratios in A2 and A3 significantly decreased 45.8%–84.7%(P<0.001, Fig. 5). Capellacci et al.(2013)investigated different sources of DISi that could affect the silicon bioavailability for marine diatom growth. They demonstrated that DISi generated in seawater by crystalline sources are highly bio-available compared to those obtained by biogenic and amorphous materials, on the other h and , the silica polymorphs involved on the ionic composition of the solution controlled the silica-water interactions(Capellacci et al., 2013). Zhu et al.(2009)reported that the cell density of S. costatum decreased significantly but less rapidly than that of P. donghaiensein the presence of low levels of nutrients in seawater, which might explain the reason that the S. costatumexhibit shorter blooms than P. donghaiense. Our results indicate that the low concentrations of nutrients, particularly in low phosphorus and silicon, in seawater rebuild the environment unsuitable by MC treatment for S. costatum blooms, which significantly deviated from the favorable chemical stoichiometry(Redfield ratio)for the growth of algae(Sañudo-Wilhelmy et al., 2004; Kemp and Villareal, 2013). However, the ways in which nutrient concentrations and compositions affect the competition among species remains unknown.
 |
| Fig. 5 The chemical stoichiometry value of nitrogen, phosphorus, and silicon in seawater of S. costatum in three comparing
systems a. the TDN/TDP ratios(in y-axis)vs TN/TP ratios(in x-axis); b. the DISi/DIN ratios(in y-axis)vs DISi/DIP ratios(in x-axis). |
Because the incubation time was limited in one month, the present study only observed the quick degradation of silicon(Fig. 3d) and Chl-a(Fig. 4)source from S. costatum detritus in A1, without observing in A2 and A3. Hamm(2002)reported that the addition of lithogenic suspensions to diatom cultures accelerated the formation of visible aggregations, and SEM images of algal-clay aggregations showed that most clay particles did not directly attach onto the diatom cells but rather formed clusters that bounded by an extracellular organic material, mostly likely attached via mucoid particles. Organic amines from algal cells can combine with Kaolin cavities(Markiewicz et al., 2013). Jardine et al.(1989)demonstrated that kaolinite is more efficient in its adsorption of organic matter than montmorillonite. The algal cytoderm contains many carboxyl groups that can react with algal-released amines, and consequently, fewer oxidizing positions would be available for microbial degradation(Liao, 2006; Lebedeva amd Fogden, 2011a, b). Simultaneously, the particles of MC also have an antimicrobial effect as well as a bacteria-virusnanoflagellate-filtering effect(Larraza et al., 2011; Salter et al., 2011). These effects could protect organic nutrient from degradation and impede the rates of decomposition. Consequently, on the one h and , MC treatment have accelerated the sinking speed of total nutrient(including inorganic and organic), on the other h and , it delayed the remineralization speed of organic nutrient and the release process of dissolved inorganic nutrient into the overlying seawater. 5 CONCLUSION
(1)The low dosage of modified clay have significant influences on the growth of S. costatum ;(>97% cells removal)with no re-blooming observed during the one-month incubation period.
(2)More importantly, major nutrients of seawater(both inorganic and organic nutrients)in the present study have been eliminated by MC treatment, and no excess inorganic nutrient release from the MC-algae matrix during one-month incubation period;
(3)The chemical stoichiometry in seawater(TN/TP, DIN/DIP, DISi/DIN, and DISi/DIP) and nutrient cycling process have been altered by MC treatment with increasing the downward flux of total nutrients, preventing the degradation rates of organic nutrients, and delaying the recycling velocity of inorganic nutrients into seawater.
Although our group has had extensive experience in HABs’ mitigation along the Chinese coast(in the Changjiang Estuary of Shanghai City, South China Sea coast of Shenzhen City, Yellow Sea coast of Qingdao City, Bohai Bay coast of Qinhuangdao City, etc.), our findings suggest that a reevaluation is necessary to determine the long-term effects of MC treatment before large-scale application. Further detailed study of MC effects is required in terms of its ability to control different HAB species. Underst and ing the effects of MC treatment can help us to determine the optimum condition of MC for HABs control in the field. 6 ACKNOWLEGEMENT
We also gratefully acknowledge the valuable suggestions and comments of Drs. FU Mei, LIN Yongxin and WU Zaixing.
| Ahammed Shabeer T P, Saha A, Gajbhiye V T, Gupta S, Manjaiah K M, Varghese E. 2014a. Simultaneous removal of multiple pesticides from water: effect of organically modified clays as coagulant aid and adsorbent in coagulation-flocculation process. Environ. Technol., 35 (20): 2 619-2 627. |
| Ahammed Shabeer T P, Saha A, Gajbhiye V T, Gupta S, Manjaiah K M, Varghese E. 2014b. Removal of poly aromatic hydrocarbons (PAHs) from water: effect of nano and modified nano-clays as a flocculation aid and adsorbent in coagulation-flocculation process. Polycycl. Aromat. Comp., 34 (4): 452-467. |
| Anderson D M. 1997. Turning back the harmful red tide. Nature, 388 (6642): 513-514. |
| Anderson D M. 2009. Approaches to monitoring, control and management of harmful algal blooms (HABs). Ocean Coast Manage., 52 (7): 342-347. |
| Anderson D M, Cembella A D, Hallegraeff G M. 2012. Progress in understanding harmful algal blooms: paradigm shifts and new technologies for research, monitoring, and management. Annu. Rev. Mar. Sci., 4 (1): 143-176. |
| Archambault M C, Bricelj V M, Grant J, Anderson D M. 2004. Effects of suspended and sedimented clays on juvenile hard clams, Mercenaria mercenaria, within the context of harmful algal bloom mitigation. Mar. Biol., 144 (3): 553- 565. |
| Armstrong R A, Lee C, Hedges J I, Honjo S, Wakeham S G. 2002. A new, mechanistic model for organic carbon fluxes in the ocean based on the quantitative association of POC with ballast minerals. Deep-Sea Res. Pt.II, 49 (1-3): 219- 236. |
| Ayyasamy P M, Shanthi K, Lakshmanaperumalsamy P, Lee S J, Choi N C, Kim D J. 2007. Two-stage removal of nitrate from groundwater using biological and chemical treatments. J. Biosci. Bioeng., 104 (2): 129-134. |
| Balzano S, Pancost R D, Lloyd J R, Statham P J. 2011. Changes in fatty acid composition in degrading algal aggregates. Marine Chemistry, 124 (1-4): 2-13. |
| Blanchemain A, Grizeau D. 1996. Eicosapentaenoic acid content of Skeletonema costatum as a function of growth and irradiance; relation with chlorophyll a content and photosynthetic capacity. J. Exp. Mar. Biol. Ecol., 196 (1- 2): 177-188. |
| Capellacci S, Battocchi C, Casabianca S, Giovine M, Bavestrello G, Penna A. 2013. Bioavailability of different chemical forms of dissolved silica can affect marine diatom growth. Marine Ecology, 34 (1): 103-111. |
| Certner R, Cho H, Gallo N, Gibbons A, Kim C, Liu T, Miller H, Parikh N, Wooten M, (Team BREATHE (Bay Revitalization Efforts Against the Hypoxic Environment)). 2011. Using Sediment Flocculation to Reduce the Impacts of Chesapeake Bay Microcystis aeruginosa Harmful Algal Blooms. Thesis submitted in partial fulfillment of the requirements of the Gemstone Program, University of Maryland, College Park. USA. p.55-107. |
| Chen J, Pan G. 2012. Harmful algal blooms mitigation using clay/soil/sand modified with xanthan and calcium hydroxide. J. Appl. Phycol., 24 (5): 1 183-1 189. |
| Churro C, Fernandes A S, Alverca E, Sam-Bento F, Paulino S, Figueira V C, Bento A J, Prabhakar S, Lobo A M, Martins L L, Mourato M P, Pereira P. 2010. Effects of tryptamine on growth, ultrastructure, and oxidative stress of cyanobacteria and microalgae cultures. Hydrobiologia, 649 (1): 195-206. |
| Conley D J, Johnstone R W. 1995. Biogeochemistry of N, P and Si in Baltic Sea sediments: response to a simulated deposition of a spring diatom bloom. Mar. Ecol. Prog. Ser., 122 : 265-276. |
| Curl H, Mcleod G C. 1961. The physiological ecology of a marine diatom, Skeletonema costatum (Grev) Cleve. J. Mar. Res., 19 (2): 70-88. |
| Desai S R, Verlecar X N, Ansari Z A, Jagtap T G, Sarkar A, Vashistha D, Dalal S G. 2010. Evaluation of genotoxic responses of Chaetoceros tenuissimus and Skeletonema costatum to water accommodated fraction of petroleum hydrocarbons as biomarker of exposure. Water Res., 44 (7): 2 235-2 244. |
| Ehrenhauss S, Witte U, Janssen F, Huettel M. 2004. Decomposition of diatoms and nutrient dynamics in permeable North Sea sediments. Cont. Shelf. Res., 24 (6): 721-737. |
| Frampton D M F, Gurney R H, Dunstan G A, Clementson L A, ToiflM C, Pollard C B, Burn S, Jameson I D, Blackburn S I. 2013. Evaluation of growth, nutrient utilization and production of bioproducts by a wastewater-isolated microalga. Bioresource Technol., 130 : 261-268. |
| Gehlen M, Bopp L, Ernprin N, Aumont O, Heinze C, Raguencau O. 2006. Reconciling surface ocean productivity, export fluxes and sediment composition in a global biogeochemical ocean model. Biogeosciences, 3 (4): 521-537. |
| Guillard R R L, Kilham P, Jackson T A. 1973. Kinetics of silicon-limited growth in the marine diatom Thalassiosira Pseudonana Hasle and Heimdal (= Cyclotella-Nana Hustedt). J. Phycol., 9 (3): 233-237. |
| Guillard R R L, Ryther J H. 1962. Studies of Marine Planktonic Diatoms: I. Cyclotella Nana Hustedt, and Detonula Confervacea (Cleve) Gran. Can. J. Microbiol., 8 (2): 229- 239. |
| Hamm C E. 2002. Interactive aggregation and sedimentation of diatoms and clay-sized lithogenic material. Limnol. Oceanogr., 47 (6): 1 790-1 795. |
| Ingalls A E, Lee C, Wakeham S G, Hedges J I. 2003. The role of biominerals in the sinking flux and preservation of amino acids in the Southern Ocean along 170°W. Deep-Sea Res. Pt. II, 50 (3-4): 713-738. |
| Ingalls A E, Liu Z F, Lee C. 2006. Seasonal trends in the pigment and amino acid compositions of sinking particles in biogenic CaCO3 and SiO2 dominated regions of the Pacific sector of the Southern Ocean along 170° W. Deep-Sea Res. Pt. I, 53 (5): 836-859. |
| Inskeep W P, Bloom P R. 1985. Extinction coefficients of chlorophyll- a and b in N, N-dimethylformamide and 80% acetone. Plant Physiol., 77 (2): 483-485. |
| Iversen M H, Ploug H. 2010. Ballast minerals and the sinking carbon flux in the ocean: carbon-specificrespiration rates and sinking velocity of marine snow aggregates. Biogeosciences, 7 (9): 2 613-2 624. |
| Iversen M H, Ploug H. 2013. Temperature effects on carbonspecific respiration rate and sinking velocity of diatom aggregates-potential implications for deep ocean export processes. Biogeosciences, 10 (6): 4 073-4 085. |
| Jardine P M, Mccarthy J F, Weber N L. 1989. Mechanisms of dissolved organic carbon adsorption on soil. Soil Sci. Soc. Am. J., 53 (5): 1 378-1 385. |
| Ji X Q, Han X T, Zheng L, Yang B J, Yu Z M, Zou J Z. 2011. Allelopathic interactions between Prorocentrum micans and Skeletonema costatum or Karenia mikimotoi in laboratory cultures. Chinese Journal of Oceanology and Limnology, 29 (4): 840-848. |
| Kemp A E, Villareal T A. 2013. High diatom production and export in stratified waters—a potential negative feedback to global warming. Progress in Oceanography, 119 : 4-23. |
| Kimura K, Tomaru Y, Nagasaki K. 2012. Ultrastructural observation of natural field phytoplankton cells by using rapid freezing and freeze substitution. Plankton & B enthos R esearch, 7 (3): 126-134. |
| Kooistra W H C F, Sarno D, Balzano S, Gu H F, Andersen R A, Zingone A. 2008. Global diversity and biogeography of Skeletonema species (Bacillariophyta). Protist, 159 (2): 177-193. |
| Krause J W, Nelson D M, Brzezinski M A. 2011. Biogenic silica production and the diatom contribution to primary production and nitrate uptake in the eastern equatorial Pacific Ocean. Deep-Sea Res. Pt. II, 58 (3-4): 434-448. |
| Larraza I, Peinado C, Abrusci C, Catalina F, Corrales T. 2011. Hyperbranched polymers as clay surface modifiers for UV-cured nanocomposites with antimicrobial activity. Journal of Photochemistry and Photobiology A : Chemistry, 224 (1): 46-54. |
| Lebedeva E V, Fogden A. 2011a. Nano-scale structure of crude oil deposits on water-wet substrates: dependence on aqueous phase and organic solvents. Colloid s Surface s A : Physicochemical and Engineering Aspects, 380 (1-3): 280-291. |
| Lebedeva E V, Fogden A. 2011b. Wettability alteration of kaolinite exposed to crude oil in salt solutions. Colloid Surface A : Physicochemical and Engineering Aspects, 377 (1-3): 115-122. |
| Lee Y J, Choi J K, Kim E K, Youn S H, Yang E J. 2008. Field experiments on mitigation of harmful algal blooms using a Sophorolipid-Yellow clay mixture and effects on marine plankton. Harmful Algae, 7 (2): 154-162. |
| Liao M. 2006. Effects of organic acids on adsorption of cadmium onto kaolinite, goethite, and bayerite. Pedosphere, 16 (2): 185-191. |
| Lin Y X, Cao X H, Song X X, Wang N, Yu Z M. 2013. Mechanisms and factors affecting the adsorption of sodium alginate onto modified clays. Chinese Journal of Oceanology and Limnology, 31 (4): 867-875. |
| Liu H J, Hu C Z, Zhao H, Qu J H. 2009. Coagulation of humic acid by PACl with high content of Al13: the role of aluminum speciation. Separation and Purification Technology, 70 (2): 225-230. |
| Loucaides S, Van Cappellen P, Roubeix V, Moriceau B, Ragueneau O. 2012. Controls on the recycling and preservation of biogenic silica from biomineralization to burial. Silicon, 4 (1): 7-22. |
| Markiewicz M, Mrozik W, Rezwan K, Thöming J, Hupka J, Jungnickel C. 2013. Changes in zeta potential of imidazolium ionic liquids modified minerals— Implications for determining mechanism of adsorption. Chemosphere, 90 (2): 706-712. |
| Maruyama S, Kim E. 2013. A modern descendant of early green algal phagotrophs. Curr. Biol., 23 (12): 1 081-1 084. |
| Matilainen A, Vepsäläinen M, Sillanpää M. 2010. Natural organic matter removal by coagulation during drinking water treatment: a review. Adv. Colloid Interfac. Sci., 159 (2): 189-197. |
| Miao C G, Tang Y, Zhang H, Wu Z Y, Wang X Q. 2014. Harmful algae blooms removal from fresh water with modified vermiculite. Environ. .Technol, 35 (3): 340-346. |
| Moriceau B, Garvey M, Ragueneau O, Passow U. 2007. Evidence for reduced biogenic silica dissolution rates in diatom aggregates. Mar. Ecol. Prog. Ser., 333 : 129-142. |
| Moriceau B, Goutx M, Guigue C, Lee C, Armstrong R, Duflos M, Tamburini C, Charriere B, Ragueneau O. 2009. Si-C interactions during degradation of the diatom Skeletonema marinoi. Deep-Sea Res. Pt. II, 56 (18): 1 381-1 395. |
| Nelson D M, Tréguer P, Brzezinski M A, Leynaert A, Quéguiner B. 1995. Production and dissolution of biogenic silica in the ocean: revised global estimates, comparison with regional data and relationship to biogenic sedimentation. Global Biogeochem. Cy., 9 (3): 359-372. |
| Officer C B, Ryther J H. 1980. The possible importance of silicon in marine eutrophication. Mar. Ecol. Prog. Ser., 3 (1): 83-91. |
| Pan G, Chen J, Anderson D M. 2011. Modified local sands for the mitigation of harmful algal blooms. Harmful A lgae, 10 (4): 381-387. |
| Pan G, Dai L C, Li L, He L C, Li H, Bi L, Gulati R D. 2012. Reducing the recruitment of sedimented algae and nutrient release into the overlying water using modified soil/sand flocculation-capping in eutrophic lakes. Environ. Sci. Technol., 46 (9): 5 077-5 084. |
| Pierce R H, Henry M S, Higham C J, Blum P, Sengco M R, Anderson D M. 2004. Removal of harmful algal cells ( Karenia brevis ) and toxins from seawater culture by clay flocculation. Harmful Algae, 3 (2): 141-148. |
| Ploug H, Iversen M H, Koski M, Buitenhuis E T. 2008. Production, oxygen respiration rates, and sinking velocity of copepod fecal pellets: direct measurements of ballasting by opal and calcite. Limnol. Oceanogr., 53 (2): 469-476. |
| Puskaric S, Mortain-Bertrand A. 2003. Physiology of diatom Skeletonema costatum (Grev.) Cleve photosynthetic extracellular release: evidence for a novel coupling between marine bacteria and phytoplankton. Journal of Plankton Research, 25 (10): 1 227-1 235. |
| Qin H J, Li S S, Li D H. 2013. An improved method for determining phytoplankton chlorophyll a concentration without filtration. Hydrobiologia, 707 (1): 81-95. |
| Rao F, Ramirez-Acosta F J, Sanchez-Leija R J, Song S X, Lopez-Valdivieso A. 2011. Stability of kaolinite dispersions in the presence of sodium and aluminum ions. Appl. Clay Sci., 51 (1-2): 38-42. |
| Reddy G B, Forbes D A, Hunt P G, Cyrus J S. 2011. Effect of polyaluminium chloride on phosphorus removal in constructed wetlands treated with swine wastewater. Water Sci. Technol., 63 (12): 2 938-2 943. |
| Salter I, Böttjer D, Christaki U. 2011. The effect of inorganic particle concentration on bacteria-virus-nanoflagellate dynamics. Environmental Microbiology, 13 (10): 2 768- 2 777. |
| Sañudo-Wilhelmy S A, Tovar-Sanchez A, Fu F X, Capone D G, Carpenter E J, Hutchins D A. 2004. The impact of surface-adsorbed phosphorus on phytoplankton Redfield stoichiometry. Nature, 432 (7019): 897-901. |
| Schroth B K, Sposito G. 1998. Effect of landfill leachate organic acids on trace metal adsorption by kaolinite. Environ. Sci. Technol., 32 (10): 1 404-1 408. |
| Siipola V, Mäntyniemi S, Lehtimäki M, Tallberg P. 2013. Separating biogenic and adsorbed pools of silicon in sediments using bayesian inference. Silicon, 5 (1): 53-65. |
| Spilling K, Lindström M. 2008. Phytoplankton life cycle transformations lead to species-specific effects on sediment processes in the Baltic Sea. Cont. Shelf Res., 28 (17): 2 488-2 495. |
| Swartzen-Allen S L, Matijevic E. 1974. Surface and colloid chemistry of clays. Chemical Reviews, 74 (3): 385-400. |
| Tallberg P, Lehtoranta J, Hietanen S. 2013. Silicate release from sand-manipulated sediment cores: biogenic or adsorbed Si? Silicon, 5 (1): 67-74. |
| Tallberg P, Räike A, Lukkari K, Leivuori M, Lehtoranta J, Pitkanen H. 2012. Horizontal and vertical distribution of biogenic silica in coastal and profundal sediments of the Gulf of Finland (northeastern Baltic Sea). Boreal Environ. Res., 17 (5): 347-362. |
| Tang D L, Di B P, Wei G F, Ni I H, Oh I S, Wang S F. 2006. Spatial, seasonal and species variations of harmful algal blooms in the South Yellow Sea and East China Sea. Hydrobiologia, 568 (1): 245-253. |
| Turner J T. 2002. Zooplankton fecal pellets, marine snow and sinking phytoplankton blooms. Aquat. Microb. Ecol., 27 (1): 57-102. |
| Unuabonah E I, Olu-Owolabi B I, Oladoja A N, Ofomaja A E, Yang Z L. 2010. Pb/Ca ion exchange on kaolinite clay modified with phosphates. J. Soil Sediment., 10 (6): 1 103- 1 114. |
| Vidoudez C, Pohnert G. 2012. Comparative metabolomics of the diatom Skeletonema marinoi in different growth phases. Metabolomics, 8 (4): 654-669. |
| Villacorte L O, Ekowati Y, Winters H, Amy G L, Schippers J C, Kennedy M D. 2013. Characterisation of transparent exopolymer particles (TEP) produced during algal bloom: a membrane treatment perspective. Desalination and Water Treatment, 51 (4-6): 1 021-1 033. |
| Wang W B, Yang C Y, Tang D S, Li D H, Liu Y D, Hu C X. 2007. Effects of sand burial on biomass, chlorophyll fluorescence and extracellular polysaccharides of manmade cyanobacterial crusts under experimental conditions. Sci. China C Life Sci., 50 (4): 530-534. |
| Wu C D, Xu X J, Liang J L, Wang Q, Dong Q, Liang W L. 2011. Enhanced coagulation for treating slightly polluted algae-containing surface water combining polyaluminum chloride (PAC) with diatomite. Desalination, 279 (1-3): 140-145. |
| Yamamoto T, Inokuchi Y, Sugiyama T. 2004. Biogeochemical cycles during the species succession from Skeletonema costatum to Alexandrium tamarense in northern Hiroshima Bay. J. Marine Syst., 52 (1-4): 15-32. |
| Yu Z M, Ma X N, Xie Y. 1995. Study of main nutrients adsorption on clays in seawater. Oceanologia et Limnologia Sinica, 26 (2): 208-214. (in Chinese with English abstract) |
| Yu Z M, Sengco M R, Anderson D M. 2004. Flocculation and removal of the brown tide organism, Aureococcus anophagefferens (Chrysophyceae), using clays. J. Appl. Phycol., 16 (2): 101-110. |
| Yu Z M, Sun X X, Song X X, Zhang B. 1999. Clay surface modification and its coagulation of red tide organisms. Chinese Sci. Bull., 44 (7): 617-620. |
| Zarchi I, Friedler E, Rebhun M. 2013. Polyaluminium chloride as an alternative to alum for the direct filtration of drinking water. Environ. Technol., 34 (9): 1 199-1 209. |
| Zhu M Y, Xu Z J, Li R X, Wang Z L, Shi X Y. 2009. Interspecies competition for nutrients between Prorocentrum donghaiense Lu and Skeletonema costatum (Grev.) Cleve in mesocosm experiments. Acta Oceanol. Sin., 28 (1): 72- 82. |
 2015, Vol. 33
2015, Vol. 33


