Institute of Oceanology, Chinese Academy of Sciences
Article Information
- WANG Renjun(王仁君), TANG Xuexi(唐学玺)
- Allelopathic effects of macroalga Corallina pilulifera on the red-tide forming alga Heterosigma akashiwo under laboratory conditions
- Chinese Journal of Oceanology and Limnology, 34(2): 314-321
- http://dx.doi.org/10.1007/s00343-015-4336-y
Article History
- Received Dec. 10, 2014
- accepted in principle Apr. 26, 2016
2 College of Marine Life, Ocean University of China, Qingdao 266003, China
Research on allopathic interactions between aquatic organisms is emerging as an interesting topic in modern chemical ecology. Allelopathic interactions between aquatic organisms can alter the structure and succession of aquatic ecosystems (Körner and Nicklish, 2002; van Donk and van de Bund, 2002).
Accordingly, the allelopathic effect of specific hydrophytes has been used for the biological control of harmful algae and waterweeds in a number of studies (Jin and Dong, 2003; Nelson et al., 2003; Wang et al., 2007, 2008, 2012a, 2012b; Zhao et al., 2009, Oh et al., 2010; Lu et al., 2011). The composition and dynamics of aquatic ecosystems are controlled by a variety of biological and non-biological factors, including physical parameters, nutrient conditions, resource competition, selective predation, and allelopathic interactions (van Donk and van de Bund, 2002; Gross, 2003). Under natural conditions, environmental factors in aquatic habitats (e.g., temperature, salinity, light, pH, and nutrients) are dynamic and constantly changing, which exerts a strong influence on the growth and physiological activities of aquatic organisms (Taylor et al., 2001), further affecting their allelopathic interactions (Gross, 2003). Dynamic changes in environmental factors can reduce or enhance allelopathic interactions between aquatic organisms (Keating, 1977). However, few studies have examined said interactions (Gross, 2003), and previous works have mainly investigated the role that environmental factors play in the occurrence of harmful marine algal blooms (Taylor et al., 2001; Yan et al., 2002). Considering the lack of research linking changes in environmental factors to allelopathic interactions between aquatic organisms, it is necessary to study the influence of environmental factors on the allelopathic effect of macroalgae on red-tide microalgae.
Heterosigma akashiwo is a major algal species that can cause harmful red tides. It has caused red tides in many countries in both northern and southern hemispheres, resulting in fish death as well as substantial economic losses (Chang et al., 1990). It is known that Corallina pilulifera can inhibit the growth of H . akashiwo (Wang et al., 2008). This study therefore examines the potential influence of changes in environmental factors (temperature, salinity, light, and pH) on the allelopathic effects of C . pilulifera on H . akashiwo . This study provides scientific evidence for the enhanced allelopathic effect of macroalgae on harmful red-tide microalgae through changes in environmental factors.
2 MATERIAL AND METHOD 2.1 MaterialThe Laboratory of Applied Microalgae Biology, Ocean University of China provided the H . akashiwo .
Corallina pilulifera was collected from coastal waters at Taipingjiao in Qingdao, China (36°07′N/120°33′E). After removal of miscellaneous algae, the fresh macroalgal samples were rinsed with distilled water to remove sediment and other impurities. The algal samples were then surface-sterilized using mixed antibiotics, rinsed 3-4 times with sterile seawater, and cultured in sterile f/2 medium (Guillard, 1975) under the following conditions: 20℃, light intensity of 70 μmol/(m2∙s), and bright-to-darkness ratio of 12 h:12 h. Natural seawater was collected from Luxun Park in Qingdao and filtered through degreasing cotton and 300 μm bolting cloth. The seawater was boiled and cooled to room temperature, then filtered through a glass cellulose membrane (0.22 μm poresize) to remove particulates. The seawater was adjusted to a pH of 8.5 and a salinity of 30 before use.
2.2 Experimental designGrowth experiments were carried out in a coculture system of macroalga and harmful red-tide microalga. While in the exponential growth phase, both H . akashiwo and C . pilulifera were simultaneously inoculated into 100 mL flasks containing 40 mL of f/2 medium. The initial density of H . akashiwo was adjusted to 8×104 cells/mL. The fresh tissue content was 0.05 g. Fresh f/2 medium was supplied daily to the co-culture system to prevent potential nutrient limitation. The control group was prepared with an individual culture of H . akashiwo under the same conditions.
The experimental treatments involved four environmental factors: temperature, salinity, light, and pH. Two-factor experiments were designed between each of the two different factors: temperaturesalinity, temperature-light, temperature-pH, light-pH, salinity-light, and salinity-pH. The effects of these six combination treatments were examined to test the potential influence of four environmental factors on the allelopathic effect of C . pilulifera on H . akashiwo, and the possible interactions between each of the factors. Each environmental factor was set at four treatment levels: temperature, 15, 20, 25, and 30℃; salinity, 10, 20, 30, and 40; light intensity, 20, 100, 200 and 400 μmol/(m2∙s); and pH, 5.5, 7, 8.5, and 10. Each treatment included three replicates. The culture broth pH was adjusted using 1 mol/L HCl and 1 mol/L NaOH. The algae can change the medium pH through photosynthesis and respiration during growth, and thus HCl and NaOH solutions were used to adjust the medium pH to the default level each day. Excluding the two experimental environmental factors, all other conditions were controlled in each treatment. The growth experiments were carried out for eight days.
Each treatment involved three parallel groups. The flasks were agitated twice at regular times each day to prevent algal growth from adhering to the flask walls. An 1-mL sample was taken from each flask every day and fixed with Lugol’s reagent. Cell counts of H . akashiwo from these samples were conducted using a hemocytometer counting chamber under an Olympus optical microscope.
2.3 Data processing and statistical analysesThe allelopathic effect of C . pilulifera on H . akashiwo was indicated by the growth inhibition rate of H . akashiwo : I=100(H c − H t)/ H c , where H c and H t denote the cell density of H . akashiwo in the control group (individual culture of H . akashiwo) and treatment groups (co-culture of C . pilulifera), respectively, at the end of the experiment.
Two-way analysis of variance (ANOVA) was performed to evaluate the influence of the four environmental factors on the allelopathic effect of C . pilulifera on H . akashiwo, as well as the interactions between each of two environmental factors.
3 RESULTThe growth of H . akashiwo was significantly affected by temperature, salinity, light intensity and pH (P<0.001) (Fig. 1). In addition, there were significant interactions among both temperature and salinity, and light intensity and pH (P<0.001). The effects of temperature on H . akashiwo growth were similar among the two-factor combined treatments, when the temperature was below 25℃, the growth rate of the algae increased as temperature increased. Growth was significantly reduced at a temperature of 10℃ or 30℃, with 25℃ found to be the optimum temperature for H . akashiwo growth (Fig. 1). In the combined treatments of salinity with temperature or pH, the average growth rate of the algae increased as salinity increased until salinity reached 30. The algae grew significantly slower when the salinity was 10 and 40, with a salinity of 30 optimal for algal growth. When examining salinity-light intensity combinations, it was found that the algae grew fastest at a salinity of 40. When pH was below 8.5, the average growth rate increased with increasing pH. The algae grew significantly slower at a pH of 5.5 or 10, with an optimal pH for H . akashiwo growth of 8.5. When light intensity was below 100 μmol/(m2∙s), the average growth of the algae increased with increasing light intensity. The algae grew significantly slower at a light intensity of 20 μmol/(m2∙s) or 400 μmol/(m2∙s), with a light intensity between 100 and 200 μmol/(m2∙s) optimal for algal growth (Fig. 1).
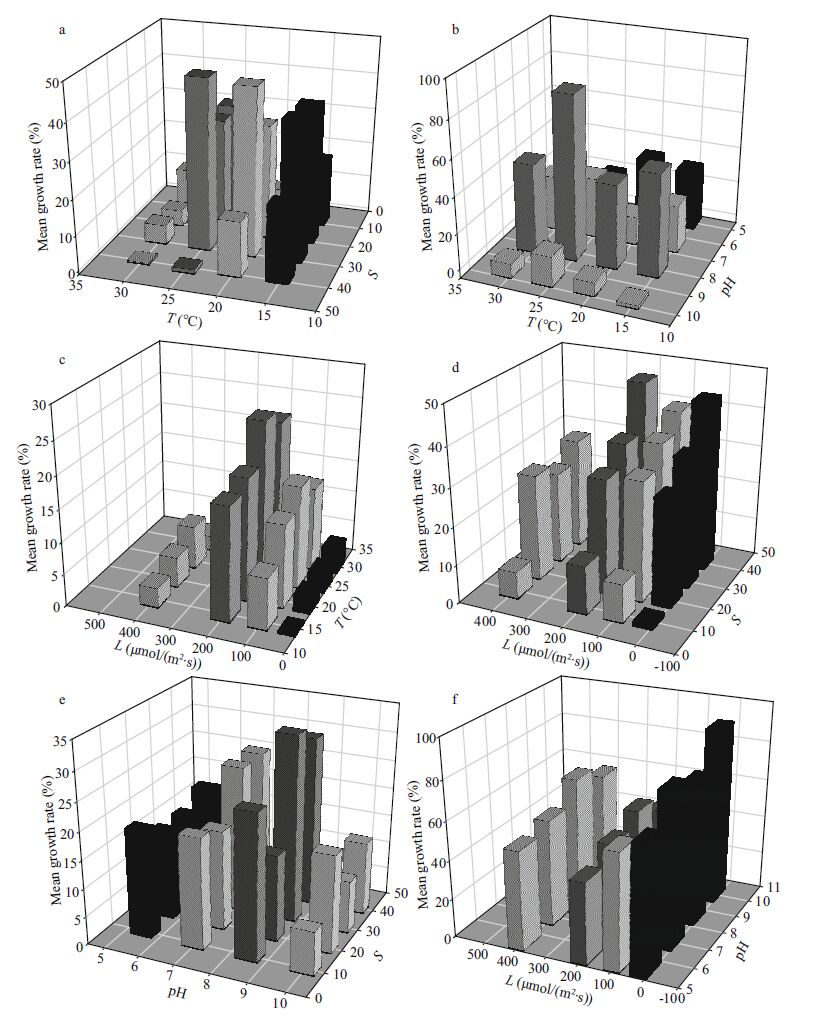
|
| Figure 1 Mean growth rates of H . akashiwo under the treatments of two-factor combinations of different temperatures (T), salinity (S), light (L) and pH |
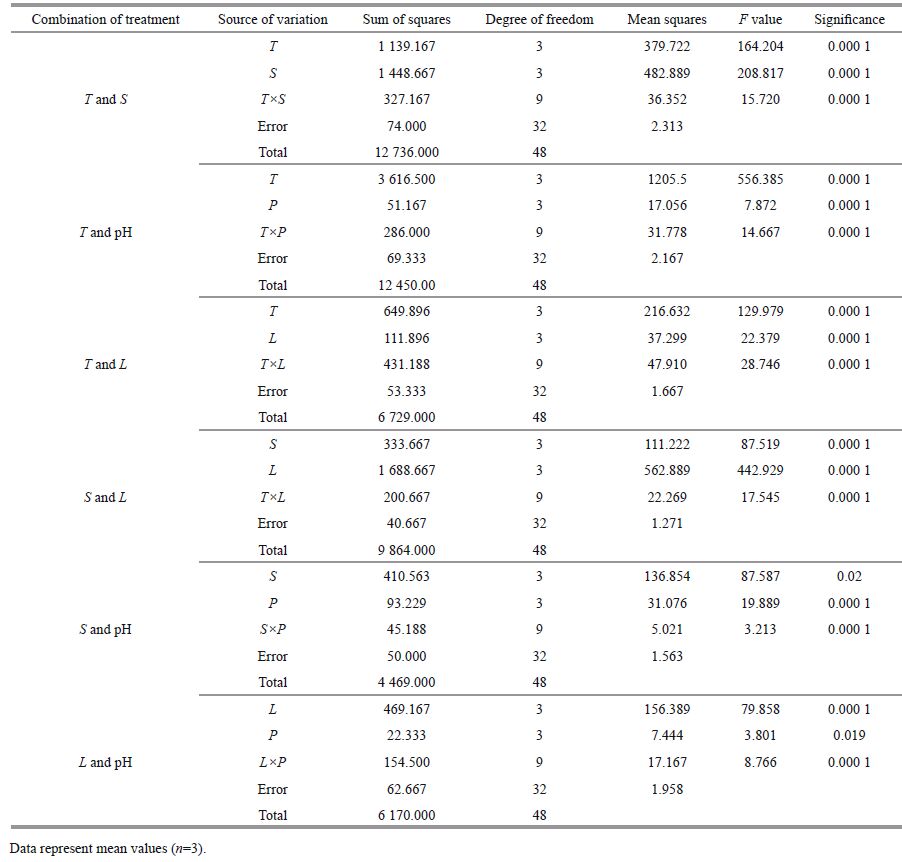
Temperature and salinity both has significant influences on the growth inhibition rate of H . akashiwo by C . pilulifera (P<0.001), with significant interactions between these two environmental factors (P<0.001) (Table 1). The growth inhibition effect of C . pilulifera on H . akashiwo was enhanced with increased salinity and decreased temperature. The greatest growth inhibition of H . akashiwo was observed at a temperature of 15℃ with a salinity of 40 (>95%, Fig. 2a).
|
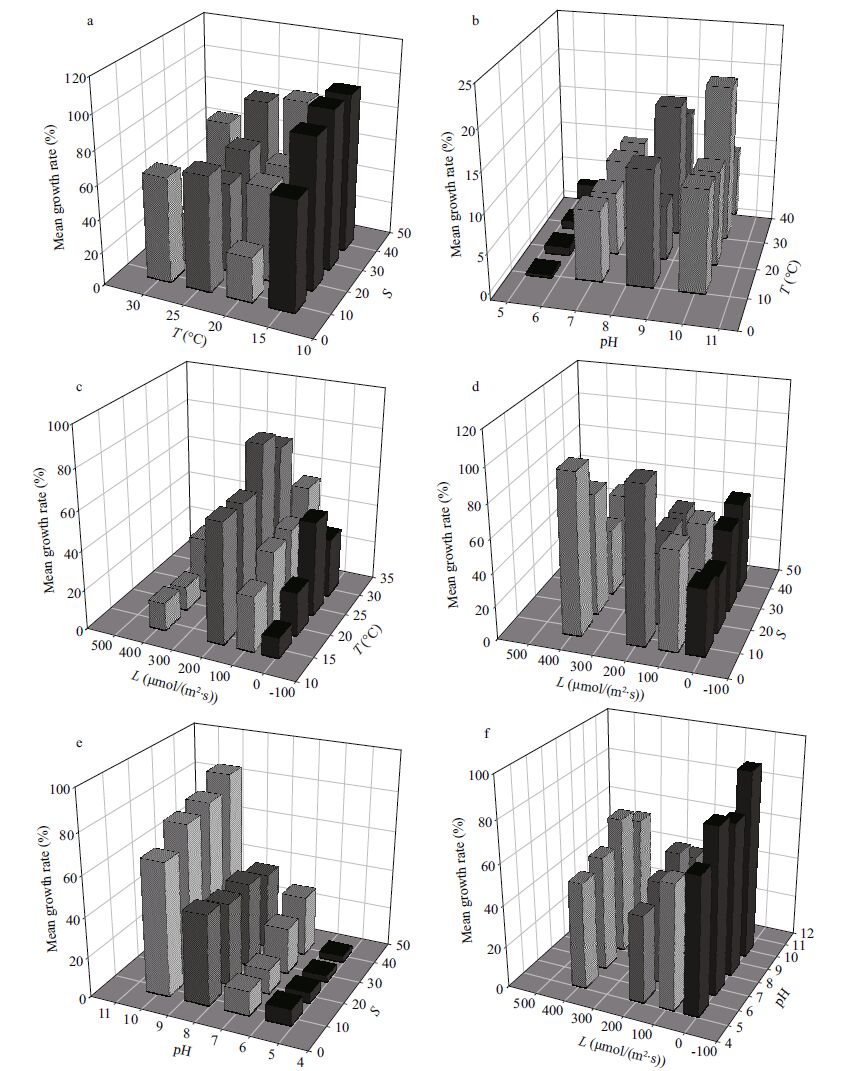
|
| Figure 2 Growth inhibition rates of H . akashiwo by C . pilulifera under the of two-factor combination treatments of temperature (T), salinity (S), light (L) and pH Data represent mean values (n =3). |
Both temperature and pH significantly influenced the growth inhibition rate of H . akashiwo by C . pilulifera (P<0.001), with significant interactions between these two environmental factors (P<0.001) (Table 1). The growth inhibition rate of H . akashiwo increased with increasing pH and peaked at a temperature of 25℃ and a pH of 10 (>90%). At pH 5.5, the growth inhibition rate of H . akashiwo was below zero, suggesting a growth-promoting effect (Fig. 2b).
3.3 Influence of the temperature-light treatment combination on the allelopathic effect of C . pilulifera on H . akashiwoTemperature and light intensity both significantly influenced the growth inhibition rate of H . akashiwo by C . pilulifera (P<0.001), with significant interactions between these two environmental factors (P<0.001) (Table 1). The growth inhibition rate of H . akashiwo increased with temperature and light intensity when they were below 25℃ and 200 μmol/(m2∙s), respectively. The largest growth inhibition rate of H . akashiwo was observed at a temperature of 25℃ with medium- to high-intensity light at 200-400 μmol/(m2∙s) (>90%, Fig. 2c).
3.4 Influence of the salinity-light treatment combination on the allelopathic effect of C . pilulifera on H . akashiwoLight intensity and salinity both significantly influenced the growth inhibition rate of H . akashiwo by C . pilulifera (P<0.001), with significant interactions between these two environmental factors (P<0.001) (Table 1). The growth inhibition rate of H . akashiwo increased with increasing light intensity. The highest growth inhibition rate of H . akashiwo was observed under high-intensity light at 400 μmol/ (m2∙s) and a salinity of 10 (>90%, Fig. 2d).
3.5 Influence of the salinity-pH treatment combination on the allelopathic effect of C . pilulifera on H . akashiwoIn the salinity-pH treatment combination, pH significantly affected the growth inhibition rate of H . akashiwo by C . pilulifera, but there was no significant effect of salinity (P<0.001). There were significant interactions between salinity and pH (P<0.001) (Table 1). The growth inhibition rate of H . akashiwo increased with increasing pH value and salinity. The highest growth inhibition rate of H . akashiwo was observed at a pH of 10 with a salinity of 40 (>90%, Fig. 2e).
3.6 Influence of the light-pH treatment combination on the allelopathic effect of C . pilulifera on H . akashiwothe growth inhibition rate of H . akashiwo by C . pilulifera (P<0.001), with significant interactions between these two environmental factors (P<0.001) (Table 1). The growth inhibition rate of H . akashiwo increased with increasing light intensity. The highest growth inhibition rate of H . akashiwo was observed under high-intensity light at 400 μmol/(m2∙s) and pH 10 (>90%, Fig. 2f).
4 DISCUSSIONAllelochemicals are compounds with which phytoplankton promotes or inhibits competitors. Under environmental stress, the production and release of these compounds increases to strengthen the competitive ability of allelopathic species. The secretion of allelochemicals is affected by many biotic and abiotic factors, including light, temperature, salinity and pH. These factors also interfere with the compound’s actions against the target alga (Granéli et al., 2008).
The strength of allelopathic effects on aquatic organisms can be related to their growth status. Under optimal growth conditions, the allelopathic effect of the toxic organism is often not as strong. In contrast, in stressful situations, the toxic organism has more effective allelopathic interactions with the target organism(s). This phenomenon may be attributed to two causes: (1) under stressful situations, the donor organism increases the production of the allelopathic substance; and (2) under stress, the target organism is more sensitive to the allelopathic substance produced by the donor organism (Gross, 2003).
Temperature is a key factor affecting growth and physiological activities of all aquatic organisms. Environmental conditions can influence the effects of allelopathic interactions between algae (Kong et al., 2000). Legrand et al. (2003) pointed out that the production of allelopathic substances by Coolia monotis was related to the temperature of the liquid medium, and that the allelopathic interactions were more intense at an optimal temperature. It has been reported that H . akashiwo can form red tides within a temperature range of 15-30℃. In Japan, H . akashiwo commonly occurs in water at 20-25℃ (Honjo, 1993). Similarly, in Dalian, China, red tides mainly occur in waters at ~22℃ (Guo, 1994). In the present study, the highest growth inhibition rate of H . akashiwo by C . parietina was at 15℃, possibly because of the high sensitivity of the red-tide microalga to the macroalga at low temperatures. Similarly, Issa (1999) reported that the production of allelopathic substances by the cyanobacteria Oscillatoria angustissima and Calothrix parietina were related to the cultivation temperature.
Heterosigma akashiwo inhabits intertidal and coastal zones, thus experiencing substantial salinity changes in natural aquatic systems. Except for the L - S combination treatment (highest inhibition rate at a salinity of 10), the growth inhibition rate of H . akashiwo by C . pilulifera was highest at a salinity of 40. As H . akashiwo lacks cell walls (Guo, 1994), high salinity likely destroys its cellular structure and physiological function, thereby increasing its sensitivity to C . pilulifera .
Variations in light distribution and intensity in seawater can affect the propagation, distribution and primary productivity of phytoplankton. Phytoplankton requires light for photosynthesis and thus for red tide formation. In the present study, the highest inhibition rate of H . akashiwo by C . pilulifera was observed under a high-intensity light of 400 μmol/(m2∙s). The growth of microalgal cells may have been inhibited by the high light intensity, whereas the growth rate of the macroalgal cells increased. As a result, the microalga was subject to a relatively large concentration of allelopathic substance per cell. The high-intensity light might also impair the physiological function of microalgal cells, increasing their sensitivity to allelopathic substances produced by C . pilulifera . UV treatments caused different light reactions of allelopathic substances produced by two freshwater macrophytes, further influencing their allelopathic activities (Farjalla et al., 2001). Furthermore, under high-intensity light, the production and secretion of allelopathic substances by the roots of Eichhornia crassipes were inhibited (Sun et al., 1989). Together these findings indicate that the intensity and type of light influence the effect of allelopathic interactions between aquatic organisms.
In our study, the highest inhibition rate of H . akashiwo by C . pilulifera was observed at pH 10, possibly because of the increased production and enhanced activity of allelopathic substances produced by the macroalga C . pilulifera at high pH. Alternatively, the cellular physiology of H . akashiwo could have been affected at high pH and thus cells were more sensitive to the allelopathic substances produced by C . pilulifera . The inhibition rate of H . akashiwo was lowest at low pH, suggesting that the acidic environment imposes a negative effect on the production of allelopathic substances and the allelopathic activity of C . pilulifera . Schimidt and Hansen investigated the influence of pH on the allelopathic effect of Chrysochromulina polylepis on Heterocapsa triquetra and found that the allelopathic effect was strongest at high pH (8.9-9.6) and was weak at pH<7.7 (Schmidt and Hansen, 2001). In addition, Ray and Bagchi (2001) found that high pH conditions increased the production of allelopathic substances by the cyanobacterium Oscillatoria laetevirens, which is consistent with our findings. Kong et al. (2000) also reported that environmental conditions influence the effect of allelopathic interactions between algae species. Furthermore, Legrend proposed that the production of allelopathic substances by the dinoflagellate Coolia monotis is related to the medium pH, and that the allelopathic interactions were more intense at an optimal pH (Sun et al., 1989). This further proves that pH influences the allelopathic effect of macroalgae on red-tide microalgae.
Further studies we have conducted on the allelopathic effects of macroalgae on harmful red tide microalga have isolated a number of unsaturated fatty acids from the macroalgae Corallina pilulifera and Sargassum thunbergii . Among them, linolelaidic acid, linoleic acid and eicosapentaenoic acid are highly algicidal, and have high concentrations in macroalgae. We propose that allelochemicals control the biocidal mechanisms underlying oxidative stress-induced apoptosis in H . akashiwo . We are still examining the effect of abiotic factors such as light, temperature, salinity and pH on the biocidal mechanisms.
5 CONCLUSIONThe four environmental factors studied (temperature, salinity, light, and pH) all significantly influenced the allelopathic effects of C . pilulifera on H . akashiwo . Low temperature (15℃), low salinity (10), high-intensity light (400 μmol/(m2∙s)), and high pH (10) treatments enhanced the allelopathic effect of C . pilulifera on H . akashiwo . The strongest allelopathic effect of C . pilulifera on H . akashiwo was observed under the following treatments: 15℃ and salinity of 40, 25℃ and pH 10, 25℃ with medium- to high-intensity light at 200-400 μmol/(m2∙s), 400 μmol/(m2∙s) and salinity of 10, 400 μmol/(m2∙s) and pH 10, and pH 10 with a salinity of 40.
6 ACKNOWLEDGMENTThe authors thank Prof. Timothy A. Nelson, XING Guangxi and YI Kexi for their contribution to this work. We also thank Dr. Edward C. Mignot for linguistic advice.
| Chang F H, Anderson C, Boustead N C, 1990. First record of a Heterosigma(Raphidophyceae) bloom with associated mortality of cage-reared salmon in Big Glory Bay, New Zealand. New Zealand Journal of Mar ine and Freshw ater Research, 24 (4) : 461 –469. Doi: 10.1080/00288330.1990.9516437 |
| Farjalla V F, Anesio A M, Bertilsson S, Granéli W, 2001. Photochemical reactivity of aquatic macrophyte leachates:abiotic transformations and bacterial response. Aquat.Microbial. Ecol., 24 (2) : 187 –195. |
| Granéli E, Weberg M, Paulo S, 2008. Harmful algal blooms of allelopathic microalgal species:the role of eutrophication. Harmful Alage, 8 (1) : 94 –102. Doi: 10.1016/j.hal.2008.08.011 |
| Gross E M, 2003. Allelopathy of aquatic autotrophs. Crit. Rev.Plant. Sci., 22 (3-4) : 313 –339. Doi: 10.1080/713610859 |
| Guillard R L, 1975. Culture of phytoplankton for feeding marine invertebrates. In:Smith W L, Chanley M H eds.Culture of Marine Invertebrate Animals. Plenum Press, New York. p : 26 –60. |
| Guo Y J, 1994. Studies on Heterosigma akashiwo(Hada) Hada in the Dalian bight, Liaoning, China. Oceanologica et Limnologia Sinica, 25 (2) : 211 –215. |
| Honjo T, 1993. Overview of bloom dynamics and physiological ecology of Heterosigma akashiwo. In:Smayda T J, Shimizu Y eds. Toxic Phytoplankton Blooms in the Sea.Elsevier Scientific, Amsterdam : 33 –41. |
| Issa A A, 1999. Antibiotic production by the cyanobacteria Oscillatoria angustissima and Calothrix parietina. Environ. Toxicol. Pharmacol., 8 (1) : 33 –37. Doi: 10.1016/S1382-6689(99)00027-7 |
| Jin Q, Dong S L, 2003. Comparative studies on the allelopathic effects of two different strains of Ulva pertusa on Heterosigma akashiwo and Alexandrium tamarense. J ournal of Experimental Marine Ecology and Biology, 293 (1) : 41 –45. Doi: 10.1016/S0022-0981(03)00214-4 |
| Keating K I, 1977. Allelopathic influence on blue-green bloom sequence in a Eutrophic lake. Science, 196 (4292) : 885 –887. Doi: 10.1126/science.196.4292.885 |
| Kong C H, Xu T, Hu F, Huang S S, 2000. Allelopathy under environmental stress and its induced mechanism. Acta Ecologic a Sinica, 20 (5) : 849 –854. |
| Körner S, Nicklish A, 2002. Allelopathic growth inhibition of selected phytoplankton species by submerged macrophytes. Journal of Phycology, 38 (5) : 862 –871. Doi: 10.1046/j.1529-8817.2002.t01-1-02001.x |
| Legrand C, Rengefors K, Fistarol G O, Granéli E, 2003. Allelopathy in phytoplankton-biochemical, ecological and evolutionary aspects. Phycologia, 42 (4) : 406 –419. Doi: 10.2216/i0031-8884-42-4-406.1 |
| Lu H M, Xie H H, Gong Y X, Wang Q, Yang Y F, 2011. Secondary metabolites from the seaweed Gracilaria lemaneiformis and their allelopathic effects on Skeletonema costatum. Biochemical Systematics and Ecology, 39 (4-6) : 397 –400. Doi: 10.1016/j.bse.2011.05.015 |
| Nelson T A, Lee D, Smith B C, Prins R, 2003. Are "green tides" harmful algal blooms? Allelopathic properties of extracts from Ulva fenestrata and Ulvaria obscura. J.Phycol., 38 (S1) : 28 –29. |
| Oh M Y, Lee S B, Jin D H, Hong Y K, Jin H J, 2010. Isolation of algicidal compounds from the red alga Corallina Pilulifera against red tide microalgae. Journal of Applied Phycology, 22 (4) : 453 –458. Doi: 10.1007/s10811-009-9478-x |
| Ray S, Bagchi S N, 2001. Nutrients and pH regulate algicide accumulation in cultures of the cyanobacterium Oscillatoria laetevirens. New Phytol., 149 (3) : 455 –460. |
| Schmidt L E, Hansen P J, 2001. Allelopathy in the prymnesiophyte Chrysochromulina polylepis:effect of cell concentration, growth phase and pH. Mar. Ecol.Prog. Ser., 216 : 67 –81. Doi: 10.3354/meps216067 |
| Sun W H, Yu Z W, Yu S W, 1989. The harness of an eutrophic water body by Water-Hyacinth. Acta Scientiae Circumstantiae, 9 (2) : 188 –195. |
| Taylor R, Fletcher R L, Raven J A, 2001. Preliminary studies on the growth of selected‘green tide’algae in laboratory culture:effects of irradiance, temperature, salinity and nutrients on growth rate. Bot. Mar., 44 (4) : 327 –336. |
| van Donk E, van de Bund W J, 2002. Impact of submerged macrophytes including charophytes on phyto-and zooplankton communities:allelopathy versus other mechanisms. Aqua. Bot., 72 (3-4) : 261 –274. Doi: 10.1016/S0304-3770(01)00205-4 |
| Wang R J, Feng L, Tang X X, Wang J H, Dong S L, 2012a. Allelopathic growth inhibition of Heterosigma akashiwo by the three Ulva spcieces(Ulva Pertusa, Ulva linza, Enteromorpha intestinalis) under laboratory conditions. Acta Oceanologica Sinica, 31 (3) : 138 –144. Doi: 10.1007/s13131-012-0214-z |
| Wang R J, Tang X X, Sun J H, 2008. Allelopathic effects of Corallina pilulifera on red tide microalgae Heterosigma akashiwo. Chinese Journal of Applied Ecology, 19 (10) : 2322 –2326. |
| Wang R J, Wang Y, Tang X X, 2012b. Identification of the toxic compounds produced by Sargassum thunbergii to red tide microalgae. Chinese Journal of Oceanology and Limnology, 30 (5) : 778 –785. Doi: 10.1007/s00343-012-1294-5 |
| Wang R J, Xiao H, Zhang P Y, Qu L, Cai H J, Tang X X, 2007. Allelopathic effects of Ulva pertusa, Corallina pilulifera and Sargassum thunbergii on the growth of the dinoflagellates Heterosigma akashiwo and Alexandrium tamarense. Journal of Applied Phycology, 19 (2) : 109 –121. Doi: 10.1007/s10811-006-9117-8 |
| Yan T, Zhou M J, Qian P Y, 2002. Growth of fish killing red tide species Raphidophyte Heterosigma akashiwo. Oceanologica et Limnologia Sinica, 33 (2) : 209 –214. |
| Zhao Y, Yu Q Y, Zhou B, Ju Q, Tang X X, 2009. Allelopathic effect of Corallina pilulifera on Heterosigma akashiwo and its responses to UV-B irradiation. Chinese Journal of Applied Ecology, 20 (10) : 2558 –2562. |
 2016, Vol. 34
2016, Vol. 34


