Institute of Oceanology, Chinese Academy of Sciences
Article Information
- REN Wei(任伟), WANG Shujun(王淑军), LÜ Mingsheng(吕明生), WANG Xiaobei(王小贝), FANG Yaowei(房耀维), JIAO Yuliang(焦豫良), HU Jianen(胡建恩)
- Optimization of four types of antimicrobial agents to increase the inhibitory ability of marine Arthrobacter oxydans KQ11 dextranase mouthwash
- Journal of Oceanology and Limnology, 34(2): 354-366
- http://dx.doi.org/10.1007/s00343-015-4376-3
Article History
- Received Dec. 13, 2014
- accepted in principle Apr. 27, 2015
2 Jiangsu Marine Resources Development Research Institute, Lianyungang 222005, China;
3 Jiangsu Key Laboratory of Marine Pharmaceutical Compound Screening, Huaihai Institute of Technology, Lianyungang 222005, China;
4 Co-Innovation Center of Jiangsu Marine Bio-Industry Technology, Huaihai Institute of Technology, Lianyungang 222005, China
Dextranase (1, 6-α-D-glucan-6-glucanohydrolase, EC3.2.1.11) catalyzes the endohydrolysis of α-(1-6)- D-glycoside linkages in random sites of dextran (Wang et al., 2014). The initial interest in enzymes which could hydrolyze dextran arose from studies aiming to reduce the viscosity of sugar syrup in industrial applications. The presence of dextran in sugar factories causes expensive microbial losses of sucrose yield. In fact, dextran plays a crucial role in dental plaque, which is a main contributor to the development of some common oral diseases. This has become one of the main driving forces to investigate dextran-hydrolyzing enzymes (Khalikova et al., 2005). Dental plaque is a common human disease resulting from a biofilm that forms on the surface of teeth (Koo et al., 2010). When food particles ferment, acid is produced through the fermentation of carbohydrates (Marotta et al., 2002; Islam et al., 2009; Dong et al., 2012). The resulting acidic condition is characterized by an overgrowth of opportunistic pathogens including Streptococcus mutans, the main culture found in dental plaque biofilm. S . mutans produces exopolysaccharides for the inhabitation of other growing bacteria such as Lactobacillus acidophilus and Aggregatibacter actinomycetemcomitans (Deng and ten Cate, 2004; Totiam et al., 2007; Aires et al., 2011; Samot et al., 2011).
Most dextranases come from fungus (Hatada et al., 2004; Chen et al., 2008) which require long production cycles and causes many challenges in food, cosmetic, and medical applications. Moreover, the dextranase applied in current commercial mouthwashes is also mainly from fungi, such as Penicillium lilacinum and Chaetomium erraticum (Marotta et al., 2002; Eggleston and Monge, 2005). We recently described marine Arthrobacter oxydans KQ11 dextranase (Jiao et al., 2014) as a dextranase that can overcome these problems and will be more stable and effective at the oral temperature and is suitable for industrial application as a dental caries-preventing agent. In addition, marine enzymes are characterized by high salt tolerance, hyperthermostability, and low ideal temperature tolerance. Therefore, the effectiveness of current commercial dextranase mouthwashes in preventing dental caries could be enhanced by replacing fungal dextranases with marine bacterial dextranases. In the future development of efficient dextranase oral caries products, marine bacterial dextranase must be greatly emphasized. However, our previous study reported that marine dextranase loosens the biofilm structure instead of killing the opportunistic pathogens (Jiao et al., 2014). Thus, it is important to find antimicrobial agents with biofilmkilling and -disrupting effects which can improve the function of marine Arthrobacter oxydans KQ11 dextranase mouthwash (designed and developed by our laboratory).
Substances which have been used in oral care products include sodium fluoride, essential oils, cetylpyridinium chloride and triclosan, to name a few (Schaeken et al., 1996; Radford et al., 1997; Giertsen, 2004; Hu et al., 2009; Pahwa et al., 2011; Mello et al., 2013). However, chlorhexidine is the main and most effective antibiofilm agent widely used in the oral care industry (Bae et al., 2006). However, reports have suggested that some secondary effects occur, namely the reduction of human taste perception and pigmentation of the teeth and tissues surfaces (Helms et al., 1995; Medlicott et al., 1999; Jamilian et al., 2008).
Zinc is an essential trace element in the human body (Ma et al., 2013), and is inexpensive, stable and environmentally friendly. Some reports state that zinc can inhibit bacteria and plaque, as well as reduce bad breath. In addition, zinc existing in the remineralization process could extend the length of time for such an antimicrobial agent to act. As zinc is released under acidic conditions, bacterial attachment and the formation of tartar will be inhibited (Abdullah et al., 2006; Chen et al., 2012). Lysozyme is an antibacterial enzyme which can hydrolyze the peptidoglycan layer of the cell walls of some Gram-positive bacteria (Pellegrini et al., 1997). In addition, lysozyme has antimicrobial properties and could inhibit the growth and lyse Gram-positive oral bacteria including S . mutans (Bae and Oh, 1990). Citric acid is an important organic acid, and is widely used as an acidulant, pH regulator, flavor enhancer, preservative and antioxidant synergist in many fields (Yılmaz et al., 2008). Besides this, it has been reported that citric acid can inhibit dental cells (Chan et al., 1999; Lan et al., 1999). Chitosan is a natural polysaccharide which is extracted from chitin and has been proven to inhibit microorganisms (Kong et al., 2010; Sarwar et al., 2014). In addition, chitosan possesses bioadhesive capabilities that could be applied in oral care products (Kockisch et al., 2005; Costa et al., 2013).
The main objective of this work was to optimize the use of zinc sulfate, lysozyme, citric acid and chitosan, which can suppress the S . mutans biofilm using response surface methodology, to increase the biofilm-killing ability of our marine Arthrobacter oxydans KQ11 dextranase mouthwash. Then, the effects of the optimized combination of the antimicrobial agents on the S . mutans biofilm were observed by scanning electron microscopy (SEM) and confocal laser scanning microscopy (CLSM). Also, a marine Arthrobacter oxydans KQ11 dextranase mouthwash containing the optimized amounts of antimicrobial agents was used to confirm the effect of the results for the purpose of designing and developing a future marine Arthrobacter oxydans KQ11 dextranase mouthwash.
2 MATERIAL AND METHOD 2.1 MaterialsZinc sulfate, citric acid and chitosan were purchased from Sinopharm Chemical Reagent Co. Ltd., Shanghai, China. Lysozyme (20 000 U/mg) was purchased from Generay Biotech Co. Ltd., Shanghai, China. The other reagents and materials were the brain heart infusion broth (BHI; Hangzhou Tianhe Microorganism Reagent Co. Ltd., Hangzhou, China), glutaraldehyde (Ted Pella, Inc, Shanghai, China), crystal violet (Tianjin Fuchen Chemical Reagent Factory, Tianjin, China), a 96-well flat-bottomed plastic tissue Costar™ culture plate with a lid (Fischer Scientific, Waltham, MA, USA) and a 24-well flatbottomed plastic tissue Costar™ culture plate with a lid (Fischer Scientific, Waltham, MA, USA). The marine Arthrobacter oxydans KQ11 dextranase and marine Arthrobacter oxydans KQ11 dextranase mouthwash (containing 6 U/mL dextranase) were designed and developed by our laboratory.
2.2 MicroorganismsS . mutans (ATCC25175) was purchased from the China General Microbiological Culture Collection Center (Beijing, China). A 250-mL flask containing 40 mL BHI was inoculated with S . mutans . The strain was grown at 37℃ for 24 h. Bacterial cells were collected by centrifugation for 10 min at 12 000× g at 4℃, then were washed two times with sterile saline, diluting the final concentration of the bacteria to OD550 =1.0.
2.3 Preparation of different concentrations ofantimicrobials BHI medium with 1%(w/v) sucrose was used to dissolve zinc sulfate, lysozyme and citric acid to the desired concentrations. A low pH was used to dissolve chitosan in BHI containing 1% glacial acetic acid to the desired concentrations, because chitosan is not soluble at high pH. After that, to completely dissolve chitosan, the solution was stirred overnight at 50℃. Then, NaOH (Sinopharm Chemical Reagent Co. Ltd., Beijing, China) was added in the above mentioned solution to obtain a final pH in the range of 6–6.5(Costa et al., 2013). For all of the solutions mentioned above, a final sterile filtration was carried out and the solutions were stored at 4℃.
2.4 Microtiter plate testFollowing the same methodology as in prior studies using the microtiter plate test (Stepanović et al., 2000; Vergara-Irigaray et al., 2008; Li et al., 2009; Wu et al., 2013), 200 μL of 2% gelatin was added to a 96-well flat-bottomed plastic tissue culture plate with a lid for 3–5 h before the test so that the biofilm firmly adhered to the bottom surface of the plate. After that, the culture plates were filled with 180 μL of BHI with 1% sucrose, containing different final concentrations of antimicrobial agents. Then, each well was inoculated with 20 μL of S . mutans . A biofilm was formed after incubation at 37℃ for 24 h under anaerobic conditions. Then, the liquid in each well was discarded, and each well was washed three times with sterile physiological saline to remove any unattached bacteria. The remaining attached cells were fixed with 200 μL of 95% ethanol (Sinopharm Chemical Reagent Co. Ltd., Beijing, China) per well. After 15 min, these plates were emptied and the wells were dried at room temperature. Then, 0.2 mL of 0.1% crystal violet was added to each well for 5 min to stain the biofilm. Excess stain was removed by rinsing offthe plate under running tap water. After that, the plate was dried at room temperature. The optical density (OD) of each well was measured at 595 nm. All assays were done in triplicate, and the mean values were determined. A 1% sucrose BHI solution inoculated with S . mutans without any antimicrobial agents was used as a control. The methodology used was a single factor test and an orthogonal test was the first step and the central composite design was the second step. % biofilm formation inhibition (Y):
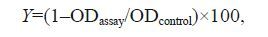 (1)
(1)Because it was not possible to obtain real optimum regions for the four different antimicrobial agents in a single step, this study used two steps:(1) the four independent variables test (single factor test: ZnSO4(A), lysozyme (B), citric acid (C), chitosan (D))(Table 1) and the orthogonal test (Table 2) were used to evaluate the system behavior as a function of the considered operating parameters to identify the proper ranges of the factors;(2) response surface methodology (RSM) with the same factors with new ranges based on the first step was applied.
Four factors with new ranges were considered to perform the subsequent central composite design: ZnSO4(A), lysozyme (B), citric acid (C) and chitosan (D), with three different levels for each of the factors. In this study, the experimental design consisted of 30 runs containing eight axis points, 16 pastries, and six repetitions of center. The experimental design and results used in this study are shown in Table 3. All the experiments were done in triplicate, and the average of inhibition (%) was taken as the dependent or response (Y). The regression and graphical analyses of the experimental data were analyzed and generated by the ‘Design Expert’ statistical package (Version 8.0.5.0, Stat-Ease Inc., Minneapolis, USA). The behavior of the system is explained by the following quadratic equation:
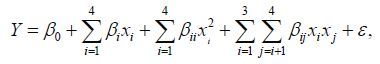 (2)
(2)where Y is the predicted response (inhibition ratio (%)), β 0 is the intercept, β i is the linear coefficient, x i is the factor variable in its coded, β i i is the quadratic coefficient for the factor I, β i j is the interaction effect and ε is the error (Muhamad et al., 2013). The significance of all terms in the polynomial was judged statistically according to the P -value which was compared with the significance level of 0.05.
2.6 Confirmation experiments 2.6.1 The effect of the optimized amounts ofantimicrobial agents on the forming biofilm To observe the effect of the optimized amounts of antimicrobial agents on the formation of biofilms, first, SEM was used (Prigent-Combaret et al., 2000; Aires et al., 2011). Sterile glass microscope slides (18 mm×18 mm) were placed in each well of a 24- well plate. Two hundred microliters of 2% gelatin was added to the 24-well plate for 3–5 h before the test to make the biofilm firmly adhere to the surface of the glass microscope slides. After the liquid was discarded, the culture of S . mutans and BHI with 1% sucrose containing the optimized amounts of antimicrobial agents were added in each well of the 24-well plates at a ratio of 1:9(v/v) and were incubated at 37℃ for 6 h, 15 h, 18 h and 21 h under anaerobic conditions. The control group only consisted of culture and BHI (containing 1% sucrose) without any antimicrobial agents. Each of the biofilms were lightly washed three times using sterile physiological saline to remove any unattached bacteria. After being washed, to fix the cells, 1 mL of 2.5% glutaraldehyde was added in each well of the 24-well plates for 3–5 h at 4℃. Then, the biofilms were washed three times with phosphate buffer; afterwards, the biofilms were dehydrated with 50%, 70%, 80%, 90% and 100% ethanol. Every ethanol concentration was continued for 15 min in a row. Finally, each sample was observed by SEM.
Second, CLSM was used to observe the effect of the optimized amounts of antimicrobial agents on the biofilm formation (Almeida et al., 2011; Cheng et al., 2012). Sterile glass microscope slides (18 mm×18 mm) were placed in each well of a 24-well plate. Two hundred microliters of 2% gelatin was added to the 24-well plate for 3–5 h before the test to make the biofilm firmly adhere to the surface of the glass microscope slides. After the liquid was discarded, the culture of S . mutans and BHI with 1% sucrose containing the following five groups in a proportion of 1:9:(1) control (BHI with 1% sucrose);(2) marine Arthrobacter oxydans KQ11dextranase mouthwash;(3) optimized amounts of antimicrobial agents;(4) marine Arthrobacter oxydans KQ11 dextranase mouthwash+optimized amounts of antimicrobial agents;(5) optimized amounts of antimicrobial agents+6 U/mL marine Arthrobacter oxydans KQ11 dextranase were added to each well of a 24-well plate and were incubated at 37℃ for 24 h under anaerobic conditions. The biofilms on the microscope slides were lightly washed three times using sterile physiological saline to remove any unattached bacteria. Afterwards, the biofilms samples were treated with 15 μL fluorescent dyes containing 30 μg/ mL fluorescein diacetate (FDA) and 50 μg/mL propidium iodide (PI) for 15 min. All of the processes were carried out in the dark. After staining, live cells and dead cells emitted green fluorescence and red fluorescence, respectively. Then, the degree of live/ dead and the thickness of the biofilms were measured by CLSM.
2.6.2 The effect of the optimized amounts ofantimicrobial agents on the formed biofilm CLSM was used to observe the effect of the optimized amounts of antimicrobial agents on the formed biofilm. Sterile glass microscope slides (18 mm×18 mm) were placed in each well of a 24- well plate. Two hundred microliters of 2% gelatin was added to the 24-well plate for 3–5 h before the test to make the biofilm firmly adhere to the surface of glass microscope slides. The culture and BHI with 1% sucrose were cultured undisturbed to form the initial biofilm. After 1 day, the biofilms were treated two times daily (5 min exposure at 10 a.m. and 4 p.m.) until the 5th day of the experiment with one of the following:(1) control (treated with sterile water);(2) marine Arthrobacter oxydans KQ11 dextranase mouthwash;(3) optimized amounts of antimicrobial agents;(4) marine Arthrobacter oxydans KQ11 dextranase mouthwash+optimized amounts of antimicrobial agents; and (5) optimized amounts of antimicrobial agents+6 U/mL marine Arthrobacter oxydans KQ11 dextranase. After treatment, biofilm samples were treated with 15 μL fluorescent dyes containing 30 μg/mL FDA and 50 μg/mL PI for 15 min. All of the processes were carried out in the dark. Then, the degree of live/dead and the thickness of the biofilms were measured by CLSM.
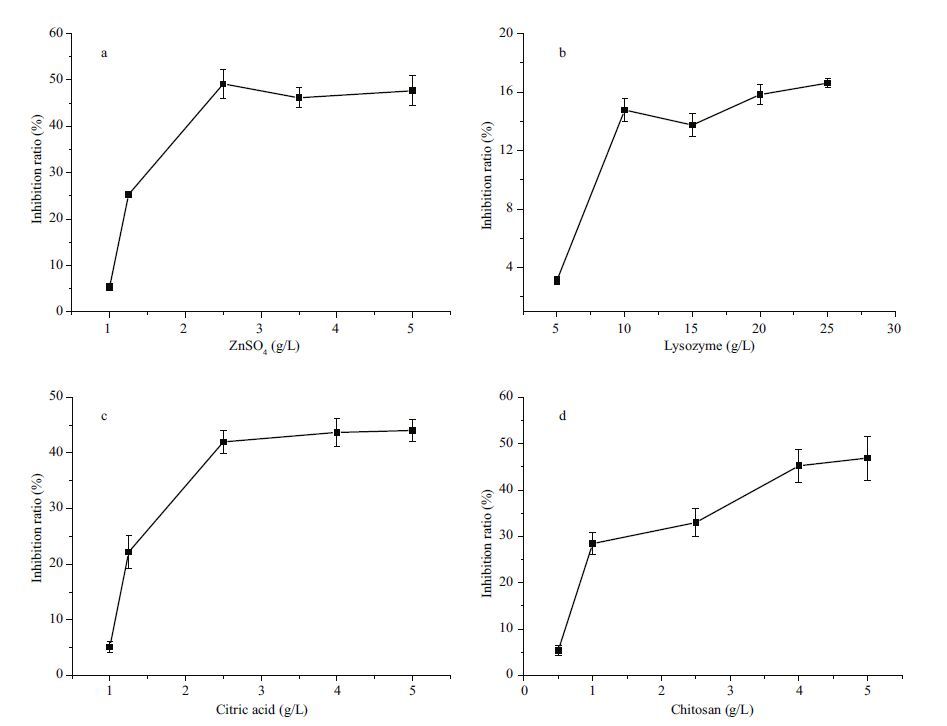
3 RESULT 3.1 The effect of different concentrations of ZnSO4 on biofilm inhibition ratioZnSO4 as an essential element has been widely studied by many researchers. The biofilm inhibition ratio affected by ZnSO4 is shown in Fig. 1a. Figure 1a indicated that the biofilm inhibition ratio increased with increasing concentration of ZnSO4 and reached a peak value at 40%–50% when the concentration of ZnSO4 was 2.5–5 g/L, and no longer changed as the inhibition ratio proceeded. This suggests that the concentration of ZnSO4 of 2.5 g/L was sufficient to obtain the perfect biofilm inhibition ratio. Therefore, 2.5 g/L ZnSO4 was considered to be the optimal level of concentration in this assay.
|
| Figure 1 Effect of different concentrations of (a) ZnSO4 ;(b) lysozyme;(c) citric acid;(d) chitosan on the biofilm 中文注解 |
Lysozyme as an alkaline enzyme can hydrolyze the mucopolysaccharides of a microorganism. The inhibition ratio of biofilm increased when the concentration of lysozyme increased from 5 to 10 g/L. As shown in Fig. 1b, when the concentration of lysozyme increased from 10 to 25 g/L, the inhibition ratio of biofilm started to maintain a dynamic equilibrium and no longer changed when the concentration of lysozyme increased. Therefore, 10 g/L of lysozyme was adopted in the present study.
3.3 The effect of different concentrations of citric acid on biofilm inhibition ratioThe effect of different concentrations of citric acid on the inhibition ratio is shown in Fig. 1c. The inhibition ratio of citric acid continued to increase with the increase of citric acid concentration (1–4 g/L). However, after the point of 4 g/L, the inhibition ratio of citric acid remained unchanged with the increasing concentration of citric acid (4–5 g/L). Thus, 4 g/L was considered as the optimal value in the present work.
3.4 The effect of different concentrations of chitosan on biofilm inhibition ratioThe dynamic change of the inhibition ratio of chitosan is shown in Fig. 1d. As shown, the value of inhibition by chitosan rose very quickly, when the concentration increased from 0.5 to 1.25 g/L. When the concentration of chitosan continued to increase to the point of 4 g/L, the rate of increase of the inhibition ratio slowed down and reached a peak value at 4 g/L. There was no change observed in the range of 4–5 g/L. This indicated that the concentration of 4 g/L chitosan was the appropriate point that could be considered to be the optimal concentration level in this experiment.
3.5 The initial optimization of inhibition ratio using the orthogonal testAccording to the preliminary results, the inhibition ratio of biofilm using the four different antimicrobial agents with new levels were measured, and an L9(4 3) orthogonal test was used to design an orthogonal test. Table 2 displays the four control factors and their selected levels. Table 4 shows the results of the inhibition ratio.
The analysis of the orthogonal test results, mainly the k and R values, were calculated and are shown in Table 4. The inhibition ratio was found to be very much dependent on the composition of the four antimicrobial agents and their amounts. According to the R values, the factors which contributed to the inhibition ratio can be ranked as: B>D>C>A.
The influence of the antimicrobial agent amount to the inhibition ratio of biofilm are ranked as: A1 >A2 >A3, B2 > B3 > B1, C1 > C3 > C2 and D3 >D1 > D2 according to the k i value; thus, the preliminary optimal composition was obtained, namely 2.5 g/L ZnSO4, 12.5 g/L lysozyme, 4 g/L citric acid and 6 g/L chitosan. It was necessary to carry out a detailed investigation, so the optimal composition was used and the value of the inhibition ratio increased by about 10% compared with the result obtained before the optimization (mean value of the orthogonal result).
3.6 Further optimization of inhibition ratio of biofilm using RSMIn this test, there were a total of 30 runs. A center composite design with three levels for all the four factors, i.e. ZnSO4, lysozyme, citric acid and chitosan, were used for this purpose. The data were analyzed by multiple regression analysis using the software Design-Expert (Version 8.0.5.0, Stat-Ease Inc., Minneapolis, USA), and the following polynomial equation was derived to represent the inhibition ratio of the biofilm.
 (3)
(3)where Y is the predicted inhibition ratio of the biofilm and A, B, C and D are the coded values for ZnSO4, lysozyme, citric acid and chitosan, respectively.
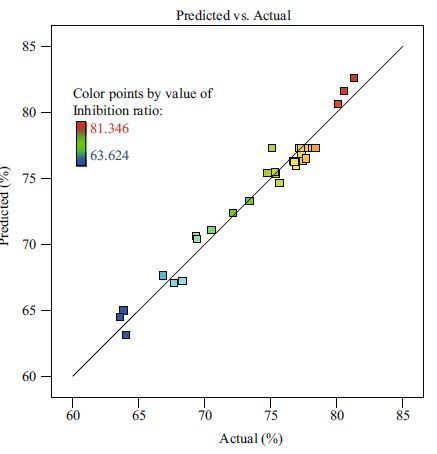
The statistical significance of the regression model was checked by the Fisher’s F -test, and the analysis of variance for the response surface quadratic model is shown in Table 5. The model was highly significant, as evident from the Fisher’s F -test with a very low probability value ([(P > F)=0.000 1]). Generally speaking, a regression model which has an R2 higher than 90% is considered to have a very high correlation (Guo et al., 2010; Jiang, 2010). At the same time, a relatively lower value of the coefficient of variation (CV=1.68%) and the value of the adjusted coefficient of determination (adj R2 =94.13%) indicated a better precision and reliability of the experiments carried out. In addition, the model presented a high coefficient of determination (R2 =96.97%) which explained about 97% of the variability in the response (Puri et al., 2002). The parity plot showed a satisfactory correlation between the experimental values and the predicted values (Fig. 2); the points cluster around the diagonal line which indicates a good fit of the model, because the deviation between the experimental and predicted values was less (Bandaru et al., 2006). All of the above indicated that the model was highly significant. The coefficient estimate and the corresponding P > F suggested that all the independent variables except ZnSO4 had a significant effect on the inhibition ratio of biofilm. The analysis also showed that there was significant interaction between lysozyme/chitosan and citric acid/chitosan.
|
| Figure 2 Parity plot showing the distribution of experimental values vs. predicted values of inhibition ratio of biofilm |
The optimum antimicrobial agent amounts, obtained by the quadratic model, for achieving the maximal inhibition ratio of biofilm was 2.16 g/L of ZnSO4, 14 g/L of lysozyme, 4.5 g/L of citric acid and 5 g/L of chitosan. The predicted optimal inhibition ratio corresponding to these values was 82.60%. To confirm the optimization results, the suggested optimal composition of antimicrobial agents was performed in triplicate. Under the suggested condition, the mean value of the inhibition ratio was found to be 84.49%, which was in agreement with the predicted value.
The 3D surface response plots were generated to show the response for any two independent variables in keeping the others at their central point. Response surface and contour plots of (a) ZnSO4 and lysozyme;(b) ZnSO4 and citric acid;(c) ZnSO4 and chitosan;(d) lysozyme and citric acid;(e) lysozyme and chitosan and (f) citric acid and chitosan are shown in Fig 6. As shown in Fig. 6a, the inhibition ratio of biofilm increased with the increasing concentration of lysozyme. However, when the concentration of ZnSO4 increased, the interaction of ZnSO4 and lysozyme declined. This phenomenon may be because a small amount of ZnSO4 can activate a particular part of lysozyme which can more effectively increase the inhibition ratio of lysozyme, but a large amount of ZnSO4 can inhibit the activity of this enzyme. Burguera-Pascu et al.(2007)also reported that low baseline levels of zinc could maintain the activity of enzyme in the saliva and in all oral hard and soft tissues. In addition, the coefficient of ZnSO4 with other factors was not significant in Fig. 3a, b, c. As shown in Figs. 3e, f, there was an interaction between lysozyme and citric acid, and citric acid and chitosan, and they were significant.

|
| Figure 3 Response surface for inhibition ratio of (a) ZnSO4 and lysozyme;(b) ZnSO4 and citric acid;(c) ZnSO4 and chitosan;(d) lysozyme and citric acid;(e) lysozyme and chitosan;(f) citric acid and chitosan |
agents on the forming biofilm To directly validate the effect of optimized antimicrobial agents on the formation of the biofilm, first, the electron microscope scan experiment was used to observe inhibition at different periods (6 h, 15 h, 18 h, 21 h, and 24 h) and the results are shown in Fig. 4. As shown in Fig. 4, large numbers of S . mutans began to link together and adhere on the glass microscope slide in the control group which resulted in the entire field of vision being covered by colonies. In addition, a three-dimensional network structure and the distribution of pores were formed at a certain period of 15–24 h (Fig. 4g, h, i, j) in the control group. As shown in Fig. 4b, c, d, e, it is clear that the optimized antimicrobial agents can effectively inhibit the forming biofilm compared with the control group.
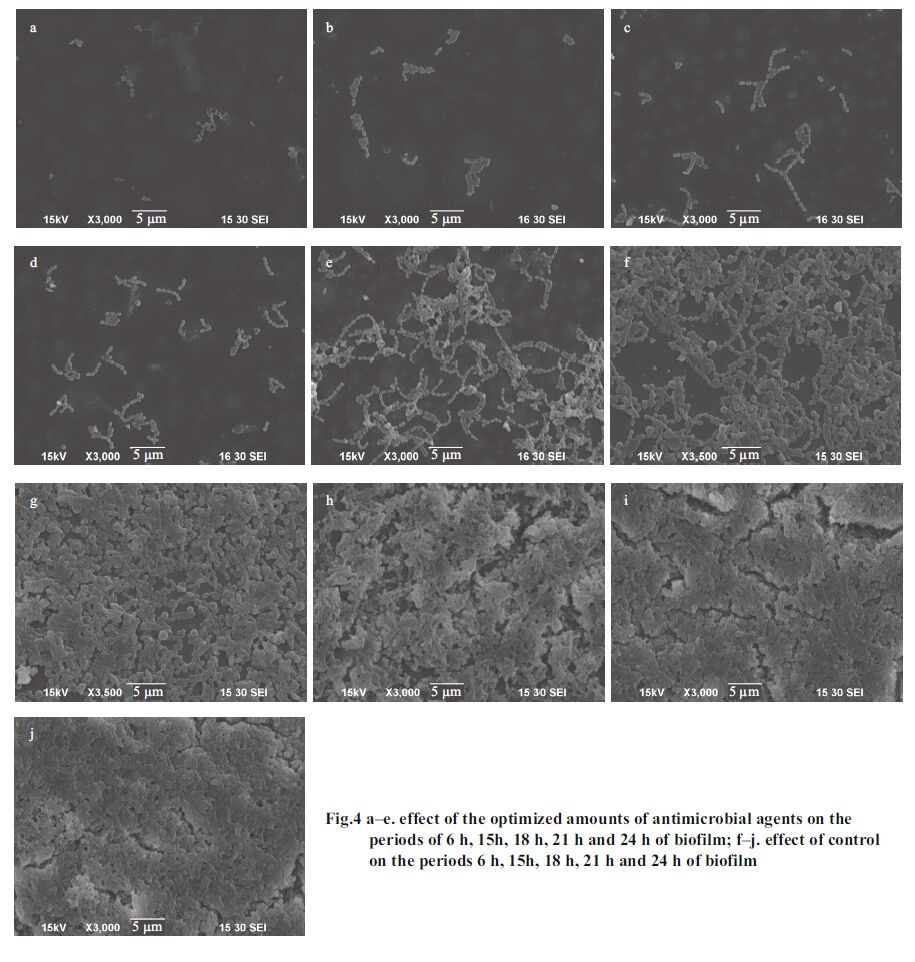
|
| Figure 4 a–e. effect of the optimized amounts of antimicrobial agents on the periods of 6 h, 15h, 18 h, 21 h and 24 h of biofilm; f–j. effect of control on the periods 6 h, 15h, 18 h, 21 h and 24 h of biofilm |
Second, the effect of the optimized amounts of antimicrobial agents on the forming biofilm was further observed by CLSM. In the current work, five groups of experiments:(1) control (only BHI with 1% sucrose);(2) marine Arthrobacter oxydans KQ11 dextranase mouthwash;(3) optimized amounts of antimicrobial agents;(4) marine Arthrobacter oxydans KQ11 dextranase mouthwash+optimized amounts of antimicrobial agents and (5) optimized amounts of antimicrobial agents+6 U/mL marine Arthrobacter oxydans KQ11 dextranase were studied by CLSM to observe the changes of the live/dead and thickness of the biofilm. After 24 h, the samples of the five groups were observed. As shown in Fig. 5, the biofilms were stained mostly green, but a few red cells were present in Fig. 5a & c, while the other groups containing the optimized amount of antimicrobial agents (Fig. 5b, d, e) were completely different, which indicated that the marine dextranase mouthwash does not have inhibitory activity. The thickness of biofilms of the groups with the optimized amounts of antimicrobial agents (optimized amounts of antimicrobial agents: 15 000 nm; marine Arthrobacter oxydans KQ11 dextranase mouthwash+optimized amounts of antimicrobial agents: 15 000 nm; optimized amounts of antimicrobial agents+6 U/mL marine Arthrobacter oxydans KQ11 dextranase: 6 000 nm) were significantly lower than the groups without any antimicrobial agents (control: 24 000 nm). All of the above indicated that the optimized amounts of antimicrobial agents were highly effective on the inhibition of the biofilm and the function of marine dextranase mouthwash could be increased significantly.
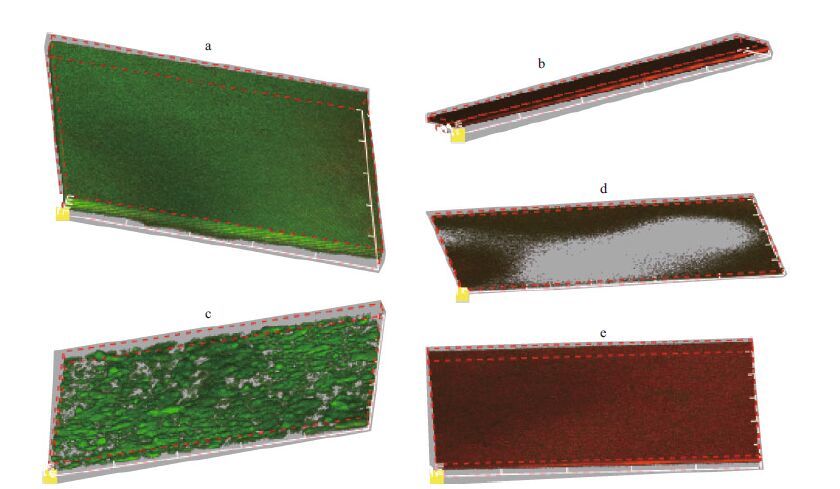
|
| Figure 5 Effect of different combination models on live/ dead and thickness of forming biofilm a–e: control, optimized amounts of antimicrobial agents; marine Arthrobacter oxydans KQ11 dextranase mouthwash; marine Arthrobacter oxydans KQ11 dextranase mouthwash + optimized amounts of antimicrobial agents; optimized amounts of antimicrobial agents + 6 U/mL marine Arthrobacter oxydans KQ11 dextranase. |
antimicrobial agents on the formed biofilm To further confirm the effectiveness of the optimized amounts of antimicrobial agents, in the current experiment, the control (BHI with 1% sucrose), the marine Arthrobacter oxydans KQ11 dextranase mouthwash, the optimized amounts of antimicrobial agents, marine Arthrobacter oxydans KQ11 dextranase mouthwash+the optimized amounts of antimicrobial agents and the optimized amount of antimicrobial agents+6 U/mL marine Arthrobacter oxydans KQ11 dextranase were used to treat the formed biofilms twice daily after they had been cultivated for 24 h, and continued for five days. The results are shown in Fig. 6; it is clear that the effect of the optimized amounts of antimicrobial agents on formed biofilm keeps the same pace with the forming biofilm which confirms the significance of the optimized amounts of antimicrobial agents and the function of marine Arthrobacter oxydans KQ11 dextranase mouthwash containing optimized amounts of antimicrobial agents.
Many reports have identified S . mutans as one of the main etiologic agent for dental caries. The ability to produce common extracellular matrices allows S . mutans to effectively colonize the tooth surface and contribute to the formation of cariogenic dental biofilms (Loesche, 1986; Koo et al., 2010). Thus, S . mutans as the major bacteria was selected to form cariogenic biofilms in our study.
Traditional methods of optimization involve changing one independent variable while fixing the others at a certain level. This single-dimensional search is laborious, time-consuming and incapable of reaching a true optimum because it does not allow for estimating interactions among experimental variables (Levin et al., 2008). RSM is a collection of statistical techniques for designing experiments, building models, evaluating the effects of factors and searching for the optimum condition (Desai et al., 2008). Using RSM, fewer experimental trials are needed as compared with studying one factor at a time. Also, significant interactions between the factors can be identified and quantified (Chen et al., 2009). RSM has been frequently used in medium optimization in microbiology (Chi and Zhao, 2003; Tang et al., 2004; Song et al., 2007; Jiang, 2010; Sansonetti et al., 2010). However, few reports have studied biofilms using RSM. Only Kumari and Sarkar (2014)used RSM to study biofilm formation in dairy chilling tanks and the optimization of clean-in-place. In the present study, RSM was used to optimize the amount of four different antimicrobial agents which could inhibit the growth of biofilm based on preliminary steps (single factor test and orthogonal test) and optimum antimicrobial agents (2.16 g/L of ZnSO4, 14 g/L of lysozyme, 4.5 g/L of citric acid and 5 g/L of chitosan), from which the desired inhibition ratio of biofilm was obtained. This is in accordance with Gu et al.(2012), who reported that mouthrinse with zinc at a concentration of 2.5 mmol/L or 5.0 mmol/L has significant antibacterial and plaque control effects on the outer and middle layers of the biofilm in vivo. From the results of the orthogonal test and RSM, it is worthwhile to mention that the influence of ZnSO4 when compared with the other factors was not so significant on the inhibition ratio of biofilm with increasing concentrations. However, lysozyme was the most important factor influencing the inhibition ratio of biofilm (P<0.000 1). As shown in Fig. 1b, when the lysozyme independently influenced the biofilm, the inhibition ratio of biofilm was very low. This phenomenon may be because a zinc ion can activate a particular part of lysozyme which can more effectively increase the inhibition ratio of lysozyme (He et al., 2002; Burguera-Pascu et al., 2007), but no significant inhibition of vitality was observed at high concentrations, which is in accordance with the report by Gu et al.(2012). Because of this reason, ZnSO4 continued to be used in the following experiment. The inhibition of citric acid agrees with a previous report that citric acid leads to dental cell deaths during an incubation period (Lan et al., 1999).
To confirm the optimization results, the qualitative analysis tests and quantitative analysis tests were used to analyze the effect of the results on biofilm using a 96-well plate, SEM and CLSM. We found that the inhibition ratio of the suggested optimal composition of antimicrobial agents was 84.49%, which was in agreement with the predicted value. In addition, the optimized amounts of antimicrobial agents were applied to the marine Arthrobacter oxydans KQ11 dextranase mouthwash (designed and developed by our laboratory), and the function of the antimicrobial agents against the forming biofilm and the formed biofilm was significantly confirmed. As shown in Fig. 5c, d, c, and Fig. 6d, it is noteworthy that the structure of the biofilms treated with marine Arthrobacter oxydans KQ11 dextranase mouthwash and marine Arthrobacter oxydans KQ11 dextranase mouthwash containing optimized amounts of antimicrobial agents was loose compared with the control, which agrees with our previous report that marine dextranase loosens the biofilm structure (Jiao et al., 2014).
| Abdullah A Z, Straff ord S M, Brookes S J, Duggal M S, 2006. The effect of copper on demineralization of dental enamel. J. Dent. Res., 85 (11) : 1011 –1015. Doi: 10.1177/154405910608501107 |
| Aires C P, Tenuta L M, Carbonero E R, Sassaki G L, Iacomini M, Cury J A, 2011. Structural characterization of exopolysaccharides from biofilm of a cariogenic streptococci. Carbohydrate Polymers, 84 (4) : 1215 –1220. Doi: 10.1016/j.carbpol.2010.12.076 |
| Almeida C, Azevedo N F, Santos S, Keevil C W, Vieira M J, 2011. Discriminating multi-species populations in biofilms with peptide nucleic acid fluorescence in situ hybridization(PNA FISH). PLoS One, 6 (3) : e14786 . Doi: 10.1371/journal.pone.0014786 |
| Bae K, Jun E J, Lee S M, Paik D I, Kim J B, 2006. Effect of water-soluble reduced chitosan on Streptococcus mutans, plaque regrowth and biofilm vitality. Clinical Oral Investigations, 10 (2) : 102 –107. Doi: 10.1007/s00784-006-0038-3 |
| Bae K, Oh H, 1990. Synergistic effect of lysozyme on bactericidal activity of magnolol and honokiol against a cariogenic bacterium, Streptococcus mutans OMZ 176. Archives of Pharmacal Research, 13 (1) : 117 –119. Doi: 10.1007/BF02857847 |
| Bandaru V V R, Somalanka S R, Mendu D R, Madicherla N R, Chityala A, 2006. Optimization of fermentation conditions for the production of ethanol from sago starch by coimmobilized amyloglucosidase and cells of Zymomonas mobilis using response surface methodology. Enzyme and Microbial Technology, 38 (1-2) : 209 –214. Doi: 10.1016/j.enzmictec.2005.06.002 |
| Burguera-Pascu M, Rodríguez-Archilla A, Baca P, 2007. Substantivity of zinc salts used as rinsing solutions and their effect on the inhibition of Streptococcus mutans. Journal of Trace Elements in Medicine and Biology, 21 (2) : 92 –101. Doi: 10.1016/j.jtemb.2006.12.003 |
| Chan C P, Jeng J H, Hsieh C C, Lin C L, Lei D, Chang M C, 1999. Morphological alterations associated with the cytotoxic and cytostatic effects of citric acid on cultured human dental pulp cells. Journal of Endodontics, 25 (5) : 354 –358. Doi: 10.1016/S0099-2399(06)81171-4 |
| Chen L, Zhou X S, Fan W M, Zhang Y X, 2008. Expression, purification and characterization of a recombinant Lipomyces starkey dextranase in Pichia pastoris. Protein Expression and Purification, 58 (1) : 87 –93. Doi: 10.1016/j.pep.2007.10.021 |
| Chen X C, Bai J X, Cao J M, Li Z J, Xiong J, Zhang L, Hong Y, Ying H J, 2009. Medium optimization for the production of cyclic adenosine 3', 5'-monophosphate by Microbacterium sp. no. 205 using response surface methodology. Bioresource Technology, 100 (2) : 919 –924. |
| Chen X, Tang Q L, Zhu Y J, Zhu C L, Feng X P, 2012. Synthesis and antibacterial property of zinc loaded hydroxyapatite nanorods. Materials Letters, 89 : 233 –235. Doi: 10.1016/j.matlet.2012.08.115 |
| Cheng L, Zhang K, Melo M A S, Weir M D, Zhou X, Xu H H K, 2012. Anti-biofilm dentin primer with quaternary ammonium and silver nanoparticles. Journal of Dental Research, 91 (6) : 598 –604. Doi: 10.1177/0022034512444128 |
| Chi Z M, Zhao S Z, 2003. Optimization of medium and cultivation conditions for pullulan production by a new pullulan-producing yeast strain. Enzyme and Microbial Technology, 33 (2-3) : 206 –211. Doi: 10.1016/S0141-0229(03)00119-4 |
| Costa E M, Silva S, Tavaria F K, Pintado M M, 2013. Study of the effects of chitosan upon Streptococcus mutans adherence and biofilm formation. Anaerobe, 20 : 27 –31. Doi: 10.1016/j.anaerobe.2013.02.002 |
| Deng D M, ten Cate J M, 2004. Demineralization of dentin by Streptococcus mutans biofilms grown in the constant depth film fermentor. Caries Research, 38 (1) : 54 –61. |
| Desai K M, Survase S A, Saudagar P S, Lele S S, Singhal R S, 2008. Comparison of artificial neural network(ANN) and response surface methodology(RSM) in fermentation media optimization:case study of fermentative production of scleroglucan. Biochemical Engineering Journal, 41 (3) : 266 –273. Doi: 10.1016/j.bej.2008.05.009 |
| Dong L P, Tong Z C, Linghu D, Lin Y, Tao R, Liu J, Tian Y, Ni L X, 2012. Effects of sub-minimum inhibitory concentrations of antimicrobial agents on Streptococcus mutans biofilm formation. International Journal of Antimicrobial Agents, 39 (5) : 390 –395. Doi: 10.1016/j.ijantimicag.2012.01.009 |
| Eggleston G, Monge A, 2005. Optimization of sugarcane factory application of commercial dextranases. Process Biochemistry, 40 (5) : 1881 –1894. Doi: 10.1016/j.procbio.2004.06.025 |
| Giertsen E, 2004. Effects of mouthrinses with triclosan, zinc ions, copolymer, and sodium lauryl sulphate combined with fluoride on acid formation by dental plaque in vivo. Caries Research, 38 (5) : 430 –435. Doi: 10.1159/000079623 |
| Gu H J, Fan D N, Gao J L, Zou W, Peng Z X, Zhao Z M, Ling J Q, LeGeros R Z, 2012. Effect of ZnCl2 on plaque growth and biofilm vitality. Archives of Oral Biology, 57 (4) : 369 –375. Doi: 10.1016/j.archoralbio.2011.10.001 |
| Guo X, Zou X, Sun M, 2010. Optimization of extraction process by response surface methodology and preliminary characterization of polysaccharides from Phellinus igniarius. Carbohydrate Polymers, 80 (2) : 344 –349. Doi: 10.1016/j.carbpol.2009.11.028 |
| Hatada Y, Hidaka Y, Nogi Y, Uchimura K, Katayama K, Li Z, Akita M, Ohta Y, Goda S, Ito H, Matsui H, Ito S, Horikoshi K, 2004. Hyper-production of an isomalto-dextranase of an Arthrobacter sp. by a proteases-deficient Bacillus subtilis:sequencing, properties, and crystallization of the recombinant enzyme. Applied Microbiology and Biotechnology, 65 (5) : 583 –592. |
| He G, Pearce E I F, Sissons C H, 2002. Inhibitory effect of ZnCl2 on glycolysis in human oral microbes. Archives of Oral Biology, 47 (2) : 117 –129. Doi: 10.1016/S0003-9969(01)00093-0 |
| Helms J A, Della-Fera M A, Mott A E, Frank M E, 1995. Effects of chlorhexidine on human taste perception. Archives of Oral Biology, 40 (10) : 913 –920. Doi: 10.1016/0003-9969(95)00062-T |
| Hu D Y, Li X, Sreenivasan P K, DeVizio W, 2009. A randomized, double-blind clinical study to assess the antimicrobial effects of a cetylpyridinium chloride mouth rinse on dental plaque bacteria. Clinical Therapeutics, 31 (11) : 2540 –2548. Doi: 10.1016/j.clinthera.2009.11.004 |
| Islam B, Khan S N, Naeem A, Sharma V, Khan A U, 2009. Novel effect of plant lectins on the inhibition of Streptococcus mutans biofilm formation on saliva-coated surface. Journal of Applied Microbiology, 106 (5) : 1682 –1689. Doi: 10.1111/jam.2009.106.issue-5 |
| Jamilian A, Ghasemi M, Gholami D, Kaveh B, 2008. Clinical effects of 2% chlorhexidine gel on patients undergoing orthodontic treatment. Orthodontic Waves, 67 (4) : 162 –166. Doi: 10.1016/j.odw.2008.07.001 |
| Jiang L F, 2010. Optimization of fermentation conditions for pullulan production by Aureobasidium pullulan using response surface methodology. Carbohydrate Polymers, 79 (2) : 414 –417. Doi: 10.1016/j.carbpol.2009.08.027 |
| Jiao Y L, Wang S J, Lv M S, Jiao B H, Li W J, Fang Y W, Liu S, 2014. Characterization of a marine-derived dextranase and its application to the prevention of dental caries. Journal of Industrial Microbiology & Biotechnology, 41 (1) : 17 –26. |
| Khalikova E, Susi P, Korpela T, 2005. Microbial dextranhydrolyzing enzymes:fundamentals and applications. Microbiology and Molecular Biology Reviews, 69 (2) : 306 –325. Doi: 10.1128/MMBR.69.2.306-325.2005 |
| Kockisch S, Rees G D, Tsibouklis J T, Smart J D, 2005. Mucoadhesive, triclosan-loaded polymer microspheres for application to the oral cavity:preparation and controlled release characteristics. European Journal of Pharmaceutics and Biopharmaceutics, 59 (1) : 207 –216. Doi: 10.1016/j.ejpb.2004.07.007 |
| Kong M, Chen X G, Xing K, Park H J, 2010. Antimicrobial properties of chitosan and mode of action:a state of the art review. International Journal of Food Microbiology, 144 (1) : 51 –63. Doi: 10.1016/j.ijfoodmicro.2010.09.012 |
| Koo H, Xiao J, Klein M I, Jeon J G, 2010. Exopolysaccharides produced by Streptococcus mutans glucosyltransferases modulate the establishment of microcolonies within multispecies biofilms. Journal of Bacteriology, 192 (12) : 3024 –3032. Doi: 10.1128/JB.01649-09 |
| Kumari S, Sarkar P K, 2014. In vitro model study for biofilm formation by Bacillus cereus in dairy chilling tanks and optimization of clean-in-place(CIP) regimes using response surface methodology. Food Control, 36 (1) : 153 –158. Doi: 10.1016/j.foodcont.2013.08.014 |
| Lan W C, Lan W H, Chan C P, Hsieh C C, Chang M C, Jeng J H, 1999. The effects of extracellular citric acid acidosis on the viability, cellular adhesion capacity and protein synthesis of cultured human gingival fibroblasts. Australian Dental Journal, 44 (2) : 123 –130. Doi: 10.1111/j.1834-7819.1999.tb00213.x |
| Levin L, Herrmann C, Papinutti V L, 2008. Optimization of lignocellulolytic enzyme production by the white-rot fungus Trametes trogii in solid-state fermentation using response surface methodology. Biochemical Engineering Journal, 39 (1) : 207 –214. Doi: 10.1016/j.bej.2007.09.004 |
| Li W, Li X Y, Wang Q, Pan Y J, Wang T, Wang H Q, Song R, Deng H B, 2014b. Antibacterial activity of nanofibrous mats coated with lysozyme-layered silicate composites via electrospraying. Carbohydrate Polymers, 99 : 218 –225. Doi: 10.1016/j.carbpol.2013.07.055 |
| Loesche W J, 1986. Role of Streptococcus mutans in human dental decay. Microbiol. Rev., 50 (4) : 353 –380. |
| Ma H Y, Darmawan E T, Zhang M, Zhang L, Bryers J D, 2013. Development of a poly(ether urethane) system for the controlled release of two novel anti-biofilm agents based on gallium or zinc and its efficacy to prevent bacterial biofilm formation. Journal of Controlled Release, 172 (3) : 1035 –1044. Doi: 10.1016/j.jconrel.2013.10.005 |
| Marotta M, Martino A, De Rosa A, Farina E, Cartenì M, De Rosa M, 2002. Degradation of dental plaque glucans and prevention of glucan formation using commercial enzymes. Process Biochemistry, 38 (1) : 101 –108. Doi: 10.1016/S0032-9592(02)00058-4 |
| Medlicott N J, Holborow D W, Rathbone M J, Jones D S, Tucker I G, 1999. Local delivery of chlorhexidine using a tooth-bonded delivery system. Journal of Controlled Release, 61 (3) : 337 –343. Doi: 10.1016/S0168-3659(99)00152-2 |
| Mello S V, Arvanitidou E, Stranick M A, Santana R, Kutes Y, Huey B, 2013. Mode of action studies of a new desensitizing mouthwash containing 0. 8% arginine, PVM/MA copolymer, pyrophosphates, and 0.05% sodium fluoride. Journal of Dentistry, 41 : S12 –S19. |
| Muhamad M H, Abdullah S R S, Mohamad A B, Rahman R A, Kadhum A A H, 2013. Application of response surface methodology(RSM) for optimisation of COD, NH3-N and 2, 4-DCP removal from recycled paper wastewater in a pilot-scale granular activated carbon sequencing batch biofilm reactor(GAC-SBBR). Journal of Environmental Management, 121 : 179 –190. Doi: 10.1016/j.jenvman.2013.02.016 |
| Pahwa N, Kumar A, Gupta S, 2011. Short term clinical effectiveness of a 0. 07% cetylpyridinium chloride mouth rinse in patients undergoing fixed orthodontic appliance treatment. The Saudi Dental Journal, 23 (3) : 135 –141. |
| Pellegrini A, Thomas U, Bramaz N, Klauser S, Hunziker P, Von Fellenberg R, 1997. Identification and isolation of a bactericidal domain in chicken egg white lysozyme. Journal of Applied Microbiology, 82 (3) : 372 –378. Doi: 10.1046/j.1365-2672.1997.00372.x |
| Prigent-Combaret C, Prensier G, Le Thi T T, Vidal O, Lejeune P, Dorel C, 2000. Developmental pathway for biofilm formation in curli-producing Escherichia coli strains:role of flagella, curli and colanic acid. Environmental Microbiology, 2 (4) : 450 –464. Doi: 10.1046/j.1462-2920.2000.00128.x |
| Puri S, Beg Q K, Gupta R, 2002. Optimization of alkaline protease production from Bacillus sp. by response surface methodology. Current Microbiology, 44 (4) : 286 –290. |
| Radford J R, Beighton D, Nugent Z, Jackson R J, 1997. Effect of use of 0. 05% cetylpyridinium chloride mouthwash on normal oral flora. Journal of Dentistry, 25 (1) : 35 –40. |
| Samot J, Lebreton J, Badet C, 2011. Adherence capacities of oral lactobacilli for potential probiotic purposes. Anaerobe, 17 (2) : 69 –72. Doi: 10.1016/j.anaerobe.2011.04.001 |
| Sansonetti S, Curcio S, Calabrò V, Iorio G, 2010. Optimization of ricotta cheese whey(RCW) fermentation by response surface methodology. Bioresource Technology, 101 (23) : 9156 –9162. Doi: 10.1016/j.biortech.2010.07.030 |
| Sarwar A, Katas H, Zin N M, 2014. Antibacterial effects of chitosan-tripolyphosphate nanoparticles:impact of particle size molecular weight. Journal of Nanoparticle Research, 16 : 2517 . Doi: 10.1007/s11051-014-2517-9 |
| Schaeken M J M, Van Der Hoeven J S, Saxton C A, Cummins D, 1996. The effect of mouthrinses containing zinc and triclosan on plaque accumulation, development of gingivitis and formation of calculus in a 28-week clinical test. Journal of Clinical Periodontology, 23 (5) : 465 –470. Doi: 10.1111/cpe.1996.23.issue-5 |
| Song X J, Zhang X C, Kuang C H, Zhu L Y, Guo N, 2007. Optimization of fermentation parameters for the biomass and DHA production of Schizochytrium limacinum OUC88 using response surface methodology. Process Biochemistry, 42 (10) : 1391 –1397. Doi: 10.1016/j.procbio.2007.07.014 |
| Stepanović S, Vuković D, Dakić I, Savić B, Švabić-Vlahović M, 2000. A modified microtiter-plate test for quantification of staphylococcal biofilm formation. Journal of Microbiological Methods, 40 (2) : 175 –179. Doi: 10.1016/S0167-7012(00)00122-6 |
| Tang X J, He G Q, Chen Q H, Zhang X Y, Ali M A M, 2004. Medium optimization for the production of thermal stable beta-glucanase by Bacillus subtilis ZJF-1A5 using response surface methodology. Bioresource Technology, 93 (2) : 175 –181. Doi: 10.1016/j.biortech.2003.10.013 |
| Totiam P, González-Cabezas C, Fontana M R, Zero D T, 2007. A new in vitro model to study the relationship of gap size and secondary caries. Caries Research, 41 (6) : 467 –473. Doi: 10.1159/000107934 |
| Vergara-Irigaray M, Maira-Litrán T, Merino N, Pier G B, Penadés J R, Lasa I, 2008. Wall teichoic acids are dispensable for anchoring the PNAG exopolysaccharide to the Staphylococcus aureus cell surface. Microbiology, 154 (3) : 865 –877. Doi: 10.1099/mic.0.2007/013292-0 |
| Wang D L, Lu M S, Wang S J, Jiao Y L, Li W J, Zhu Q, Liu Z P, 2014. Purification and characterization of a novel marine Arthrobacter oxydans KQ11 dextranase. Carbohydrate Polymers, 106 : 71 –76. Doi: 10.1016/j.carbpol.2014.01.102 |
| Wu W S, Chen C C, Chuang Y C, Su B A, Chiu Y H, Hsu H J, Ko W C, Tang H J, 2013. Efficacy of combination oral antimicrobial agents against biofilm-embedded methicillin-resistant Staphylococcus aureus. Journal of Microbiology, Immunology and Infection, 46 (2) : 89 –95. Doi: 10.1016/j.jmii.2012.03.009 |
| Yılmaz S, Ünal F, Yüzbaşıoğlu D, Aksoy H, 2008. Clastogenic effects of food additive citric acid in human peripheral lymphocytes. Cytotechnology, 56 (2) : 137 –144. Doi: 10.1007/s10616-008-9137-0 |
 2016, Vol. 34
2016, Vol. 34


