Institute of Oceanology, Chinese Academy of Sciences
Article Information
- HE Dong(何冬), LIU Jiao(刘娇), HAO Qiang(郝锵), RAN Lihua(冉莉华), ZHOU Bin(周斌), TANG Xuexi(唐学玺)
- Interspecific competition and allelopathic interaction between Karenia mikimotoi and Dunaliella salina in laboratory culture
- Chinese Journal of Oceanology and Limnology, 34(2): 301-313
- http://dx.doi.org/10.1007/s00343-016-4320-1
Article History
- Received Nov. 17, 2014
- accepted in principle Jul. 26, 2016
2 State Key Laboratory of Satellite Ocean Environmen Dynamics, Second Institute of Oceanography, State Oceanic Administration(SOA), Hangzhou 310012, China
Allelopathy is a widespread ecological/ physiological phenomenon, which is considered an effect of chemicals/toxic secondary metabolites that are produced or released from algae species or plants (Granéli et al., 2008; Corcoran et al., 2014). The primary effects include influences on other organisms in the vicinity or associated with the algae or plants, even affecting themselves (Dakshini, 1994). Rice (1984) reported that the releasing process might be active or inactive, and the subsequent influence could come in multiple forms, such as direct or indirect, harmful or beneficial. It is very important to the composition of species, community structure, biomass and growth dynamics, especially in algal bloom (Fistarol et al., 2003, 2004; Yamasaki et al., 2009).
In natural aquatic ecosystems, allelopathy is often not the only, or leading, method of interspecies interactions. A shadowed effect or competition for space and nutrients are much more common, indicating that lighting and nutrients are extremely necessary. In many cases, all types of interactions occur together; therefore, other processes may hinderthe ecology advantages gained through allelopathy (Sukenik et al., 2002). As a result, it is highly desirable to differentiate allelopathy from resource competition and investigate its extent in a controlled system.
In the previous study, we focused on allelopathic interactions between different species and the context of toxin production; however, the influences of both biotic and abiotic factors and the ecological relevancy of allelopathy should be considered. Resource competition, predator-prey interactions and other biotic-associated factors may result in allelochemicals acting as mediators of species-specific biological interactions (Tillmann et al., 2007). Beyond that, allelochemical properties always change in the presence of nutrient limitation as well as due to multiple stresses (Reigosa et al., 1999; Fistarol et al., 2003; Granéli and Johansson, 2003; Mulderij et al., 2005). This illustrates that all of these factors will have a combined impact on the allelopathic effect.
Approximately 40 harmful phytoplankton species have been described to exhibit allelopathy toward other algae (Granéli et al., 2008), including K arenia mikimotoi, which is one of the most notorious red-tide dinoflagellates associated with killing marine fauna (Gentien et al., 2007; Brand et al., 2012). Some reports have verified its allelopathic action and interspecies competition using a variety of approaches (Uchida et al., 1999; Ji et al., 2011; Dang et al., 2015). There have been several devastating blooms of K . mikimotoi reported worldwide, especially in Scotland (Davidson et al., 2009), Ireland (Silke et al., 2005), Hong Kong, China (Li et al., 2009; Wang and Wu, 2009) and Japan (Yang et al., 2000).
As the interactions of allelopathy could be one of the key factors promoting the dominance of marine harmful algal bloom, it is important to determine the role of allelopathy in the succession, biochemistry and mode of action of allelochemicals, factors affecting their production, evolutionary aspects of allelopathy, and potential use of allelochemicals in the biological control of HABs (Legrand et al., 2003).
2 MATERIAL AND METHOD 2.1 Algal species and experimental conditionsWe obtained the following two strains of algal species: K . mikimotoi and D . salina from the Ecology Lab, Marine Life College, Ocean University of China, Qingdao, China. Both strains were aseptically grown in f/2 medium. The seawater used in the experiment was autoclaved at 121℃ for 20 min after filtering with the 0.45 μm micro pore filtering film. The normal culture condition was 20±0.1℃ at a light intensity of 80 μmol/(m2·s) under a 12 h:12 h light/dark cycle in illuminating incubators. All cultures were shaken several times a day at a set time. The salinity of the seawater was 30.
All microalgae were cultured to the exponential phase before they were inoculated in the subsequent experiments. Monoculture, which was used for controls in the interspecific competition experiment and experimental group in the allelopathy experiment, was inoculated with one strain into Erlenmeyer flasks containing fresh f/2-enriched seawater, and the total experiment volume was 150 mL. The co-culture systems used as the experimental group in the interspecific competition experiment were inoculated with both two strains (1:1 size/density) in the 150 mL f/2-enriched seawater in the Erlenmeyer flasks. Measured by micrometer to measure cell size, the average cell length of K . mikimotoi is 20-28 μm, average width is 16-30 μm. The average cell length of D . salina is 18-28 μm, average width is 9.5-14 μm.
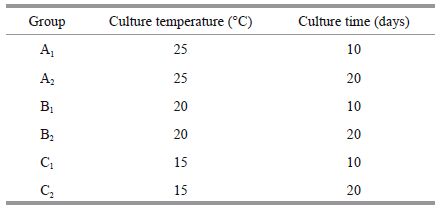
2.2 Interaction between K . mikimotoi and D . salina in bi-algal culture under different temperature conditionsWe inoculated K . mikimotoi (at a density of 5×104 cells/mL) and D . salina (at a density of 4×104 cells/mL) to keep their size/density ratio at a proportion of 1 to 1. The treatment temperatures were 15℃, 20℃, and 25℃. The light intensity was 80 μmol/(m2·s) under a 12 h:12 h light/dark cycle. As controls, both K . mikimotoi and D . salina were cultured individually. Three replicate flasks were used for each group. All cultures were shaken several times a day at a set time. Sample (0.5 mL) was used to monitor the growth of microalgae with a hemocytometer under an optical microscope every 24 h. The experiment lasted for 14 days. To obtain an accurate growth value, controls were cultured for 20 days until they reached the plateau phase.
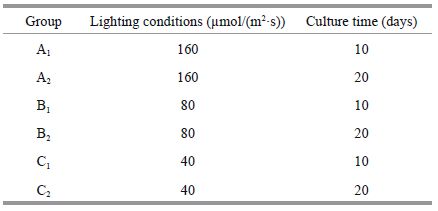
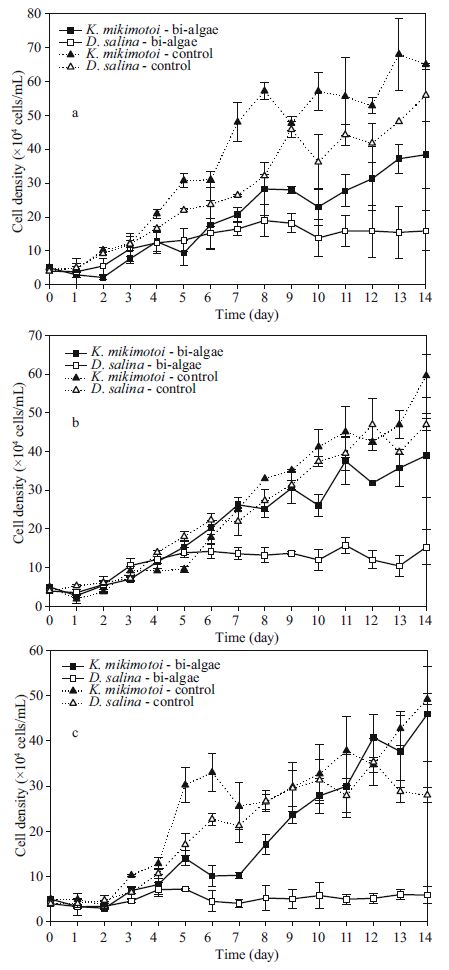
2.3 Interaction between K . mikimotoi and D . salina in bi-algal culture under different lighting conditionsThe treatment lighting conditions were 40, 80, and 160 μmol/(m2·s) with a 12-h photoperiod. The setting temperature was 20℃. All other conditions and procedures were the same as those described in Section 2.2.
The K . mikimotoi culture filtrates were prepared as follows: six groups were cultured to reach the exponential phase (10 days) and plateau period (20 days) at 15℃, 20℃, and 25℃ under 80 μmol/(m2·s) with a 12-h photoperiod. The group number and respective culture condition are described below.
The K . mikimotoi was inoculated at a density of 5×104 cells/mL. Then, the culture medium was filtered through 0.22 μm micro pore filtering film twice to remove microalgae cells. It was re-enriched with f/2 nutrient solution. D . salina was immediately inoculated to the filtrate medium at a density of 4×104 cells/mL. The total volume was maintained at 150 mL. Afterwards, three treatments were cultured in the incubator at 20℃, under 80 μmol/(m2·s), with a 12-h photoperiod. The monoculture D . salina was used as control, and we set three replicate flasks for each test. All cultures were shaken several times a day at a set time. Sample (0.5 mL) was used to monitor the growth of the microalgae with a hemocytometer under an optical microscope every 24 h. Additionally, we observed and photographed the medium and sediments.
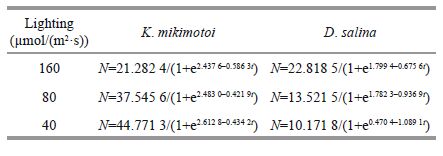
2.5 Allelopathy of K . mikimotoi and its effects on D . salina under different lighting conditionsThe K . mikimotoi culture filtrates were prepared as follows: six groups were cultured to reach the exponential phase (10 days) and plateau period (20 days) at 20℃ under 40, 80, and 160 μmol/(m2·s) with a 12-h photoperiod, respectively. The group number and respective culture conditions are shown below.
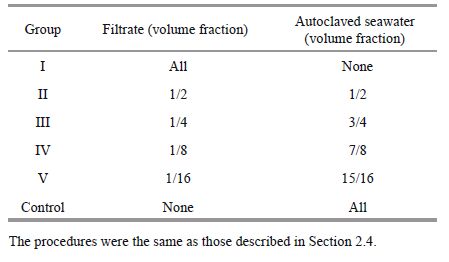
2.6 Allelopathy of K . mikimotoi and its effect on D . salina at different concentrations and biological activitiesThe K . mikimotoi culture filtrates were prepared as follows. The algae were cultured at 20℃ under 80 μmol/(m2·s) with a 12-h photoperiod to the exponential phase (10 days). Cell-free filtrates from each K . mikimotoi culture were diluted with filtered autoclaved seawater to give a dilution series of 100%, 50%, 25%, 12.5% and 6.25% in each group as below. As a control, filtered autoclaved seawater was used. Another group filter was autoclaved at 121℃ for 20 minutes. The group number and respective filtrate concentration were as summarized below.
2.7 Statistical analysisGrowth curve and characteristics were determined through logistic regression, and the Lotka-Volterra model was used to calculate the competitive parameters between two algae species. The relative growth rate was measured after inoculation following Guillard (1975) wherein
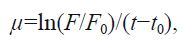
where F and F 0 represent the cell densities at time t and the initial time, t0 . ANOVA was used to test if the regression was significant (P<0.05). Analyses were conducted using Sigmaplot 10.0 and SPSS 17.0 for Windows.
|
| Figure 1 The growth of D . salina and K . mikimotoi in bi-algae cultured at (a) 25℃ with 80 μmol/(m2·s), (b) 20℃ with 80 μmol/(m2·s), (c) 15℃ with 80 μmol/(m2·s) |

|
After 14 days in culture, D . salina was significantly suppressed (P =0) by K . mikimotoi in all experimental groups compared to the controls for the growth curves in bi-algae culture and the estimated parameters of the Lotka-Volterra model.
The carrying capacity and turning point date of the D . salina population in bi-algae cultures had sharply decreased in different temperature levels (P =0) compared by controls. K . mikimotoi populations were also negatively impacted by interspecies competition. Similarly, K . mikimotoi in the 20℃ group was also promoted in the initial stage and arrived at the turning point much earlier (P =0.015<0.05), but there was not an obvious change in the growth of K . mikimotoi at 25℃ and 15℃. A distinct digression tendency of the carrying capacity was observed in the 25℃ (P =0.006<0.01) and 20℃ (P =0.004<0.01) groups but not in the 15℃ group (P =0.984>0.05).
After one-way ANOVA, it was determined that temperature factor had a significant influence on the carrying capacity (P =0.018<0.05) andT P (P =0) of K . mikimotoi in bi-algae culture, but such an effect was not observed in the intrinsic rate of increase (P =0.068>0.05). The same was true with D . salina (P =0.007<0.01, P =0.006<0.01, P =0.593>0.05). Furthermore, the two-way ANOVA results showed that there was a significant interaction between the temperature conditions and interspecific competition to K . mikimotoi (P =0.018<0.05) and D . salina (P =0).
3.2 Interaction between K . mikimotoi and D . salina in bi-algal culture at different lighting conditionsComparing the 3 lighting intensity conditions, the higher intensity had entirely different features. After 14 days in culture, K . mikimotoi had evident advantages in the numbers in the two lower lighting intensity experimental groups compared to the controls considering the growth curves of K . mikimotoi and D . salina in bi-algal culture and the estimated parameters of the Lotka-Volterra model. However, the two strains under 160 μmol/(m2·s) maintained a precarious state of equilibrium.
Moreover, both the carrying capacity and T P of the D . salina population in the experimental groups were sharply decreased (P =0) in the three lighting intensity levels compared to controls.

|
| Figure 2 The growth of D . salina and K . mikimotoi in bi-algae cultured at 20℃ with 160 (a), 80 (b), 40 (c) μmol/ (m2·s) |
A similar phenomenon was also shown in the K . mikimotoi populations. An initial positive influence was found, and there was a more obvious increase in the cell density as the lighting intensity decreased. However, the growth was still inhibited in the later growth period. The carrying capacity dropped sharply (all values, P<0.01) compared to the controls, and all experimental groups reached a turning point in advanced ( P 160=0.005<0.01, P 80=0.015<0.05, P 40=0.005<0.01).
|
The one-way ANOVA result illustrated that the lighting factor had a significant influence on the carrying capacity (P =0.003<0.01) of K . mikimotoi, but such an effect was not observed in the intrinsic rate of increase (P =0.158>0.05) andT P (P =0.103>0.05). On the other hand, a huge impact was found for main parameters of D . salina (P =0), demonstrating the existence of competition inhibition. Furthermore, the two-way ANOVA results showed that there was a significant interaction between the temperature conditions and interspecific competition to D . salina (P =0). However, their interactions did not appear to be significant in K . mikimotoi (P =0.103>0.05).
Based on the results above, we could speculate on the presence of allelopathy between two strains of microalgae. To investigate the effect further, we performed a serious of experiments as described below.
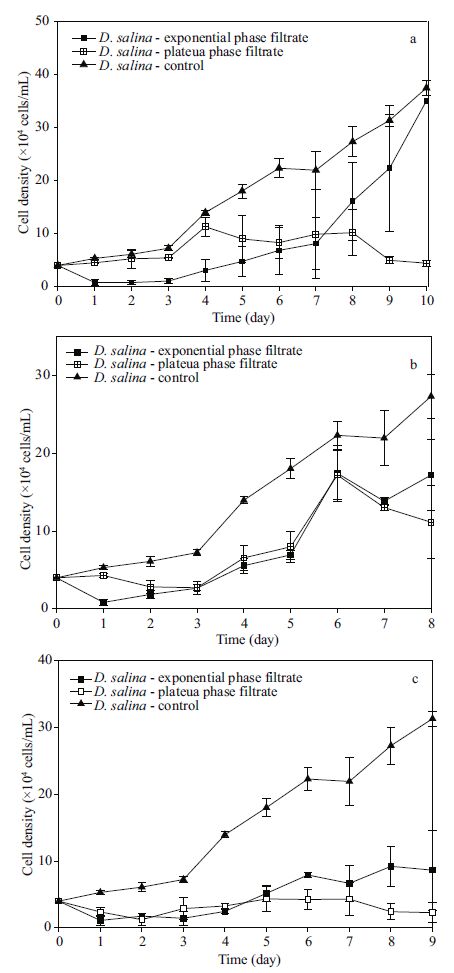
3.3 Allelopathy of K . mikimotoi cultured at different temperatures and its effects on D . salina 3.3.1 Different filtrate groups in the exponential growth period and their effectsThe filtrates of K . mikimotoi in the exponential growth period cultured at 3 different temperature conditions all exhibited significantly negative effects (P =0) on the growth of D . salina .
It was clearly recognized that most D . salina quickly died in the first 24 to 48 h, and this phenomenon was more apparent as the former culture temperate decreased. Compared to the controls, the relative growth rates of all experimental groups in the first three days were negative; they gradually increased to a peak and then remained stable. These findings could result in great disparity in the carrying capacity and turning point date between the experimental group and its control (P =0).

|
| Figure 3 The growth of D . salina in filtrates media of K .mikimotoi cultured at (a) 25℃ with 80 μmol/(m2·s) (b) 20℃ with 80 μmol/(m2·s) (c) 15℃ with 80 μmol/ (m2·s) |
In the one-way ANOVA analysis results, the temperature had a significant influence on the allelopathy of K . mikimotoi (P =0).
During the culture period, flocculent sediments were found in all filtrate media. They had a membranous shape at the second day and broke up into cotton-like matter later. At the end, microscope evidence showed that many D . salina cells with low activity were attached to the sediments.
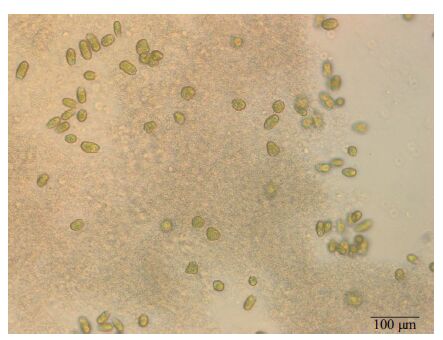
|
| Figure 4 D . salina and flocculent sediments in filtrates medium of K . mikimotoi |
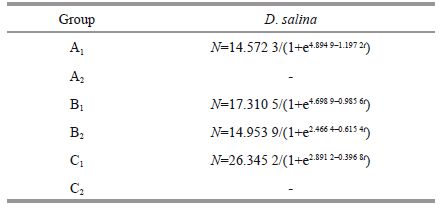
During the entire time of culture, the cell densities of the experiment group kept lower than controls, and the suppressing results were very significant (P =0). Due to the negative impact, the growth of A 2 andC2 could not be modeled by logistic regression, but a similar trend was determined by the curves. The cell densities dropped at the beginning and then increased for a short period. Finally, the A 2 andC2 groups became feeble and died. When the temperature was mild (20℃), the D . salina population always achieved positive growth, except on the second and third days. They quickly reached their density mass of 17.19× 10 4 cells/mL around day 6, which is not significantly lower than the control group (P =0.161>0.05); afterwards, they gradually declined. In this case, there were significant differences according to the comparison on the estimated parameters (all values, P<0.01) between B 2 and its control.
The same sediments were found and more microalgae cells were adhered, making some of the flocculent turn light green.
|
| Figure 5 The growth of D . salina in filtrates medium of K .mikimotoi cultured at 20℃ with 160 (a), 80 (b), 40 (c) μmol/(m2·s) |
The filtrate of K . mikimotoi, cultured at 3 different lighting conditions, had different effects on the growth of D . salina .
The filtrate of K . mikimotoi that grew to the exponential phase had a strong inhibitory effect on D . salina for a short time. In the first group, the cell density fell to 0.777 8×104 cells/mL (P =0) after one day in culture, and the specific growth rate was -1.64 cells/day. This condition lasted for 4 days; then, D . salina grew slowly. There was a large gap in cell density between the experimental and control groups until the ninth day. Afterwards, inhibition was not strong anymore. Growth of B 1 was the same as described in Section 3.3.1. The C 1 group was suppressed at the very beginning according to signal cell density decrease (P =0.041<0.05). Afterwards, the density differences were significant (all values P<0.01).
Based on the result of t-test, the carrying capacity of D . salina in groups A 1, B 1 and C 1 dropped by 29.81% (P =0.064>0.05), 83.47% (P =0), and 91.75% (P =0), respectively, but the intrinsic growth rates had grown noticeably larger (P =0, P =0.015<0.05, P =0.015<0.05), making all three strains arrive at the turning point much earlier (P =0.022<0.05, P =0, P =0).
The sediments and color changes were also the same as in Section 3.3.1.
3.4.2 Different filtrates in the plateau period and their effectsAt the beginning, growths of the A 2 and B 2 groups were promoted by the potential allelopathy, and each value was significantly different from controls (P =0.003<0.01, P =0.007<0.01). Conversely, the growth of C2 was identical to its control (P =0.595>0.05). In the latter period, three treatments strains entered recession with a reduction in the cell density. The gap between them and the control was clearly expanding and reached statistically significant differences (all values P<0.01). Because of the strong negative impact, the growth of C2 could not modeled by logistic regression, and its final cell density after 2 weeks in culture was only 0.638 9×104 cells/mL, which was so far below the control (P =0). Even in the high (P =0) and middle (P =0.004<0.01) lighting intensity groups, there was a large cell density gap.
|
| Figure 6 The growth of D . salina in the diluent filtrate medium of K . mikimotoi |
One-way ANOVA showed that the carrying capacity (P =0) andT P (P =0) were significantly affected by the lighting condition; however the intrinsic growth rate was only somewhat influenced (P =0.049<0.05).
The sediments and color changes were also the same as Section 3.3.2.
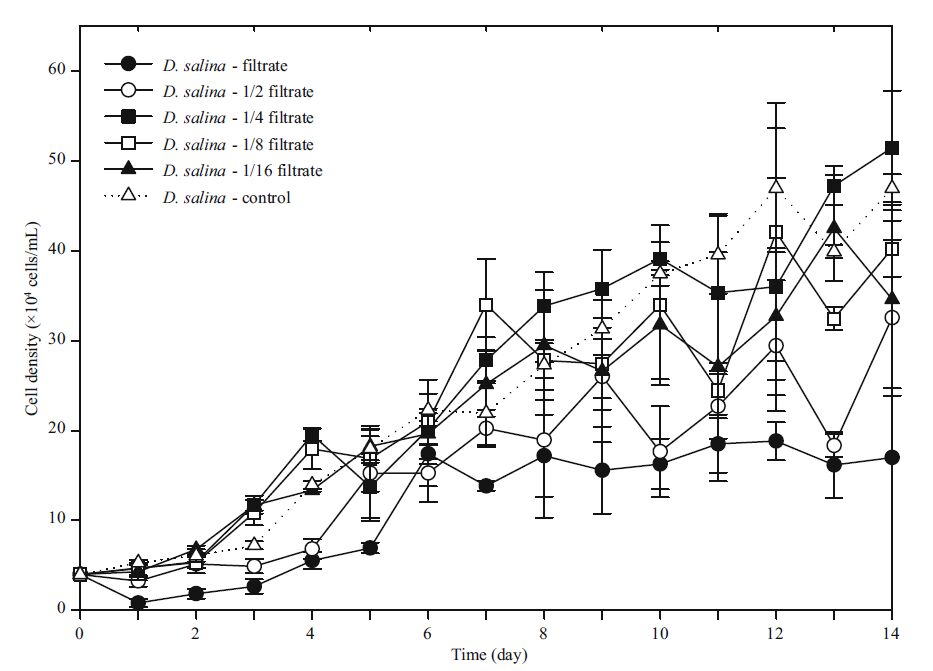
3.5 Allelopathy of the filtrates of K . mikimotoi with different concentrations or biological activities and their effects on D . salina 3.5.1 Filtrates with different concentrations and their effectsAfter comparing the cell density in each experimental group to control, there were some main findings as described below. The potential allelopathic substances group I could slightly promote its growth in the first 24 h, having a slightly higher density than control (P =0.259>0.05). Half-concentration filtrate was more effective in promoting the growth of group Ⅱ. The densities of control did not those in group Ⅱ until the 11 th day; afterwards, they lacked significant differences (all values P >0.05). In the course of further dilution, the same effect was observed in groups Ⅳ and Ⅴ, and there was a similar inhibition phenomenon. In the first 7 days, the cell density of two experimental groups remained ahead of their control before a negative impact appeared. On the 13 th day, control had overtaken group Ⅳ (P =0), whose cell density was only 32.44×104 cells/mL. For group Ⅴ, a significant strength in numbers (P =0.025<0.05) was found after 14 days in culture.
|
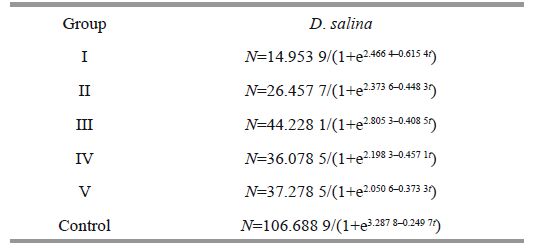
Consequently, logistic recession can be adopted to measure the growth character and gain the following regressions.
|
| Figure 7 The growth of D . salina in inactivated filtrates medium of K . mikimotoi |
As depicted with one-way ANOVA, the fold of dilution had an extremely significant impact (P =0) on the allelopathy to D . Salina . This was indicated by the results of multiple comparison that there was an obvious difference (P =0) between each experimental and control groups. Analyzed one-by-one, groups I and Ⅱ had very similar carrying capacities (P =0.51>0.05), indicating that the negative impact played a similar role in both the undiluted and halfdiluted mediums. However, the large-scale differences between groups Ⅲ to Ⅴ and the control group were very significant (P =0, P =0.001<0.05, P =0.001<0.05), as the inhibition effect weakened with decreasing concentration. Specifically, the carrying capacity of group Ⅲ was much higher than the others (P =0, P =0, P =0.016<0.05, P =0.025<0.05). The main differences (P =0.012<0.05) of the intrinsic growth rate were found between the low-concentration and highconcentration groups.
In addition, there was a significant difference (all values P =0) inT P among all experimental groups and compared with the control, respectively. In particular, group Ⅲ reached its turning point much earlier than others (P =0.004<0.01).
In the trial, the state of sediments and its color were monitored. With the increase in the dilute ratio and concentration of filtrate, sediments appeared to be less, especially in the last two groups. Finally, the color of microalgae suspension in group Ⅱ was yellowish-green, differing from the bright green of the control group. In contrast, group Ⅲ matched the color of the control group and grew very well. In addition, the lowest two groups appeared to be slightly yellowish-green in color and had observable microalgae filaments.
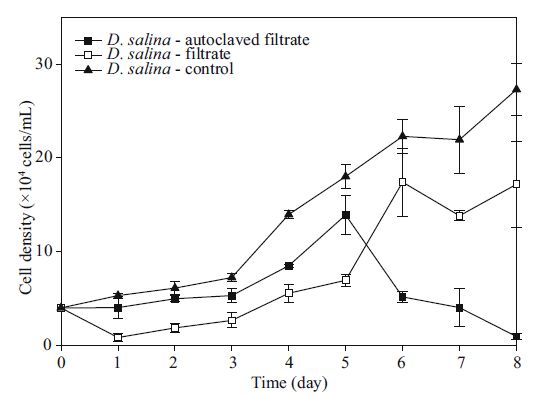
3.5.2 Filtrates with different biological activities and their effectsAt the beginning of the culture stage, D . salina in automatic filtrate medium had a short stasis with a specific growth rate of 0.006 9/d. Starting the next day, both cell density and specific growth rate increased continuously. The maximum appeared on the 5 th day, and the cell density and specific growth rate were 0.249 4/d and 13.916 7×104 cells/mL, respectively. Soon after, the population declined and fell to a very low level.
Changes in the parameters were compared between the automatic and normal cell-free filtrate groups. The results indicated that the short-term inhibition was more efficacious in non-automatic filtrate, and there was a larger cell density during the first 5 days (all values P<0.05). There was no significant difference between the maximum cell density of the automatic group and the carrying capacity of the non-automatic groups (P =0.07>0.05).
Furthermore, we compared the automatic group with the control cultured in f/2 medium. There was no significant difference between these groups in the first 3 days (all values P<0.05), and then the cell density of the automatic group was greater than the control in day 4 to 5 (P =0.035<0.05, P =0.036<0.05). Later, D . salina in automatic filtrate decreased sharply and was almost non-existent in the end, whereas the control entered an exponential growth stage and strongly expanded through the density gap (P =0).
There was an abundance of white flocculent sediments in the automatic filtrate on the second day, as has been described previously. The suspension in medium was turbid and appeared off-white compared with the control group. In the end, the liquid was nearly colorless and transparent, while some lowactivity D . salina were attached to the sediments or settled at the bottom of Erlenmeyer flasks.
4 DISCUSSIONIn our preliminary test, continuous-enrichment cultures were evaluated under the same conditions, and they had similar growth trends and competition results as in the normal groups. Therefore, nutrient limitation cannot possibly explain the growth inhibition in bi-algae culture, which is further proof of allelopathy.
The above results indicated that inhibition was caused by allelopathic interaction. Tough direct cellto- cell contact was not required to cause inhibition because strains in filtrate culture groups still had higher carrying capacities and longerT P dates than the respective D . salina strain in the bi-algae culture system with the same external conditions, suggesting physical contact might enhance the inhibition.
Although K . mikimotoi seems to tolerate a wide range of salinities, irradiances and temperatures, environmental factors still play a major role in both the growth rates and phytoplankton allelopathic effects. The advantage of K . mikimotoi in interspecific competition increased substantially when the temperature was increased and lighting density decreased. According to the negative impact of bialgae culture, D . salina would only grow rapidly over a surprisingly short time, during the very early stages, before reaching a plateau phase of low cell density.
Allelopathy of K . mikimotoi responding to various temperatures remains fairly uniform according to our experiment, and it enhances along with decreases in temperature to afford an advantage over potential competitors. Likewise, the temperature has a marked and species specific effect on toxin content of other microalgae species, such as Oscillatoria agardhii (Sivonen 1990), Pyrodinium bahamense var. compressum (Usup et al., 1994), Prorocentrum donghaiense and Scrippsiella trochoidea (Wang and Tang, 2008), Cylindrospermopsis raciborskii (Antunes et al., 2012) and Pseudo-nitzschia australis (Thorel et al., 2014).
Similar allelopathy based on irradiation is complex in our research because D . salina will increase dramatically under high light stress. Some observations provide additional evidence that the allelopathic potential of exudates from microalgae is based on the effect of irradiance, as mentioned by Mulderij (2005), Fistarol (2003) and Monti and Cecchin (2012). Related studies on macroalgae (Nan et al., 2008) demonstrate the potential impact of light.
In this case, further investivation is needed to determine how the light particularly affects allelopathic mechanisms, especially HAB ones. In addition to the environmental factors, the growth phase is a significant biotic reason for producing allelochemical compounds in spite of the initial cell density of donor species. The effect is straindependent, as determined by simultaneously evaluating other environmental elements. We observed clear distinctions between the exponential growth and plateau phases. Normally, exudates from K . mikimotoi in the exponential growth phase have a stronger short-term toxic effect and we speculated that the low temperature and high lighting intensity could accelerate the creation and secretion process of allelochemical compounds in K . mikimotoi in this growth phase. However, the allelopathic substances released by K . mikimotoi in the plateau phase could maintain stable biological activity for a longer time, which would be more significant at a lower temperature and lighting intensity condition.
Other studies support the findings related to the growth phase; however, the conclusion is likely to depend on the species. Suikkanen (2004) found that a culture filtrate of N . spumigena was more hepatotoxic in the stationary phase. Meanwhile Arzul (1999) compared the allelopathic properties in three toxic Alexandrium and reported that allelopathic activity was observed to be more toxic in the stationary phase than in the log phase. Furthermore, Lee (2000) and Tong (2011) also showed that the cell toxin content of Microcystis aeruginosa and Dinophysis acuminataat in various growth stages were different. Granéli and Flynn (2006) concludes by discriminating between the toxin expression events that are truly cell-cycle dependent. However, very few studies combine both environmental factors and growth phases together to estimate their integrated influences on allelopathic interaction.
A further study on the short time-scale allelopathic effect showed that the filtrate of K . mikimotoi was low-promoting and high-repressing for D . Salina . It was concluded that the 25%-concentration filtrate had the most effective promoting influence at the beginning period. In the long run, the inhibition effect was always present in the filtrate irrespective of its actual concentration. From 25% to 100%, as the concentration increased, the allelopathy was also stronger. However, in the lower range, there was no statistically significant difference. Some previous studies have focused on the different proportion of added filtrate of microalgae species, but the dose effect relationship of allelopathic substance varies from species to species. Li (2011) made up several Skeletonema costatum filtrate mediums with concentrations varying from 0% to 100%. She found out that the auto-allelopathic effect of S . costatum became stronger as the concentration increased. Yang (2001) observed that allelopathic chemicals in cellfree filtrate of Chattonella marina had no obvious inhibitive effect on S . costatum, and suppression only occurred in the high concentration filtrates. Compared with these filtrate concentration experiments, more studies have focused on the effects of different initial cell densities of alllelopathic algae (Uchida et al., 1999; Wang et al., 2006).
The results of 3.5.2 indicated that the short-term inhibition was more efficacious in the non-automatic filtrate, and automatic ones had a better function.
According to the result, we concluded that allelopathic substances released by K . mikimotoi were not completely destroyed by high temperature and pressures. The automatic filtrate also had a strong inhibition function.
According to the results above, changes in the toxin content are closely related to the disturbed physiology in general and there are different cellular activities and structures. The variety of different quantities or chemical compositions of substances released by K . mikimotoi might explain the different restraining levels, which was determined by previous culture conditions.
In addition, we obtained that allelopathic compounds released from K . mikimotoi, which seem highly water-soluble and can be rapidly degradable or long-term effective, as determined by the interspecific impact. Sometimes, they are not so damaging and may temporarily inhibit some functions in the target species’ ecophysilogy without causing death. This type of re-establishment is reported by Nan (2008) in his research on the allelopathic effects of Ulva lactuca on three red tide microalgae, which may be from volatizing, natural degradation and inactivation or decomposition by the microorganism that is attached or even by their constant coexistence (Kubanek et al., 2005). However, most often, the competing species will not instantaneously interact until they are mature and have advantageous against potential competitors (Tapaswi and Mukhopadhyay, 1999). This suggests that the allelopathy is an adaptation for gaining a competitive advantage (Granéli et al., 2008).
Few allelochemicals have been identified so far. The ichthyotoxicity of K . mikimotoi is due to the production of hemolysin (Tatum and Canobell, 2006), fatty acid (all-cis-octadecapentaenoic acid), glycoglycerolipids (1-acyl-3-digalactosylglyercol) (Yasumoto et al., 1990; Gentien et al., 2007) and superoxide (Marshall et al., 2005). Using a cytotoxicity assay, the Gymnocin A and B toxins have been suggested as a potential cause of mortality in marine vertebrates and invertebrates (Satake et al., 2002, 2005). Although the involvement of toxins in the phenomena is easily perceived, sophisticated quantitative analysis and structural confirmation are still very much required.
Finally, we suspect that intracellular bacteria might produce the sediments, and there is likely a complex interaction between bacteria and Karenia lethality. By comparisons of the microalgae suspension color and state of sediments at the end of experiments, we concluded that for a stronger inhibition effect, there were more sediments and a lighter color.
5 CONCLUSIONThe results of our study demonstrate that K. mikimotoi had detrimental allelopathic effects on D. salina, which did not require direct cell-to-cell contact. However, the outcome was somewhat affected by both abiotic and biotic factors. The allelopathy was positively correlated with temperature decrease, while the irradiance, growth phase, and concentration and bioactivity of allelochemicals had complex effects. Therefore, the allelochemicals might be a mixture with light/temperature-sensitive components, and the extracellular metabolites may contribute to the formation and/or lasting of harmful algae blooms.
| Antunes J T, Leão P N, Vasconcelos V M, 2012. Influence of biotic and abiotic factors on the allelopathic activity of the cyanobacterium Cylindrospermopsis raciborskii strain LEGE 99043. Microbial Ecology, 64 (3) : 584 –592. |
| Arzul G, Seguel M, Guzman L, Erard-Le Denn E, 1999. Comparison of allelopathic properties in three toxic Alexandrium species. Journal of Experimental Marine Biology and Ecology, 232 (2) : 285 –295. |
| Brand L E, Campbell L, Bresnan E, 2012. Karenia:the biology and ecology of a toxic genus. Harmful Algae, 14 : 156 –178. |
| Corcoran A A, Richardson B, Flewelling L J, 2014. Effects of nutrient-limiting supply ratios on toxin content of Karenia brevis grown in continuous culture. Harmful Algae, 39 : 334 –341. |
| Dakshini K M M, 1994. Algal allelopathy. The Botanical Review, 60 (2) : 182 –196. |
| Dang L X, Li Y, Liu F, et al, 2015. Chemical response of the toxic dinoflagellate Karenia mikimotoi against grazing by three species of Zooplankton. Journal of Eukaryotic Microbiology, 62 (4) : 470 –480. |
| Davidson K, Miller P, Wilding T A, Shutler J, Bresnan E, Kennington K Swan S, 2009. A large and prolonged bloom of Karenia mikimotoi in Scottish waters in 2006. Harmful Algae, 8 (2) : 349 –361. |
| Fistarol G O, Legrand C, Granéli E, 2003. Allelopathic effect of Prymnesium parvum on a natural plankton community. Marine Ecology:Progress Series, 255 : 115 –125. |
| Fistarol G O, Legrand C, Selander E, Hummert C, Stolte W, Granéli E.:effect on a natural plankton community and on algal monocultures, 2004. Allelopathy in Alexandrium spp. Aquatic Microbial Ecology, 35 (1) : 45 –56. |
| Gentien P, Lunven M, Lazure P, Youenou A, Crassous M P, 2007. Motility and autotoxicity in Karenia mikimotoi(Dinophyceae). Philosophical Transactions of the Royal Society Lond B:Biological Sciences, 362 (1487) : 1 937 –1 946. |
| Granéli E, Flynn K. 2006. Chemical and physical factors influencing toxin content. In:Granéli E, Turner J eds.Ecology of Harmful Algae. Springer, Berlin Heidelberg.p.229-241. |
| Granéli E, Johansson N, 2003. Increase in the production of allelopathic substances by Prymnesium parvum cells grown under N-or P-deficient conditions. Harmful Algae, 2 (2) : 135 –145. |
| Granéli E, Weberg M, Salomon P S, 2008. Harmful algal blooms of allelopathic microalgal species:the role of eutrophication. Harmful Algae, 8 (1) : 94 –102. |
| Guillard R R. 1975. Culture of phytoplankton for feeding marine invertebrates. I n:Smith W L, Chanley M H eds.Culture of Marine Invertebrate Animals. Springer, US.p.29-60. |
| Ji X Q, Han X T, Zheng L, et al, 2011. Allelopathic interactions between Prorocentrum micans and Skeletonema costatum or Karenia mikimotoi in laboratory cultures. Chinese Journal of Oceanology and Limnology, 29 (4) : 840 –848. |
| Kubanek J, Hicks M K, Naar J, Villareal T A, 2005. Does the red tide dinoflagellate Karenia brevis use allelopathy to outcompete other phytoplankton?. Limnology and Oceanography, 50 (3) : 883 –895. |
| Lee S J, Jang M H, Kim H S, Yoon B D, Oh H M, 2000. Variation of microcystin content of Microcystis aeruginosa relative to medium N:P ratio and growth stage. Journal of Applied Microbiology, 89 (2) : 323 –329. |
| Legrand C, Rengefors K, Fistarol G O, Granéli E, 2003. Allelopathy in phytoplankton-biochemical, ecological and evolutionary aspects. Phycologi a, 42 (4) : 406 –419. |
| Li H, 2011. The effect of allelopathy on the species competition between Skeletonema costatum and Prorocentrum donghaiiense. Ocean University of China, Qingdao, China . |
| Li J, Glibert P M, Zhou M, et al, 2009. Relationships between nitrogen and phosphorus forms and ratios and the development of dinoflagellate blooms in the East China Sea. Marine Ecology Progress Series, 383 : 11 –26. |
| Marshall J A, Ross T, Pyecroft S, Hallegraeff G, 2005. Superoxide production by marine microalgae. Marine Biology, 147 (2) : 541 –549. |
| Monti M, Cecchin E, 2012. Comparative growth of three strains of Ostreopsis ovata at different light intensities with focus on inter-specific allelopathic interactions. Cryptogamie Algologie, 33 (2) : 113 –119. |
| Mulderij G, Mooij W M, Smolders A J P, van Donk E, 2005. Allelopathic inhibition of phytoplankton by exudates from Stratiotes aloides. Aquatic Botany, 82 (4) : 284 –296. |
| Nan C R, Zhang H Z, Lin S Z, Zhao G Q, Liu X Y, 2008. Allelopathic effects of Ulva lactuca on selected species of harmful bloom-forming microalgae in laboratory cultures. Aquatic Botany, 89 (1) : 9 –15. |
| Reigosa M J, Sánchez-Moreiras A, González L, 1999. Ecophysiological approach in allelopathy. Critical Reviews in Plant Sciences, 18 (5) : 577 –608. |
| Rice E L, 1984. Allelopathy. 2nd edn. Academic Press, New York : 422p . |
| Satake M, Shoji M, Oshima Y, Naoki H, Fujita T, Yasumoto T, 2002. Gymnocin-A, a cytotoxic polyether from the notorious red tide dinoflagellate, Gymnodinium mikimotoi. Tetrahedron letters, 43 (33) : 5 . |
| Satake M, Tanaka Y, Ishikura Y, Oshima Y, Naoki H, Yasumoto T, 2005. Gymnocin-B with the largest contiguous polyether rings from the red tide dinoflagellate, Karenia(formerly Gymnodinium) mikimotoi. Tetrahedron Letters, 46 (20) : 3 . |
| Silke J, O'Beirn F, Cronin M. 2005. Karenia mikimotoi:an exceptional dinoflagellate bloom in western Irish waters, summer 2005. Marine Environment and Health Series, No. 21, http://hdl.handle.net/10793/240. |
| Sivonen K, 1990. Effects of light, temperature, nitrate, orthophosphate, and bacteria on growth of and hepatotoxin production by Oscillatoria agardhii strains. Applied and Environmental Microbiology, 56 (9) : 2 658 –2 666. |
| Suikkanen S, Fistarol G O, Grané li E, 2004. Allelopathic effects of the Baltic cyanobacteria Nodularia spumdigena, Aphanizomenon flos-aquae and Anabaena lemmermannii on algal monocultures. Journal of Experimental Marine Biology and Ecology, 308 (1) : 85 –101. |
| Sukenik A, Eshkol R, Livne A, Hadas O, Rom M, Tchernov D, Vardi A, Kaplan A, 2002. Inhibition of growth and photosynthesis of the dinoflagellate Peridinium gatunense by Microcystis sp. (cyanobacteria):a novel allelopathic mechanism. Limnology and Oceanography, 47 (6) : 1 656 –1 663. |
| Tapaswi P K, Mukhopadhyay A, 1999. Effects of environmental fluctuation on plankton allelopathy. Journal of Mathematical Biology, 39 (1) : 39 –58. |
| Tatum N, Canobell L, 2006. A modified assay to determine hemolytic toxin variability among Karenia clones isolated from the Gulf of Mexico. Harmful Algae, 5 (5) : 592 –598. |
| Thorel M, Fauchot J, Morelle J, Raimbault V, Le Roy B, Miossec C, Kientz-Bouchart V, Claquin P, 2014. Interactive effects of irradiance and temperature on growth and domoic acid production of the toxic diatom Pseudo-nitzschia australis(Bacillariophyceae). Harmful Algae, 39 : 232 –241. |
| Tillmann U, John U, Cembella A, 2007. On the allelochemical potency of the marine dinoflagellate Alexandrium ostenfeldii against heterotrophic and autotrophic protists. Journal of Plankton Research, 29 (6) : 527 –543. |
| Tong M M, Kulis D M, Fux E, Smith J L, Hess P, Zhou Q X, Anderson D M, 2011. The effects of growth phase and light intensity on toxin production by Dinophysis acuminata from the northeastern United States. Harmful Algae, 10 (3) : 254 –264. |
| Uchida T, Toda S, Matsuyama Y, et al, 1999. Interactions between the red tide dinoflagellates Heterocapsa circularisquama and Gymnodinium mikimotoi in laboratory culture. Journal of Experimental Marine Biology and Ecology, 241 (2) : 285 –299. |
| Usup G, Kulis D M, Anderson D M, 1994. Growth and toxin production of the toxic dinoflagellate Pyrodinium bahamense var. compressum in laboratory cultures.Natural Toxins, 2 (5) : 254 –262. |
| Wang J H, Wu J Y, 2009. Occurrence and potential risks of harmful algal blooms in the East China Sea. Science of the Total Environment, 407 (13) : 4 012 –4 021. |
| Wang Y, Tang X X, 2008. Interactions between Prorocentrum donghaiense Lu and Scrippsiella trochoidea(Stein) Loeblich Ⅲ under laboratory culture. Harmful Algae, 7 (1) : 65 –75. |
| Wang Y, Yu Z M, Song X X, et al, 2006. Interactions between the bloom-forming dinoflagellates Prorocentrum donghaiense and Alexandrium tamarense in laboratory cultures. Journal of Sea Research, 56 (1) : 17 –26. |
| Yamasaki Y, Shikata T, Nukata A, Ichiki S, Nagasoe S, Matsubara T, Shimasaki Y, Nakao M, Yamaguchi K, Oshima Y, Oda T, Jenkinson I R, Asakawa M, Honjo T, 2009. Extracellular polysaccharide-protein complexes of a harmful algal mediate the allelopathic control it exerts within the phytoplankton community. The ISME Journal, 3 (7) : 808 –817. |
| Yang C Y, Zhao N N, Xia C H, Liu S J, Zhou S W, 2001. Effects of Chattonella marina cell-free filtrate on bloom microalgae and co-culture of it and microalgae. Marine Environmental Science, 30 (6) : 798 –803. |
| Yang Z B, Takayama H, Matsuoka K, Hodgkiss I J, 2000. Karenia digitata sp. nov.(Gymnodiniales, Dinophyceae), a new harmful algal bloom species from the coastal waters of west Japan and Hong Kong. Phycologia, 39 (6) : 463 –470. |
| Yasumoto T, Underdal B, Aune T, Hormazabal V, Skulberg O M, Oshima Y, 1990. Screening for hemolytic and ichthyotoxic components of Chrysochromulina polylepis and Gyrodinium aureolum from Norwegian coastal waters. I n:Granéli E, Sundström B, Edler L et al eds.Toxic Marine Phytoplankton; Fourth International Conference. Elsevier Science Publishing Co., Inc, New York, USA; Amsterdam, Netherlands : 436 –440. |
 2016, Vol. 34
2016, Vol. 34