Institute of Oceanology, Chinese Academy of Sciences
Article Information
- XU Qiang(许强), GAO Fei(高菲), YANG Hongsheng(杨红生)
- Importance of kelp-derived organic carbon to the scallop Chlamys farreri in an integrated multi-trophic aquaculture system
- Chinese Journal of Oceanology and Limnology, 34(2): 322-329
- http://dx.doi.org/10.1007/s00343-015-4332-2
Article History
- Received Dec. 5, 2014
- accepted in principle Apr. 5, 2016
2 Yellow Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences, Qingdao 266071, China
Integrated multi-trophic aquaculture (IMTA) has proved effective to reduce organic load to marine environment and accepted as a sustainable aquaculture mode to harvest seafood products (such as bivalves, seaweeds et al.) (Chopin et al., 2001; Neori et al., 2004; FAO, 2006; Whitmarsh et al., 2006; Ridler et al., 2007; Allsopp et al., 2008). Suspension-feeding bivalves and seaweeds are important cleaners widely used in IMTA systems. Previous studies have confirmed the mutual benefit between seaweed and bivalves regarding nutrient and organic matter utilization in the IMTA system (Fang et al., 1996; Wei et al., 2002). However, quantitative studies are still lacking on the exact amount of seaweed-derived organic matter utilized by the co-cultured bivalves.
The contribution of detrital organic matter from seaweed (mostly kelp) to bivalves (such as mussels and scallops) has been quantified in natural seaweed beds for decades (Newell and Field, 1983; Newell, 1984; Duggins et al., 1989; Bustamante and Branch, 1996; Xu and Yang, 2007). Bustamante and Branch, 1996 estimated, using stable carbon and nitrogen isotopes, that the contribution of kelp-derived carbon to the mussel Mytilus galloprovincialis was as high as 60%. Unlike natural ecosystems, an IMTA system is highly modified to provide maximum value for humans. Taking the kelp-bivalve IMTA system in China as an example, the bivalves are usually cultured at an extremely high density for maximum product output. A sufficient particulate organic matter (POM) supply is vital to guaranteeing the growth of a large bivalve population. The biomass of kelp in the system increases gradually and then drops to almost zero because of harvest after 6 months. This certainly results in dramatic variations of detritus amounts in the water, and impacts an important potential food source for cultured bivalves. A detailed study on pelagic detritus dynamics and its subsequent impact on the cultured bivalve’s diet composition is still lacking.
The present study was carried out in Sungo Bay, North China Sea, a representative IMTA farm for the kelp Saccharina japonica and the scallop Chlamys farreri . The exact contribution of kelp-derived detritus to POM and scallops was quantified during an entire kelp culture cycle using stable isotope analysis. The aim was to evaluate the trophic importance of kelp and reveal how the kelp support such a large-scale IMTA ecosystem.
2 MATERIAL AND METHOD 2.1 Study areaSungo Bay is a semi-open bay in North China (37°01′-37°09′N, 122°24′-122°35′E) with an area of about 132 km 2 . The bay is separated into three parts that use different aquaculture styles (Fig. 1). Exchange between the bay and the Yellow Sea is driven by a semi-diurnal tide (tidal range 2 m), across a broad (10 km) mouth. The average depth of the bay is 7.5 m (max. 15 m), and the water volume is about 1×10 9 m 3 (Zhao et al., 1996). There are no major freshwater inflows. The water temperature ranges from 1.8℃ in February to 24.9℃ in August (average, 13℃). The salinity averages 31.76 with little variation.

|
| Figure 1 Map of Sungo Bay (Weihai, China) |
All samples were collected in the IMTA area with co-cultured kelp and scallops in 2006 (site a). Samples were collected intermittently throughout the kelp culture cycle on: February 24 (fast growth), April 27 (ready for harvest), May 30 (kelp cultured only at the bay mouth, site b) and August 9 (1 month after kelp harvest).
During the first two sampling periods, five kelp fronds were collected by cutting them off the long line and on May 30, five kelp samples were collected in the kelp monoculture area at the bay mouth. Twoyear- old scallops were collected from three sites in the kelp-bivalve co-culture area. Phytoplankton samples (n =2) were obtained by hauling a plankton net (50-μm mesh) horizontally in the subsurface water until enough sample was acquired. Phytoplankton and water samples (n =3) were prefiltered with a 200-μm mesh net to eliminate large zooplankton and debris, and then transferred into plastic bottles. All samples were taken to the laboratory within 1 hour and processed immediately.
Particulate organic matter (POM), Chlorophyll a (Chl a), particulate organic carbon (POC) and nitrogen (PON) parameters were monitored simultaneously to quantify the organic materials in the water. POM samples were acquired by filtering 1 L of water through a Whatman GF/C filter (pre-combusted under 450℃ for 6 h). Chl a samples were collected on a GF/C filter by filtering 1 L of water and adding 5 mL of concentrated alkali magnesium carbonate. POC/ PON samples were acquired by filtering 250 mL of water through a 25-mm pre-combusted GF/C filter. Filters were kept at -20℃ in darkness until analysis.
2.3 Sample pretreatmentKelp and scallop samples were preserved on ice and taken back to the laboratory within 6 h. Kelp blades were cleaned thoroughly with grit-filtered seawater, and the length and wet weight were measured. Because the kelp was too long (over 4 m), it was partially sampled as follows: the blade was cut into five equal pieces, then the middle one-third of each piece was collected and combined into one representative sample per kelp blade. Kelp detritus is mostly released from the tip as it grows, so the frond tip (20 cm long) was sampled and analyzed separately. Kelp tissue was cleaned with Milli-Q water and freeze-dried for 48 h. Scallops were cleaned with seawater after fouling organisms on the shells were scraped off with a knife. Of these, 12-30 individuals were randomly selected for a condition index (CI) measurement, and other scallops were dissected. Adductor tissue was selected for stable carbon isotope determination (four individuals were pooled as one sample and three replicates were set. Tissues were rinsed thoroughly with Milli-Q water and then freezedried for 48 h.
2.4 Quantification of detritus and phytoplanktonPOM was analyzed gravimetrically. Chl a was measured according to the standard procedure of acetone extraction and spectrometry analysis. POC and TN were measured on a CHN element analyzer (model 240c, PerkinElmer Co., Wellesley, Massachusettes, USA) after acidification with concentrated HCl to eliminate carbonates.
2.5 Stable isotope analysisFreeze-dried kelp and scallop tissues were ground to fine powder with a mortar and pestle (precombusted under 500℃ for 3 h; washed with ethanol and air-dried between samples). Samples were sealed in combusted glass vials with lab tape and stored below -20℃ until analysis.
To eliminate possible carbonates, net-towed phytoplankton samples were acidified with 1 N HCl at 4℃ and shaken occasionally until bubbling ceased. They were then dried at 60℃ for 24 h.
The isotopic ratio was measured on a EuroEA3000 (EuroVector Co., Milan, Italy) element analyzer and an Iso-Prime stable isotope mass spectrometer (GV Instruments Co., Manchester, UK). Data were expressed in the standard δ unit notation:
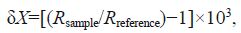
were reported relative to the Pee Dee Belemnite standard.
2.6 Statistics 2.6.1 Quantification of organic carbon sources in POM and scallop tissueIn a kelp ecosystem, particulate organic carbon is mostly derived from detritus and phytoplankton. We estimated the detritus carbon in the water using the following equations:
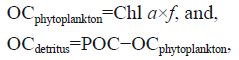
where f =60 (Horne, 1969). A POC/Chl a ratio above 100 was also used to indicate significant amounts of detritus in the POM (Zeitzschel, 1970).
The carbon contributions of kelp and phytoplankton to cultured scallops were estimated using a simple linear, two-source mixing model (Phillips and Koch 2002):

where the subscripts X, Y, and M represent two food sources (kelp and phytoplankton) and the mixture (scallop), respectively; f represents the fractional contribution of carbon from each food source to the scallop, and Δ 13 C tissue- X, Y is the trophic enrichment from two sources. In a previous study on cultured scallops and phytoplankton in Jiaozhou Bay (Qingdao, China) without kelp cultivation, the Δ 13 C from phytoplankton to a scallop’s adductor muscle was +1.7‰. Another laboratory polyculture experiment showed that the Δ 13 C from kelp detritus to a scallop’s adductor was +0.9‰ (Xu et al., 2010). Therefore, these two values were selected as the representative trophic enrichments of 13 C from kelp and phytoplankton to scallop growth.
2.6.2 Carbon contribution assessmentThe total contribution of kelp-derived organic carbon to scallops harvested each year was combined with the annual yield of scallops in Sungo Bay and evaluated using the following equation:
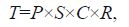
where T represents the carbon contribution of the kelp (ton C/a); P represents the annual yield of scallops; S represents the ratio of the dry mass of a commercial scallop’s soft body to its total wet weight; C is the carbon content of scallop’s soft tissue; and R is the average contribution of kelp-derived organic carbon, which was calculated using the following equation:
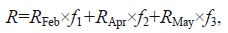
where R Feb, R Apr, and R May are the contributions of kelp-derived organic carbon during 3 months, respectively; f 1, f 2, and f 3 are weight coefficients, meaning the ratio of days in February, April and May, respectively, to total days in all of the 3 months.
2.6.3 Statistical methodSignificant differences between samples were determined by one way-analysis of variance followed by a Tukey’s test. A student’s t-test was used to check for differences between two means. Significant correlation between temporal changes of two parameters was examined with a Pearson correlation test. In all cases, a 95% significance level was adopted. Analyses were performed using the SPSS 21.0 statistical package (IBM Co., New York, USA).
3 RESULT 3.1 Brief characterization of phytoplankton and detritusThe dynamics of Chl a, POM, POC and PON in the IMTA area are shown in Table 1. Chl a concentration increased gradually from Feb to Aug; POM and POC levels were also elevated during the sampling period. The POC in August was twice the value measured in May and the C:N ratio of POM was extremely high (19.27). The C:N ratio of healthy phytoplankton is considered to be 6 (Redfield ratio), whereas that of fresh kelp fronds is much higher (18.24, current study, May). Microscopic examination found that there were large brown amorphous particles in the net-towed sample in August, probably from degraded kelp debris.
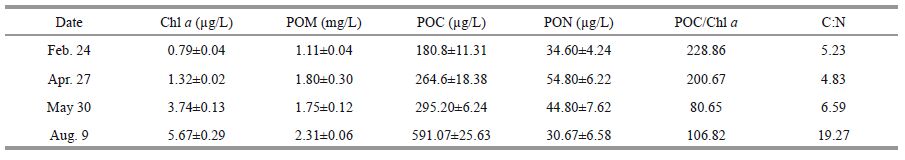
|
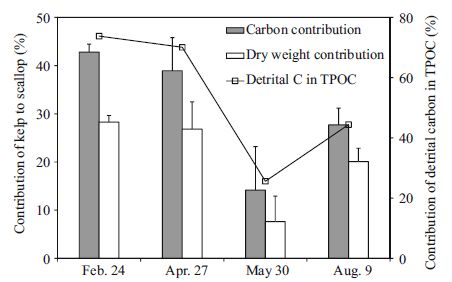
The organic carbon sources and its dynamic changes are presented in Table 2 and Fig. 2. The detritus carbon ranged from 70.1% to 73.8% of the total POC in February and April during scallop and kelp polyculture, whereas in May and August the detritus carbon proportions dropped to 25.6% and 44.4% after kelp harvest. The POC/Chl a ratio dropped below 100 only in May, and varied in accordance with the detrital carbon proportion (Pearson correlation, one-tailed, F =0.977, P =0.011). In August, measured phytoplankton carbon was 55.6% of the total POC.
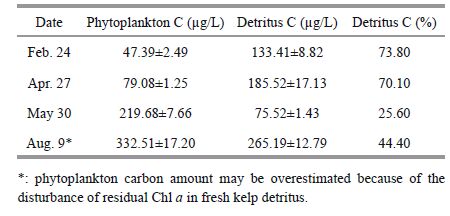
|
|
| Figure 2 Temporal contributions of detrital carbon to TPOC and the contribution of kelp-derived organic carbon to scallop tissue during one cycle of kelp cultivation in Sungo Bay (Weihai, China) |
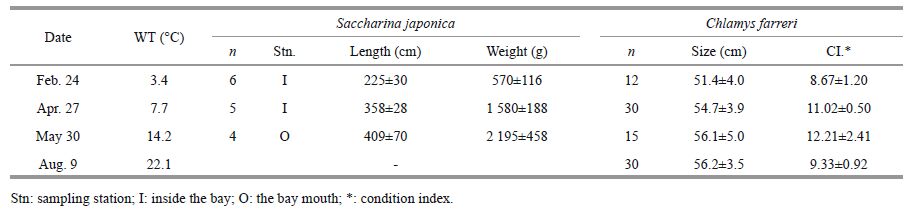
Rapid growth of scallops and kelp were found during the entire experimental period (Table 3). The δ 13 C values of the scallops, phytoplankton and kelp are shown in Table 4. Scallops in May and August were isotopically lighter than during the previous two sampling dates. The phytoplankton isotopic data for August is missing because the net-towed sample was full of kelp detritus. The δ 13 C value of the sample can represent the isotopic composition of fresh kelp detritus in August. Kelp frond samples in April were isotopically lighter than the other 2 months, whereas the frond tip sample in May was significantly heavier. There were significant differences between the δ 13 C value of the kelp frond and tip except for in the February sample.
|

|
The carbon contributions of phytoplankton and kelp to the scallop tissue were estimated based on the isotopic data in Table 4. The δ 13 C value of phytoplankton in May (-21.89‰) was selected to represent the exact isotopic value of phytoplankton in the whole sampling period, because of little kelp detritus in the water after kelp harvest. Results of the mixing model showed that the scallop C . farreri obtained up to 42.8% of its organic carbon from kelp during the polyculture period in February and April. After kelp harvest in May, however, the contribution declined to 14.1% (Fig. 2). A strong significant correlation was found between the kelp-derived carbon proportion in the scallop tissue and the detritus carbon percentage in the water (Pearson correlation, one-tailed, F =0.993, P= 0.003).
To evaluate the overall contribution of kelp carbon to scallops, the annual yield of scallops (17 000 tons wet weight in 2013), the weight ratio of dry mass to wet soft tissue of scallop (0.055, May sample, present research), and the carbon content of scallop soft tissue (0.447 5, Zhou et al., 2002) were used. After calculation, the average contribution of kelp-derived organic carbon was 33.3%. Estimated results showed that for the given amount of harvested scallops (418.4 t organic carbon), cultured kelp contributed about 139.3 t organic carbon. Kelp’s carbon content is about 26% of its dry weight (data from sample in May), so the scallop incorporated at least 535.8 t dry mass from the kelp.
4 DISCUSSION 4.1 Pelagic detritus dynamics and the impact of kelpKelp detritus is mainly released by the erosion of the frond tip when it grows. Zhang et al. (2012) studied the growth and loss of cultured kelp in Sungo Bay and found that the erosion rates (in accordance with detritus release rates) were positively correlated with temperature (R =0.787, n =23, P<0.01) before May. In Sungo Bay, considering the total mariculture area of S . japonica at about 7 500 km 2, it is estimated that the annual gross production and total loss (91.5% as detritus release) in terms of carbon were 58 652 t and 36 150 t, respectively, and the detritus carbon produced from erosion was 33 077 t, or 44.1 kg/ha (Zhang et al., 2012). In the present study, although phytoplankton was also a potential source of detritus in the water, the relationship between the detritus proportion and kelp growth confirmed that kelp detritus had strong control over the pelagic detritus pool.
In February, before the phytoplankton bloom, the detritus proportion stayed very high (over 70%) in the TPOC. Kelp-derived detritus made a major contribution to the TPOC in winter with cold water temperatures. In April, the phytoplankton grew faster as indicated by the elevated Chl a concentration. The detritus release process also sped up and caused increasing detritus in the water. Zhang et al. (2012) found a maximum erosion rate of 20.4 g/day on Apr. 25 and kelp-derived detritus was still predominant in the TPOC.
After the kelp harvest in May, phytoplankton bloomed inside the bay without kelp to uptake the nutrients in the water (mainly N and P). The detritus amount dropped sharply to below 80 μg/L. The POC/ Chl a ratio decreased to 80.65, indicating that detritus was no longer dominant in the SPM. At this time, the detritus was probably derived mainly from dead phytoplankton and the kelp cultured at the bay mouth.
Kelp-derived suspended particulate matter may be transported by ocean currents and utilized tens of kilometers downstream from the kelp beds (Kaehler et al., 2006). In August, a large amount of fresh kelp debris was found in the SPM. Previous research noted that the rate of kelp breakage showed a significant positive correlation with kelp length and took place during June and July (Zhang et al., 2012). These fragmented kelps eroded rapidly under high water temperatures in August and released large amounts of detritus into the water (Liu et al., 2003).
The detritus carbon amount was very high in August, and the C:N ratio was extremely high (19.27), which is very similar to that of fresh kelp tissue (Zhang et al., 2012). When kelp detritus is released into the water at an appropriate speed, it can be colonized by microbes that cause faster decay, and the C:N ratio drops to about six within 1 month (Xu et al., 2010). In August, however, a large amount of fresh kelp debris was released to the water in such a short time that it could not be sufficiently degraded by microbes.
4.2 Temporal variation of kelp-derived carbon contribution to scallopFilter-feeding bivalves in marine ecosystems are confronted with a wide range of living and non-living materials (Shumway and Parsons, 2006). The seston consists of plankton with a wide range of sizes and palatability, organic detritus derived from macrophytes, as well as material re-suspended from the benthos, fecal pellets and microorganisms (Passow et al., 1994; Crocker and Passow, 1995). The complex of organic particles around the bivalves results in wide variation in their diet. In the present study, temporal changes in detrital vs. phytoplanktonic POM proportions were found in the IMTA area, which were mirrored in the diet composition of the scallops.
Phytoplankton grows slowly during winter and early spring in temperate oceans. A previous study found that the phytoplankton biomass was only 12 800 cells/L at the center of Sungo Bay when the water temperature was 3-4℃ (Liu et al., 2003). Such a low concentration of phytoplankton is unable to meet the dietary demand of intensively cultured bivalves. Cultured kelp acted as another important POM pool and contributed a large proportion of detritus to the water, which supplied additional food to the bivalves. Results from the mixing model showed that as much as 42.8% of the scallop’s tissue carbon came from kelp in February. The trophic importance of kelp-derived organic materials to invertebrates during the cold season was also seen in natural habitats. During the dark winter period when phytoplankton were absent, the predominant kelp, L . solidungula, contributed over 50% of the organic carbon to gastropods and chitons (herbivores) and an ascidian (nonselective suspension feeder) in an isolated kelp bed community on Alaska’s north Arctic coast (Dunton and Schell, 1987).
In March and April, although the phytoplankton bloomed (Liu et al., 2003), kelp-derived detritus was still predominant in the POM and contributed over 50% of the scallop’s diet. When the kelp was harvested in May, phytoplankton became predominant in the water and the detritus carbon proportion dropped to 26% in the TPOC. Correspondingly, the proportion of the scallops’ diet composed of kelp detritus declined to 14.1%.
In August, rapid release of fresh kelp detritus resulted in a poor-quality particulate food source with an extremely high C:N ratio (19.27). The C:N ratio of C . farreri tissue is around 4 (Zhou et al., 2002), so the detritus food source was N-limited for scallops. Generally, nitrogen limitation can limit carbon utilization by the animal, which must be compensated by increased feeding amount on low quality of food. This elevates the basal metabolic level and is unfavorable for the animal (Cruz-Rivera and Hay, 2000). Slightly degraded kelp detritus may have high values of toxic polyphenols (17.5 mg/gdw in S . japonica ; Yan, 1996). Poor-quality food and high metabolic rates caused by elevated temperatures in summer resulted in severe self-consumption by the scallop indicated by a lower condition index (9.3). The ammonium excretion and oxygen consumption rates were extremely high in July and August (4.946 μmol N/(gdw∙h) and 2.629 mg O/(gdw∙h), Mao, 2005). In recent years, cultured scallops in Sungo Bay experienced summer mass mortality, and this may relate to the malnutrition caused by poorquality food sources.
5 CONCLUSIONThe present study found that kelp is an important component of the kelp-bivalve IMTA ecosystem. Kelp was an important contributor to the detritus pool and seasonally determined the detritus level in the water. It provided an important supplemental food source to co-cultured scallops, especially when phytoplankton levels were insufficient. Commercial scallops obtained as much as 42.8% of their carbon from the kelp during the cold season. Results suggest that kelp in the IMTA ecosystem could supply an additional food source to cultured bivalves.
6 ACKNOWLEDGEMENTWe would like to thank Xunshan Fishery Ltd. for providing experimental areas. We are grateful to the Ecosystem Research Station of Jiaozhou Bay for their help in sample analysis. Finally, we would like to dedicate this paper in memory of Mr. XU Xinling for his help in the field sampling in Sungo Bay.
| Allsopp M, Johnston P, Santillo D, 2008. Challenging the Aquaculture Industry on Sustainability. 2nd edn. Greenpeace International, Netherlands : 24p . |
| Bustamante R H, Branch G M, 1996. The dependence of intertidal consumers on kelp-derived organic matter on the west coast of South Africa. J. Exp. Mar. Biol. Ecol., 196 (1-2) : 1 –28. Doi: 10.1016/0022-0981(95)00093-3 |
| Chopin T, Buschmann A H, Halling C, Troell M, Kautsky N, Neori A, Kraemer G P, Zertuche-González J A, Yarish C, Neefus C, 2001. Integrating seaweeds into marine aquaculture systems:a key toward sustainability. J.Phycol., 37 (6) : 975 –986. Doi: 10.1111/jpy.2001.37.issue-6 |
| Crocker K M, Passow U, 1995. Differential aggregation of diatoms. Mar. Ecol. Prog. Ser., 117 : 249 –257. Doi: 10.3354/meps117249 |
| Cruz-Rivera E, Hay M E, 2000. Can quantity replace quality? Food choice, compensatory feeding, and fitness of marine mesograzers. Ecology, 81 (1) : 201 –219. Doi: 10.1890/0012-9658(2000)081[0201:CQRQFC]2.0.CO;2 |
| Duggins D O, Simenstad C A, Estes J A, 1989. Magnification of secondary production by kelp detritus in coastal marine ecosystems. Science, 245 (4914) : 170 –173. Doi: 10.1126/science.245.4914.170 |
| Dunton K H, Schell D M, 1987. Dependence of consumers on macroalgal(Laminaria solidungula) carbon in an arctic kelp community:δ13C evidence. Mar. Biol., 93 (4) : 615 –625. Doi: 10.1007/BF00392799 |
| Fang J G, Sun H L, Yan J P, Kuang S H, Li F, Newkirk G F, Grant J, 1996. Polyculture of scallop Chlamys farreri and kelp Lamin a ria japonica in Sungo Bay. Chinese J.Oceanol. Limnol., 14 (4) : 322 –329. Doi: 10.1007/BF02850552 |
| F AO, 2006. State of World Aquaculture, FAO Fisheries Technical Paper No. 500. FAO Fisheries and Aquaculture Department, Rome : 134p . |
| Horne R A, 1969. Marine Chemistry. Wiley-Interscience, New York. p : 77 –198. |
| Kaehler S, Pakhomov E A, Kalin R M, Davis S, 2006. Trophic importance of kelp-derived suspended particulate matter in a through-flow sub-Antarctic system. Mar. Ecol. Prog.Ser., 316 : 17 –22. Doi: 10.3354/meps316017 |
| Liu H, Fang J G, Dong S L, Wang L C, Lian Y, 2003. Annual variation of major nutrients and limiting factors in Laizhou Bay and Sanggou Bay. J. Fish. Sci. China, 10 (3) : 227 –234. |
| Mao Y Z, 2005. Effects of Bivalve Raft Culture on Environment and Their Ecological Regulation in Sanggou Bay, China. Ocean University of China, Qingdao, China. p : 63 –68. |
| Neori A, Chopin T, Troell M, Buschmann A H, Kraemer G P, Halling C, Shpigel M, Yarish C, 2004. Integrated aquaculture:rationale, evolution and state of the art emphasizing seaweed biofiltration in modern mariculture. Aquaculture, 231 (1-4) : 361 –391. Doi: 10.1016/j.aquaculture.2003.11.015 |
| Newell R C, Field J G, 1983. Relative flux of carbon and nitrogen in a kelp-dominated system. Mar. Biol. Lett., 4 (4) : 249 –257. |
| Newell R C, 1984. The biological role of detritus in the marine environment. In:Fasham M J R ed. Flows of Energy and Materials in Marine Ecosystems:Theory and Practice.Springer , US. p : 317 –344. |
| Passow U, Alldredge A L, Logan B E, 1994. The role of particulate carbohydrate exudates in the flocculation of diatom blooms. Deep-Sea Res. I:Oceanogr. Res. Paper., 41 (2) : 335 –357. Doi: 10.1016/0967-0637(94)90007-8 |
| Phillips D L, Koch P L, 2002. Incorporating concentration dependence in stable isotope mixing models. Oecologia, 130 (1) : 114 –125. Doi: 10.1007/s004420100786 |
| Ridler N, Wowchuk M, Robinson B, Barrington K, Chopin T, Robinson S, Page F, Reid G, Szemerda M, Sewuster J, Boyne-Travis S, 2007. Integrated multi-trophic aquaculture(IMTA):a potential strategic choice for farmers. Aquaculture Economics & Management, 11 (1) : 99 –110. |
| Shumway S, Parsons J, 2006. Scallops:Biology, Ecology and Aquaculture. 2nd edn. Elsevier, Amsterdam . |
| Wei W, Fang J G, Dong S L, Liu Y, 2002. Preliminary studies on mutually beneficial mechanism in the polyculture of scallop(Chlamys farreri) and kelp(Laminaria a japonica). Marine Fisheries Research, 23 (3) : 20 –25. |
| Whitmarsh D J, Cook E J, Black K D, 2006. Searching for sustainability in aquaculture:an investigation into the economic prospects for an integrated salmon-mussel production system. Mar. Policy, 30 (3) : 293 –298. Doi: 10.1016/j.marpol.2005.01.004 |
| Xu Q, Gao F, Yang H S, 2010. Microorganism colonization in different decomposing phases of kelp(Laminaria japonica). J. Fish. China, 34 (12) : 1853 –1859. |
| Xu Q, Yang H S, 2007. Food sources of three bivalves living in two habitats of Jiaozhou bay(Qingdao, China):indicated by lipid biomarkers and stable isotope analysis. J. Shellfish Res., 26 (2) : 561 –567. Doi: 10.2983/0730-8000(2007)26[561:FSOTBL]2.0.CO;2 |
| Yan X J, 1996. Quantitative determination of phlorotannins from some Chinese common brown seaweeds. Studia Marina Sinica, 37 : 61 –65. |
| Zeitzschel B, 1970. The quantity, composition and distribution of suspended particulate matter in the Gulf of California. Mar. Biol., 7 (4) : 305 –318. Doi: 10.1007/BF00750823 |
| Zhang J H, Fang J G, Wang W, Du M R, Gao Y P, Zhang M L, 2012. Growth and loss of mariculture kelp Saccharina japonica in Sungo Bay, China. J. Appl. Phycol., 24 (5) : 1209 –1216. Doi: 10.1007/s10811-011-9762-4 |
| Zhao J, Zhou S L, Sun Y, Fang J G, 1996. Research on Sanggou Bay aquaculture hydro-environment. Mar. Fish. Res., 17 (2) : 68 –79. |
| Zhou Y, Yang H S, Liu SL, He Y Z, Zhang F S, 2002. Chemical composition and net organic production of cultivated and fouling organisms in Sishili Bay and their ecological effects. J. Fish. China, 26 (1) : 21 –27. |
 2016, Vol. 34
2016, Vol. 34


